Compared with “healthy” vessels, the extracellular matrix of atheromatous plaques includes increased content of type-EIIIA-domain–containing fibronectin. Apolipoprotein E-null mice engineered to produce fibronectin lacking this alternatively spliced exon exhibit reduced atherosclerosis. Diminished hypercholesterolemia and reduced macrophage foam cell formation may contribute to this phenotype.
Variations in the molecular composition of the extracellular matrix (ECM) play important roles during vascular remodeling in pathophysiologic processes (ie, vasculogenesis and angiogenesis, wound healing, and vascular obstructive lesion formation).1 Fibronectin is a ligand for fibrin, heparin, collagen, and several integrins implicated in the recruitment of blood leukocytes to the arterial endothelium.2 While several splice-variant forms of fibronectin have been identified, the 2 major forms are plasma fibronectin (pFN), which is produced by hepatocytes, and cellular fibronectin (cFN), which is produced locally by different cell types and deposited and assembled into the ECM.2 A single gene encodes for distinct fibronectin moieties depending on alternative RNA splicing of exons encoding for the V/CS-1 segment (also known as the V exon), extra type III repeat segments (known as ED-A and ED-B in humans, or EIIIA and EIIIB in rodents), and the (V + C) region. pFN lacks extra type III repeat segments, whereas cFN contains variable amounts of these alternative domains.
Although a causal link between fibronectin and cardiovascular pathobiology has been difficult to establish because null mutations for fibronectin cause embryonic lethality,3 evidence exists implicating fibronectin in cardiovascular pathobiology2,4,5 : (1) EIIIA-positive fibronectin (EIIIA-FN) up-regulation is associated with clinical and experimental hypertension; (2) expression of EIIIA-FN, EIIIB-FN, and fibronectin lacking both alternative exons is rapidly induced after myocardial infarction in both humans and animal models; (3) EIIIA-FN and EIIIB-FN forms, which are not normally present in “healthy” vessels, are induced in neointimal lesions; (4) elevated plasma levels of circulating cFN have been described in clinical syndromes with vascular damage; and (5) in vivo studies using blocking antibodies have implicated the alternative fibronectin V/CS-1 segment in leukocyte recruitment during murine atherosclerosis.
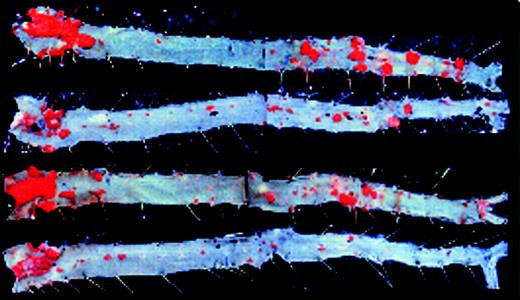
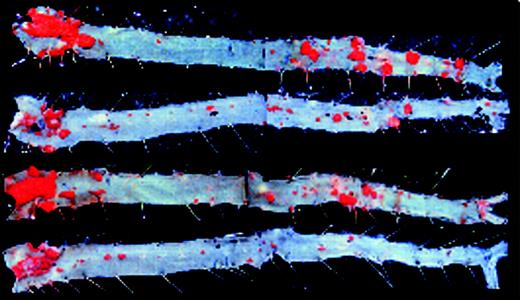
Recently, analysis of fibronectin conditional knock-out mice revealed a role of pFN in thrombus initiation, growth, and stability.6 Now, Tan and colleagues (page 11) directly test the function of EIIIA-FN in atherosclerosis by engineering EIIIA-null (EIIIA–/–) mice lacking the EIIIA exon. They crossed EIIIA–/– mice with atherosclerosis-prone apolipoprotein E–null (ApoE–/–) mice and found as much as a 67% reduction in aortic atherosclerosis in doubly deficient fat-fed EIIIA–/–ApoE–/– mice compared with ApoE–/– controls. An intriguing observation that deserves further examination is that EIIIA–/–ApoE–/– females display significant protection at all time points assayed (8, 12, and 16 weeks of fat feeding), while males are protected only after 16 weeks. Compared with ApoE–/– controls, both male and female EIIIA–/–ApoE–/– mice displayed diminished total plasma cholesterol levels, a reduction that is specific to the very low density lipoprotein fraction. Increased EIIIA-FN expression was found in both the plasma and in endothelial cells and macrophages within atherosclerotic lesions of ApoE–/– mice. Moreover, in vitro foam cell formation by ApoE–/– macrophages was associated with increased EIIIA-FN mRNA expression, and lipid accumulation in EIIIA–/–ApoE–/– macrophages was reduced by 31% compared with ApoE–/– controls.FIG1
EIIIA–/–ApoE–/– mice are protected from atherosclerosis. See the complete figure in the article beginning on page 11.
EIIIA–/–ApoE–/– mice are protected from atherosclerosis. See the complete figure in the article beginning on page 11.
Collectively, the study by Tan and colleagues convincingly demonstrates an atherogenic role of EIIIA-FN and suggests that this form of fibronectin is functional in both plasma lipoprotein metabolism and in macrophage foam cell formation. Future studies are warranted to elucidate additional systemic mechanisms and processes within the vessel wall by which EIIIA-FN may contribute to atherosclerosis. For example, the possibility that EIIIA-FN may interact with specific lipoprotein fractions and may affect leukocyte recruitment should be investigated. Moreover, because endothelial and smooth muscle cells cultured on different ECM components display significant differences in proliferation, migration, and apoptosis, comparing the phenotypic properties of vascular cells cultured on EIIIA-FN, EIIIB-FN, and fibronectin lacking both EIII segments may shed significant insight into the role of distinct variants of fibronectin in atherosclerosis.