Abstract
Regulatory T cells participate in immunologic homeostasis by active suppression of inappropriate immune responses. Regulatory T lymphocytes expressing CD4 and CD25 antigens and naturally present in the peripheral blood were the first to be phenotypically characterized. However, their small number and antigen nonspecific suppression has prompted efforts to identify and dissect antigen-specific regulatory T cells. In this review we discuss how antigen-specific regulatory T cells can be identified, the cellular and molecular mechanisms underlying their induction and activity, and the challenges facing their potential clinical application.
Introduction
It is axiomatic that the immune system, with its remarkable ability to respond to antigenic stimuli, must possess equally potent mechanisms to regulate the form, extent, and duration of this reaction. The ability to exploit such regulatory mechanisms would afford novel therapeutic opportunities in autoimmune disorders, allergies, and transplantation. We are only now beginning to understand the complexity of the control methods available to the immune system, and it is the purpose of this review to discuss a method of increasing interest, namely antigen inducible (as opposed to naturally occurring) regulatory T lymphocytes.
Regulatory (“suppressor”) T cells were first described in 1971 by Gershon and Kondo1 who demonstrated their ability to transfer antigen-specific tolerance to naive animals. This concept rapidly lost credibility2 because of 3 serious problems. First, it was impossible to identify specific surface markers associated with suppressor T cells. Second, when methods for studying T-cell receptor (TCR) genes became available, suppressor T cells did not appear to have functional gene rearrangements. Finally—and most damagingly—suppressor T cells were said to be governed in their activity by the murine I-J locus, which was hypothesized to code for soluble suppressor factors. Conventional mouse genetics mapped this I-J gene within the I complex of the major histocompatibility complex (MHC) region. Embarrassingly, when hybrid DNA technology was used to analyze this locus in the early 1980s, it showed that the putative I-J region simply did not exist. Although many investigators subsequently dismissed the entire concept of suppression as an artifact of the complex and poorly reproducible biologic assays used to demonstrate its presence, several experimental observations remained difficult to interpret without postulating some form of active down-regulation of the immune response.3
Natural versus inducible regulatory T cells
In 1995, a phenotypic description of one class of regulatory T cells finally became available. Sakaguchi et al4 showed that when CD4+ T cells from normal Balb/C mice were depleted of the fraction expressing the CD25+ (interleukin-2 receptor [IL2R] α-chain) marker and injected into Balb/C athymic nude mice, all recipients developed multiple autoimmune diseases. In addition, the deleterious effects of CD4+CD25– T cells could be prevented by the coadministration of CD4+CD25+ T cells. Since then, CD4+CD25+ T cells, which occur naturally in peripheral blood and originate from the thymus,5 have been the subject of intense scrutiny and have been shown to contribute to peripheral self-tolerance in rodents6-9 and humans.10-13 Their ability to actively transfer unresponsiveness in vitro and in vivo distinguishes them from other mechanisms of peripheral tolerance including T-cell anergy,14 T-cell depletion,15 and immunologic ignorance.16 Their characteristics include a low proliferative capacity after allogeneic or polyclonal stimulation, an inhibition of CD4+CD25–10,11 and CD8+17 immune responses in a cell-to-cell contact and dose-dependent manner. They also express higher levels of CD45RO and cytotoxic T-lymphocyte antigen-4 (CTLA-4) than do CD4+CD25– T cells. In further characterization of the phenotype and function of these cells, Shimizu et al18 and McHugh et al19 demonstrated the surface expression of the glucocorticoid-induced tumor necrosis factor (TNF) receptor, which appears to be implicated in regulatory function. While these CD4+CD25+ T cells evidently have the potential for therapeutic use, several limitations have precluded their successful exploitation. Only small numbers of cells can be obtained, and their proliferative potential appears limited. Moreover, only a minority of CD4+CD25+ T lymphocytes have suppressive activity20 and this function is antigen nonspecific,21 since these cells fail to interact with antigen-presenting cells (APCs) and hence cannot recognize MHC-bound peptides. Instead, they inhibit T-cell responses by T-cell–to–T-cell interaction.22 This lack of specificity reduces their therapeutic potential in humans. These shortcomings have prompted an effort to discover mechanisms able to generate so-called inducible regulatory T cells (Trs). A description of these antigen-induced and antigen-specific Trs, the processes involved in their induction, the evidence for their antigen specificity, and the mechanisms by which they induce suppression are the subjects of the remainder of this review.
Some preliminary definitions
There is as yet no uniformly agreed upon definition or terminology for inducible regulatory T cells, which have been described as having either suppressor or regulatory functions. “Suppressor” is the historical term, first used in 1971 by Gershon and Kondo1 , but the more-recent term “regulatory” is generally preferred because of the years of skepticism surrounding I-J–regulated suppressor T cells. The fundamental property defining a Tr is its ability to transfer immune unresponsiveness in vivo from one animal to another, or in vitro from one culture to another. The relationship of surrogate measures (such as phenotype and patterns of cytokine production) to the active transfer of suppression remains uncertain. The transfer of unresponsiveness is commonly assessed in vitro by the inhibition of proliferation or cytotoxicity. In vivo, the end points are inhibition of autoimmune phenomena, allergic reactions, or allograft rejection, characterized by dose dependence and antigen specificity. Some investigators further separate T regulatory cells into subsets according to the cytokines they produce. Tr1s secrete IL10 and only small amounts of IL2, IL4, and transforming growth factor β (TGFβ), in contrast to T helper 3 (Th3) regulatory cells, which secrete high levels of TGFβ but only small amounts of IL10 and IL4. Whether these distinctions represent developmental or functional differences, or both, remains unclear. The antigen-specific responses of Trs have been termed cell-contact dependent, as they require physical contact between the Trs and APCs and the responding T cells. However, some systems have been reported to be cell-contact independent and appear to rely on soluble factors.
Unlike naturally occurring antigen nonspecific Trs, inducible and antigen-specific Trs have not yet been assigned a surface phenotype based solely on CD4/CD8 or CD25 and CD28 molecules. Instead, the regulatory activity may be distributed among multiple cell fractions including those defined as CD4+CD25+,23,24 CD4+CD25–,25,26 CD8+,27,28 CD8+CD28–,29 or even CD4–CD8–CD25+CD28–.30 This heterogeneity likely reflects both multiple routes of induction and disparate physiologic functions.
Mechanisms by which antigen-specific regulatory T cells are induced
Mucosal (oral, tracheal, nasal) or cutaneous delivery of the antigen
Oral delivery of antigens is a well-established mechanism of tolerance induction, whose physiologic purpose likely relates to prevention of harmful immune responses to food proteins and bacterial antigens present in the mucosal flora.31-33 Low doses of oral antigens induce Trs, whereas high doses result in clonal anergy or deletion.31,32 In several murine models of autoimmune disease, administration of the self-antigen by the oral route induces antigen-specific Trs (of the Th3 subgroup) that are CD4+ or CD8+.31,34,35 While these Th3 cells secrete high levels of TGFβ, the contribution of this cytokine to the suppression observed is unclear. Administration of human insulin either orally36 or by aerosol37 to nonobese diabetic (NOD) mice induces CD4+ or CD8+ Trs, respectively, that render the animals hyporesponsive to this protein when injected by an otherwise immunostimulatory route. Similarly, in a murine model of allergic encephalomyelitis, the epicutaneous administration of the allergen induces antigen-specific CD4+ Trs that suppress immunity in a cell-contact–dependent manner.38
Tolerance to alloantigens can also be induced by this approach. In rats, oral exposure to alloantigen generates CD8+ Trs within kidney grafts that will prolong graft survival when transferred to naive animals.39 Similarly, intratracheal delivery of either allogeneic splenocytes40 or allogeneic peptides41 prolongs the survival of allogeneic cardiac grafts by inducing donor-specific Trs that secrete IL4 and IL10. The activity of the Trs induced by this approach may be further enhanced by using antibodies to block the CD40-CD40 ligand (L) pathway (see next section), under which conditions there may be indefinite survival of allogeneic cardiac grafts in mice.42
In summary, efforts to induce antigen-specific Trs by mucosal or cutaneous delivery of the antigen have made it clear that regulation can be achieved by several different cell populations functioning toward the same regulatory end, albeit through different means. Mucosal surfaces and skin provide a favorable environment for the induction of antigen-specific Trs, although the mechanism(s) by which exposure to unique subsets of APCs or to a particular cytokine/chemokine milieu promotes this outcome remains unknown.
Suboptimal costimulatory signals during T-cell recognition of antigen
When T cells recognize their specific peptides through the antigen-specific TCR, several additional receptor-ligand interactions transduce additional signals and stabilize the binding process, permitting T-cell activation and clonal expansion. The CD3 T-cell surface molecule, composed of 3 proteins (γ, δ, ϵ) noncovalently associated with the TCRαβ heterodimer, allows the T cell to engage its specific antigen. The CD8 and CD4 proteins bind to nonpolymorphic regions of MHC class I and class II molecules, respectively, and together with CD3, transduce signals initiating T-cell activation during antigen recognition. CD4 and CD8 are 2 of a series of molecules that support the interaction between T cells and their antigen-presenting target cells. Important costimulatory signals include CD40 ligand (CD154), which is expressed on activated CD4 T cells after antigen recognition and binds to CD40 on APCs such as dendritic cells (DCs). After engagement of CD40, there is up-regulation of other costimulatory molecules, including CD80 and CD86, followed by the initiation of a cascade of signaling events that induce full T-cell activation and proliferation. The TCRs CD28 and CTLA-4 are members of the immunoglobulin supergene family. Both bind the same ligands, CD80 and CD86. CTLA-4 has a higher binding avidity and is expressed on activated and memory T cells, acting as a potent negative regulator of the T-cell response; whereas CD28 is expressed on naive and activated T cells and induces activation, IL2 secretion, and proliferation. Thus, CD28 mediates the differentiation of naive cells into effector cells, while CTLA-4 is thought to play a role in terminating T-cell response and in the induction of self-tolerance.
Blocking some of these critical ancillary signals at the time of TCR engagement by antigen may lead to the induction of Trs. This effect was first clearly shown by Waldmann's group with nonlytic monoclonal antibodies (mAbs)43,44 to CD4 and CD8. In a series of mouse transplant models (skin, heart, bone marrow), such antibodies induced CD4+ Trs that transferred tolerance to naive recipients. The alloantigen-specific regulatory activity was found in both the CD25+ and CD25– subpopulations.26,45 Suppression was maintained only if Trs were continuously exposed to alloantigens in the donor graft. Similarly, Belghith et al23 showed that nondepleting, nonmitogenic CD3-specific antibody induced Trs that prevented the onset of diabetes in a NOD mouse model. These Trs were antigen-specific, predominantly CD4+CD25+, and mediated their suppressive activity by the secretion of TGFβ.
Interruption of the CD40-CD40L pathway may also lead to induction of antigen-specific Trs. Indeed, treatment with anti–CD40L mAb yielded donor-specific CD4+ Trs that inhibited both naive and primed alloreactive T cells in vitro and in vivo.46,47 Such regulatory activity requires cell-to-cell contact.46 A CD40L blockade also prevented the development of diabetes in a murine model in which lymphocytic choriomeningitis virus (LCMV) proteins were expressed in pancreatic islet cells; adoptive transfer of the Trs prevented the induction of diabetes in LCMV-transgenic recipients. These Trs were of an unusual phenotype, expressing both CD11c, a DC-associated marker, and NK1.1, a marker of natural killer cells; a further illustration of the wide variety of regulatory mechanisms utilized by the immune system.48 It is likely that blockade of other costimulatory molecules on APCs will have a similar effect, and certainly interruption of both CD40 and CD86 binding can induce human alloantigen-specific Trs in vitro.49 Of note, inactivation of the downstream signal transduction components of these costimulatory molecules can produce effects that are essentially identical to those achieved by obstructing the receptors themselves. For example, blockade of nuclear factor κ B (NFkB) nuclear translocation will generate antigen-specific CD4 Trs,50 raising the possibility of developing targeted small molecules for Tr induction.
The above findings indicate that Trs are induced by blocking the individual molecular components required for optimal T-cell activation. Thus, in the absence of all such costimulatory interactions, Tr development might be even more strongly favored. Immature dendritic cells (iDCs) are a class of APCs expressing low levels of MHC antigens and the costimulatory molecules CD40, CD80, and CD86. They also secrete low levels of immunostimulatory cytokines such as IL12.51 Human iDCs can be generated in vitro by culturing monocytes for 5 to 6 days in the presence of IL4 and granulocyte macrophage–colony-stimulating factor (GMCSF). If allogeneic iDCs are added52 to naive cord blood–derived CD4+ T cells, Tr cells are generated that are hyporesponsive to repeat allostimulation—even with mature allogeneic DCs—and suppressive in adoptive transfer experiments. These CD4+ Trs express CTLA-4 and secrete IL10. iDC-mediated inhibition requires cell-to-cell contact, but unlike “classically” induced Trs, it is not specific for the inducing alloantigens; however, such lack of specificity is not an invariable consequence of using iDCs to induce Trs, and more likely represents the consequence of using naive cord blood–derived CD4+ T cells as Tr precursors. Hence, when iDCs were loaded with an influenza matrix peptide and injected subcutaneously into 2 healthy volunteers, it was possible to obtain CD8 Trs from the circulation that secreted IL10 and inhibited the proliferation of autologous T cells in a cell contact–dependent and antigen-specific manner.28,53 Similarly, Jiang et al54 have reported the in vitro induction of human allopeptide-specific CD4 Trs after peptide presentation by iDCs. These Trs suppressed T-cell responses in an antigen-specific, dose-dependent manner, and required cell contact for their activity. As a final example, when Steinbrink et al55 treated iDCs with IL10 to further diminish expression of costimulatory molecules, they generated alloantigen- and peptide-specific human CD4+ and CD8+ Trs, both of which acted in a cell contact– and dose-dependent manner. Allogeneic irradiated CD2-depleted peripheral blood mononuclear cells (PBMCs) can also function as immature APCs. These cells induce alloantigen-specific Trs from the CD8+CD28– T-cell population of the stimulated PBMCs, and again function in a cell contact– and dose-dependent manner.29
Similar observations have been made with immature murine DCs (imDCs), suggesting that this is a generalizable phenomenon. The DEC-205 receptor is involved in antigen endocytosis and is present almost exclusively on imDCs. Mahnke et al24 coupled ovalbumin (OVA) to a DEC-205 mAb and injected it into Balb/C mice seeded with T cells expressing an OVA-specific transgenic TCR. This protocol induced antigen-specific CD4+CD25+ Trs which appeared to originate from a CD4+CD25– T-cell population and suppressed the T-cell response in a cell contact– and dose-dependent manner.
Although exposure to immature DCs generally favors induction of Trs, whereas exposure to mature DCs induces both helper and cytotoxic T cells, there is at least one exception. When stimulated by IL3 and CD40L, so-called plasmacytoid T cells in human tonsils mature into plasmacytoid dendritic cells56 (PDCs, or DC2, to distinguish them from monocyte-derived DC1). These apparently mature PDCs express high levels of CD40, CD80, and CD86 while having limited phagocytic and endocytic ability.56 They can produce alloantigen-specific CD8 Trs in vitro. Unlike most antigen-specific Trs however, these cells mediate their inhibitory function by producing IL1027 and the effects can be blocked with IL10 mAb.
Hence, suboptimal costimulation during antigen presentation, however achieved, induces murine and human Trs both in vitro and in vivo. It has been suggested that interaction with iDCs represents an important physiologic mechanism for sustaining or reestablishing tolerance to self-antigens, for example, after bacterial or viral infections.57
Positive signaling by ligand-receptor interaction
Besides the lack of signals, discussed in the previous section, it is evident that Trs can be induced by certain positive signals to T cells. Engagement of CD2 by leukocyte function–associated antigen 3 (LFA-3, or CD58) expressed on DCs supports intercellular adhesion and transduces signals enhancing TCR-mediated activation. Wakkach et al58 demonstrated that engagment of CD2 on human naive CD4 T cells leads to their differentiation into Tr1 and that HLA-DR1–specific Trs were generated from HLA-DR1–negative donors when LFA-3 was coexpressed with HLA-DR1 on APCs. Stimulation of the T-cell surface molecule ICOS (inducible costimulatory molecule)56,57 and Notch during antigen presentation has also been shown to induce antigen-specific Trs.58-64
The ICOS molecule has been implicated in the regulation of the Th2 memory response. After activation by its ligand, ICOS binds phosphoinositide-dependent kinase 3 (PI3K) and activates phosphoinositide-dependent kinase 1 (PIK1) and protein kinase B (PKB). ICOS engagment enhances production of interferon γ (IFNγ), IL4, and IL10.59 The tolerogenic properties of the ICOS–ICOS-ligand pathway have been demonstrated both in vitro and in a murine model of asthma,60 where intranasal exposure to allergen (ovalbumin) leads the DCs to induce antigen-specific CD4+ Trs (as described in “Mucosal (oral, tracheal, nasal) or cutaneous delivery of the antigen”). Both the overexpression of ICOS-ligand on DCs and the secretion of IL10 were critical for the induction of Trs, since anti-IL10, anti-ICOS, and anti–ICOS-L mAbs all blocked the phenomenon.
It has also become evident, in mice and in humans, that the transmembrane receptor Notch and its ligands can contribute to the induction of Trs. Notch plays a critical role in cell fate decisions during embryogenesis and is widely expressed on hematopoietic cells, including T cells, during postnatal life; its ligands are expressed on hematopoietic stromal cells. The Notch pathway contributes to the survival, proliferation, and fate choices of hematopoietic progenitor cells61 and exerts a major role influence on the lineage commitment of lymphoid cells.62 Binding of Notch ligands such as Jagged in humans and Serrate in mice induces proteolytic cleavage in the intracellular portion of the Notch receptor, releasing the intracellular domain of Notch (ICN), or “activated Notch,” which moves into the nucleus and binds to the transcription factor CBF1.63 The importance of Notch and its ligands in the induction of Trs was first suggested by Hoyne et al,64,65 who demonstrated that overexpression of the Notch-ligand Serrate on DCs during the phase of antigen recognition induced naive CD4 T cells to differentiate into Trs capable of transferring antigen-specific inhibition to naive recipient mice. More recently, we confirmed that such activity can be replicated with human cells in vitro.66,67 Using Epstein Barr virus (EBV)–transformed B cells (lymphoblastoid cell lines) as APCs that overexpressed Jagged-1, a human counterpart of Serrate, we found that activation of the Notch pathway during antigen presentation induced CD4+ and CD8+ Trs. In an alloantigen model based on CD45RA (naive) T cells, the Trs secreted high levels of TGFβ67 while in a viral (EBV) model based on memory T cells, they secreted high levels of IL10.66 The Trs were specific for the stimulating alloantigen and viral antigen in these models, and worked by direct cell contact in a dose-dependent manner. Hence, activation of Notch during antigen recognition can induce naive T cells to differentiate into antigen-specific Trs and may also recruit or reprogram EBV-specific memory T cells to acquire antigen-specific Tr activity.
Positive signaling by cytokines
While cell-surface receptor-ligand systems evidently have a crucial role in the induction of Trs, increasing evidence suggests that soluble mediators such as IL10 and TGFβ also contribute to this phenomenon. Groux et al68,69 generated antigen-specific CD4+ Tr1s by simulating ovalbumin-specific naive CD4 T cells from DO11-10 transgenic mice, using a combination of IL10, splenic APCs, and OVA. These Tr1s inhibited colitis in a murine model, and the same approach successfully generated human alloantigen-specific Tr1s. Since these Trs were generated in an APC-dependent system, IL10 may have acted by modulating costimulatory molecules on APCs (see preceding section), or by acting directly on T cells. IL10 certainly down-regulates expression of the costimulatory molecules CD40, CD80, and CD86 on DCs,70-72 possibly to levels that are suboptimal for the induction of helper (Th) or cytotoxic (Tcyt) T lymphocytes, but are ideal for the induction of Trs. There is less evidence that Trs are induced by direct action of IL10 on CD4+ T cells. Instead, most of the data suggest that after exposure to IL10 in an APC-free system, CD4+ T cells become anergic but not suppressive.73-75
Although the mechanism of action of IL10 remains to be determined, this cytokine plays a major role in Tr induction in a large number of animal models, and it may afford a mechanism by which potentially immunogenic microorganisms establish persistent infection and evade Th or Tcyt responses. For example, Bordetella pertussis organisms interact with murine DCs to induce IL10 secretion and generate antigen-specific Tr1s from naive T cells.76 These Tr1s, in turn, secrete IL10 to further enhance inhibition. In tumor immunology, IL10 has been identified as a candidate for inhibiting responses to tumor associated antigens.77 The combination of IL10 and TGFβ may be either additive or synergistic for Tr induction. While such an effect has not been formally demonstrated, the combination was shown to induce alloantigen-specific murine CD4+ Trs in vitro that were able to suppress a murine model of graft-versus-host disease.78 The induced Trs secreted IL10, but the suppression was cell-contact dependent.
Although IL10 and TGFβ have drawn most of the attention to date, it is clear that other peptides and soluble proteins may also induce antigen-specific Trs. In a model of autoimmune uveoretinitis, Taylor and Namba79 showed that antigen presentation with neuropeptide α-melanocyte–stimulating hormone induced antigen-specific CD4+ Trs that in turn suppressed T-cell responses in a TGFβ-dependent manner. Other molecules not generally considered to be part of the immunologic repertoire may also contribute: for example, administration of high doses of antithrombin III (500 U/kg) led to indefinite survival of fully allogeneic murine cardiac grafts by inducing Trs.80 It is possible that antithrombin III at these nonphysiologic doses has the properties of an as-yet-unidentified protease inhibitor that would normally perform this function.
In summary (Table 1; Figure 1), a range of soluble and cell-surface ligands can actively induce functionally distinct subpopulations of antigen-specific Trs.
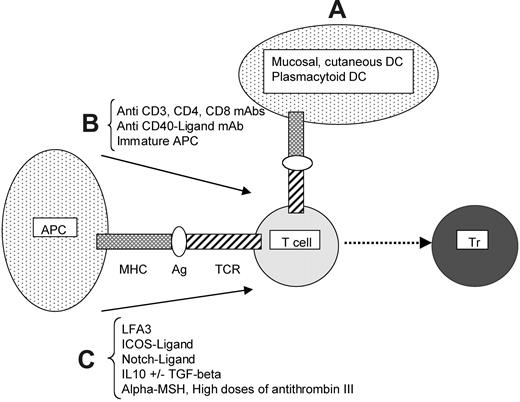
The induction of antigen-specific regulatory T cells. The following 3 strategies can be distinguished. (A) Antigen presentation by dendritic cells (DCs) especially dedicated to the induction of regulatory T cells (Trs; mucosal or cutaneous DCs or plasmacytoid DCs). The molecular mechanisms underlying the tolerogenic properties of these DCs remain to be elucidated. (B) Antigen presentation by antigen-presenting cells (APCs) with suboptimal costimulation, achieved through use of CD3, CD4, CD8, or CD40-ligand monoclonal blocking antibodies or immature APCs. (C) Antigen presentation by mature APCs with an additional regulatory signal provided by either cell-surface molecules such as LFA-3, ICOS-ligand, Notch-ligand, or soluble factors, such as IL10 with or without TGFβ, α-MSH (melanocyte-stimulating hormone), or high doses of antithrombin III.
The induction of antigen-specific regulatory T cells. The following 3 strategies can be distinguished. (A) Antigen presentation by dendritic cells (DCs) especially dedicated to the induction of regulatory T cells (Trs; mucosal or cutaneous DCs or plasmacytoid DCs). The molecular mechanisms underlying the tolerogenic properties of these DCs remain to be elucidated. (B) Antigen presentation by antigen-presenting cells (APCs) with suboptimal costimulation, achieved through use of CD3, CD4, CD8, or CD40-ligand monoclonal blocking antibodies or immature APCs. (C) Antigen presentation by mature APCs with an additional regulatory signal provided by either cell-surface molecules such as LFA-3, ICOS-ligand, Notch-ligand, or soluble factors, such as IL10 with or without TGFβ, α-MSH (melanocyte-stimulating hormone), or high doses of antithrombin III.
Cellular basis of antigen-specific regulation by Trs
As illustrated in Figure 2A-B, the antigen-specific regulation of T-cell immunity encompasses 3 stages: antigen presentation, Tr activation, and recognition of target cells by Trs.
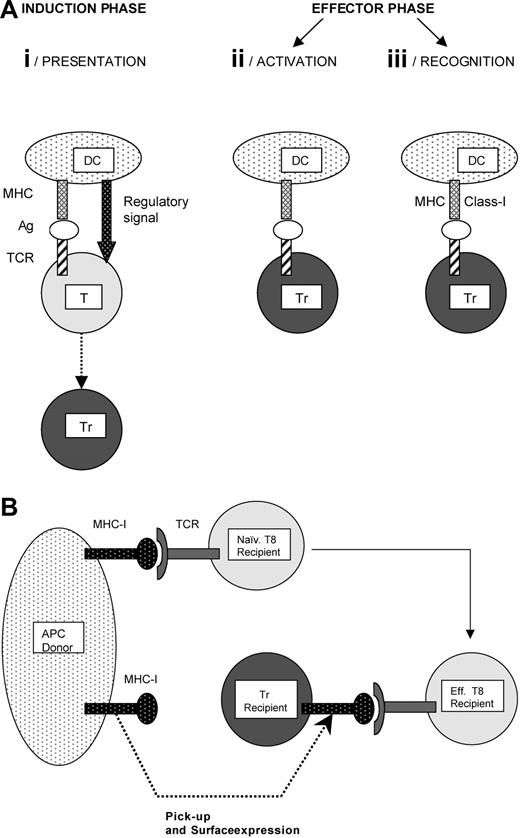
Antigen-induced regulatory T cells: cellular basis of the antigen specificity. (A) Antigen specificity is ensured at 3 stages: presentation, activation, and recognition. (i) Presentation. A dendritic cell (DC) is presented to a conventional T cell (T) combined with a regulatory signal provided by, or accompanying the DC. The regulatory signal induces differentiation of the antigen-specific conventional T cell into an antigen-specific regulatory T cell (Tr). (ii) Activation. The Tr must encounter the same antigen again in order to become activated and fully competent to suppress the specific immune response. This antigen-driven activation ensures that suppression is limited to a particular target. (iii) Recognition. Two mechanisms support the antigen-specificity target cell (APC or T cell) recognition by Trs. First, some Trs specifically recognize MHC class I antigens on allogeneic APCs. The second mechanism is illustrated in Figure 2B. (B) Antigen-specific recognition of CD8 effector T cells by regulatory T cells. This mechanism has been described in a murine model of skin allotransplantation.30 The Trs acquire allogeneic MHC class I molecules from APCs and express them on their surface. This transfer allows the Trs to recognize autologous cytotoxic CD8 effector T cells bearing a TCR specific for the transferred MHC class I molecule.
Antigen-induced regulatory T cells: cellular basis of the antigen specificity. (A) Antigen specificity is ensured at 3 stages: presentation, activation, and recognition. (i) Presentation. A dendritic cell (DC) is presented to a conventional T cell (T) combined with a regulatory signal provided by, or accompanying the DC. The regulatory signal induces differentiation of the antigen-specific conventional T cell into an antigen-specific regulatory T cell (Tr). (ii) Activation. The Tr must encounter the same antigen again in order to become activated and fully competent to suppress the specific immune response. This antigen-driven activation ensures that suppression is limited to a particular target. (iii) Recognition. Two mechanisms support the antigen-specificity target cell (APC or T cell) recognition by Trs. First, some Trs specifically recognize MHC class I antigens on allogeneic APCs. The second mechanism is illustrated in Figure 2B. (B) Antigen-specific recognition of CD8 effector T cells by regulatory T cells. This mechanism has been described in a murine model of skin allotransplantation.30 The Trs acquire allogeneic MHC class I molecules from APCs and express them on their surface. This transfer allows the Trs to recognize autologous cytotoxic CD8 effector T cells bearing a TCR specific for the transferred MHC class I molecule.
The first step requires the presentation of a peptide/antigen by an APC to a conventional T cell, accompanied by a “regulatory signal,” which may be the absence of an appropriate cosignal, the presence of immature or plasmacytoid DCs, or the presence of specific cell-surface or soluble molecules. The Trs generated by mucosal tolerance likely use one or more of these mechanisms, but since the “regulatory signal” has not yet been elucidated, it is possible that additional mechanisms exist. It is still unknown if during antigen recognition in a Tr-generating environment, T cells activate their Tr programs in response to specific signals or if T-cell subsets already expressing such a program simply respond to positive growth and differentiation signals.
Once induced, Trs become functional when they encounter the same peptide/antigen. The requirement for this second encounter ensures that inhibitory activities are antigen-specific and limited to the desired area55,68,74,81 by making activation of functional Trs a local phenomenon depending on antigen recognition. This concept of local secondary activation is supported by observations in transplant-tolerance models of Tr localization in the tolerated allograft.82,83
The third step requires antigen-specific recognition of the target cell by Trs. The cellular basis of this phenomenon remains controversial and may be context dependent. One mechanism depends on alloantigen-specific recognition of MHC class I molecules on target DCs by Trs.84 Another mechanism was demonstrated in a model in which mice were transfused with allogeneic-donor specific T cells prior to receiving a skin graft from the same strain. In that system, the Tr activity prevented graft rejection30 by specifically recognizing the effector CD8 T cells. In vitro investigation of this specific recognition (Figure 2B) showed that Trs acquire allogeneic MHC class I molecules from neighboring APCs and express them on their surface, thus allowing specific recognition and targeting of autologous CD8 effector T cells bearing the TCR specific for the transferred MHC molecule.
Mechanisms of suppression
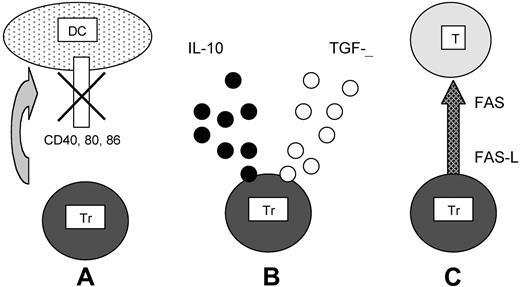
Antigen-induced regulatory T cells: 3 mechanisms of suppression. (A) After antigen-specific recognition of the target DCs, Trs inhibit CD40, CD80, and CD86 up-regulation. (B) After recognition of its specific antigen, Tr secretes IL10 and/or TGFβ. (C) Tr activates the FAS–FAS ligand cytolytic pathway to kill the effector target T cell (T) after its specific recognition.
Antigen-induced regulatory T cells: 3 mechanisms of suppression. (A) After antigen-specific recognition of the target DCs, Trs inhibit CD40, CD80, and CD86 up-regulation. (B) After recognition of its specific antigen, Tr secretes IL10 and/or TGFβ. (C) Tr activates the FAS–FAS ligand cytolytic pathway to kill the effector target T cell (T) after its specific recognition.
The first, down-regulation of CD40, CD80, and CD86 costimulatory molecules on DCs, has been characterized by Suciu-Foca et al22 who showed that alloantigen-specific and MHC class I–restricted CD8+CD28– Trs inhibit CD40-mediated CD80 and CD86 up-regulation on APCs.84 This defective costimulation was responsible for the lack of T-cell reactivity.22,85 Recently, the same investigators demonstrated that CD8+CD28– T cells induce up-regulation of immunoglobulin (Ig)–like transcripts 3 and 4 on APCs, leading to decreasing expression of CD80. These data provide much-needed insight into the molecular mechanisms underlying the inhibitory effects of CD8+CD28– Trs.86
The second mechanism relies on IL10 and/or TGFβ secretion by antigen-activated Trs.27,31,68,74 The effects of IL10 on most DCs are mediated by the Jak/Stat system with a crucial early role for the Jak1 kinase, which phosphorylates (on tyrosine) and activates the transcription factor Stat3.72 This in turn inhibits secretion of the proinflammatory mediators IL1β, IL6, IL12, and TNFα by monocytes, macrophages, and immature DCs and down-regulates expression of MHC class II, CD80, and CD86 molecules on these cells. IL10 also affects the function of CD4+ T cells by inhibiting NFkB activation and subsequently IL2 production.72 The effects of TGFβ are mediated by the intracytoplasmic phosphorylation/activation of the Smad complex. After its transport to the nucleus, the molecule associates with transcriptional modulators and inhibits T-cell proliferation.87 TGFβ also inhibits the differentiation of naive T cells and the maturation of DCs.87
In the third mechanism, initially recognized in a mouse model of skin allotransplantation, CD4–CD8– Trs specifically kill the effector CD8+ T cells responsible for rejection, using the FAS–FAS ligand pathway.30 This pathway was later found to be important in other Tr models.88,89 So far, the FAS–FAS ligand pathway is the only cytolytic mechanism demonstrated to be active in cell contact–dependent suppression. Whether the perforin/granzyme system participates similarly in the cytolytic effects of Trs remains uncertain.
Finally, we would stress that none of the above mechanisms are mutually exclusive, and that in real life, as opposed to carefully designed reductionist models, all may operate simultaneously.
Future challenges
The major issue facing investigators of antigen-induced Trs is whether or not our accumulated knowledge represents any advance over what was known in the 1970s and 1980s. From the “half-empty” perspective, we still lack a firm phenotypic means of identifying antigen-specific Trs. This is a crucial deficiency, as it means that we must still depend on complex and variable biologic assays to evaluate Tr induction and activity. It is also evident that multiple mechanisms may underlie the biologic end points of inhibition of proliferation and cytotoxicity ex vivo, or of graft tolerance or inhibition of autoimmune disease and allergy in vivo. This in turn greatly increases the complexity of our experimental strategies and limits how definitive our conclusions can be. Hence, the statement that “so far, no one has had a pure population of suppressor T cells in a test tube”2 remains as true today as in 1988. From the “half-full” viewpoint, we now possess many powerful tools that can be used to analyze the molecular and cellular basis of the regulatory T-lymphocyte phenomenon and results to date have identified several potentially `drugable' pathways that are clearly capable of inducing at least some of the activities attributed to Trs. Substantial issues remain before Trs can be introduced clinically. If, for example, investigators wished to use Trs to prevent graft-versus-host disease (GVHD) after allogeneic stem cell transplantation, they would first have to devise a robust and optimized protocol and validatable assays to show their functionality and specificity prior to infusion or induction in vivo. Since assays of antigen-specific regulatory T cells are currently long and complex, this is a challenging demand, and one that will likely require introduction of reliable surrogate assays. These may have to incorporate proteomic analyses rather than the cell-surface phenotyping now current. Moreover, regulatory T cells will mostly be used in environments, for example, after stem cell transplantation, in which the patient is already on immunosuppressive therapy for the underlying disease. We do not know how such immunosuppression will affect the in vivo persistence or function of antigen-specific Trs. These challenges notwithstanding, there is little doubt that the enormous potential value of antigen-specific Trs in the treatment of human disease mandates their continued investigation.
Prepublished online as Blood First Edition Paper, March 16, 2004; DOI 10.1182/blood-2004-01-0182.
Supported by a grant from the “Association pour la Recherche sur le Cancer” (ARC), France (S.V.), and grants from “Comitato Maria Letizia Verga per lo studio e la cura della leucemia del bambino,” Monza, Italy, and “Fondazione Cassa di Risparmio di Genova ed Imperia-Associazione Cristina Bassi contro le leucemie acute dell'adulto,” Genova, Italy (E.B.).