This report provides new evidence that accelerated apoptosis of neutrophils and their precursors is an important mechanism for neutropenia in systemic lupus erythematosus.
In this issue of Blood, Matsuyama and colleagues (page 184) report a novel mechanism for the pathogenesis of neutropenia in systemic lupus erythematosus (SLE). SLE is a relatively common disorder with a prevalence of 50 cases per 100 000 population and a female-male ratio of approximately 10:1. Neutropenia, defined as a blood neutrophil count less than 1.8 × 109/L, occurs in about 50% of individuals with SLE. Although usually mild (ie, 1.0-1.8 × 109/L), SLE-related neutropenia is considered to be an important factor predisposing these individuals to bacterial infections. Several mechanisms have been identified for the pathogenesis of neutropenia in SLE. Approximately two thirds of patients have antineutrophil autoantibodies (usually immunoglobulin G [IgG]).1 Neutropenia is more common in individuals with either anti-Ro autoantibodies.2 Several previous studies have implicated Fas (CD95)–mediated apoptosis of circulating neutrophils, monocytes, and lymphocytes of patients with SLE.3,4 Marrow hypoplasia and Fas-mediated apoptosis of CD34+ hematopoietic progenitor cells are additional factors contributing to SLE-associated cytopenias.5 FIG1
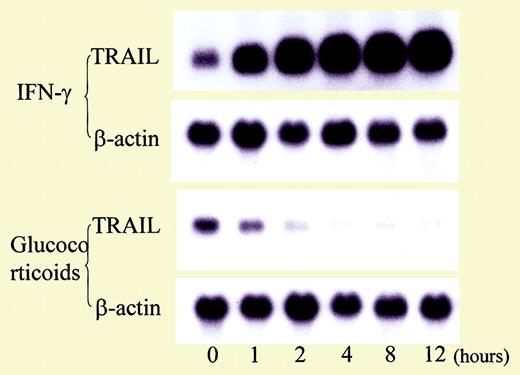
Influence of cytokines and glucocorticoid on TRAIL expression in T cells. See the complete figure in the article beginning on page 184.
Influence of cytokines and glucocorticoid on TRAIL expression in T cells. See the complete figure in the article beginning on page 184.
The report by Matsuyama et al provides further insight into the pathogenesis of neutropenia in SLE by describing abnormalities in another mediator of apoptosis, tumor necrosis factor–related apoptosis-inducing ligand (TRAIL). In this study involving 28 patients with SLE and 8 healthy controls, serum TRAIL levels were inversely proportional to blood neutrophil counts. Expression of TRAIL receptor 3, a decoy receptor for TRAIL, also was lower in neutropenic patients than in patients or controls without neutropenia. In vitro, TRAIL induced apoptosis in neutrophils. Corticosteroid therapy reduced expression of TRAIL on T cells and enhanced expression of Fas-associating protein with death domain–like interleukin-1β–converting enzyme (FLICE)–inhibitory protein (FLIP), an antiapoptotic protein, by neutrophils. TRAIL may also play an important role in immunoregulation, and dysregulation of TRAIL or its cognate receptor may contribute to the development of systemic autoimmune diseases, such as SLE.6 For example, mice deficient in TRAIL have been previously shown to be susceptible to the development of accelerated autoimmune disease.
This study by Matsuyama et al also adds to the growing evidence that neutropenia in SLE is chiefly attributable to accelerated apoptosis of mature neutrophils. It suggests to us that careful studies of the pathogenic mechanisms responsible for the cytopenias associated with SLE may provide the basis for development of novel and effective therapeutic approaches for clinical management of this severe autoimmune disorder.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal