Abstract
Cytotoxic T lymphocytes (CTLs) specific for hematopoietic-restricted minor histocompatibility antigens (mHags) are important reagents for adoptive immunotherapy of relapsed leukemia after allogeneic stem cell transplantation. However, expansion of these CTLs to therapeutic numbers is often hampered by the limited supply of antigen-presenting cells (APCs). Therefore, we evaluated whether cell-sized latex beads coated with HLA/mHag complexes HLA-A2/HA-1 or HLA-A2/HA-2 and recombinant CD80 and CD54 molecules can replace professional APCs. The artificial antigen-presenting constructs (aAPCs) effectively stimulated HA-1– and HA-2–specific CTL clones as shown by ligand-specific expansion, cytokine production, and maintenance of cytotoxic activity, without alteration of CTL phenotype. Furthermore, HA-1–specific polyclonal CTL lines were enriched as efficiently by aAPCs as by autologous HA-1 peptide-pulsed dendritic cells. Thus, aAPCs coated with HLA/mHag complexes, CD80, and CD54 may serve as tools for in vitro enrichment of immunotherapeutic mHag-specific CTL lines.
Introduction
The successful application of donor lymphocyte infusions for the treatment of relapsed leukemia after allogeneic stem cell transplantation illustrates the feasibility of adoptive immunotherapy of hematologic malignancies.1 To minimize graft-versus-host disease, we earlier proposed the use of minor histocompatibility antigens (mHags) HA-1 and HA-2 as immunotherapeutic reagents.2 HA-1 and HA-2 display hematopoietic-restricted tissue distribution and relevant expression on leukemic cells and their progenitors.3-6 Moreover, cytotoxic T lymphocytes (CTLs) directed against these mHags do not cause graft-versus-host disease in an ex vivo skin explant model,7 and coincide with complete remission of relapsed leukemia and multiple myeloma after HLA-matched HA-1– or HA-2–mismatched donor lymphocyte infusions.8
HA-1– and HA-2–specific CTLs can be generated in vitro using peptide-pulsed or mHag-transduced autologous dendritic cells (DCs) as antigen-presenting cells (APCs).9,10 However, expansion of CTLs is difficult due to the limited availability of donor-derived DCs. To date, several reports indicate effective stimulation of T cells by HLA/peptide ligands expressed on artificial antigen-presenting constructs (aAPCs) such as liposomes11,12 or microbeads.13-16 We developed aAPCs that can be manufactured under good manufacturing practice conditions and used to expand mHag-specific CTLs for adoptive immunotherapy. Hereto, cell-sized latex microbeads coated with HLA-A2/HA-1 or HLA-A2/HA-2 complexes, CD80, and CD54 were investigated for their potential to efficiently stimulate HA-1– and HA-2–specific CTL clones and lines while keeping their antigen-specific cytolytic properties.
Study design
mHag-specific CTL clones and polyclonal CTL lines, CD4+ T helper cells, and DCs
In vivo and in vitro generation of mHag-specific CTL clones, polyclonal CTL lines, and DCs is documented in detail elsewhere.9,17 CD4+ T-helper cells (CD4+Th cells) were generated by culturing peripheral blood mononuclear cells (PBMCs) for 14 days in Iscove modified Dulbecco medium (IMDM) containing 10% pooled human serum (HS) and 0.5% standard Dutch diphtheria/pertussis/tetanus/polio vaccine (National Institute of Public Health and the Environment, Bilthoven, The Netherlands), and 20 U/mL interleukin 2 (IL-2; Cetus, Emeryville, CA) from day 6 onward.
Functional assays
All functional assays were performed in IMDM plus 10% HS. Stimulator cells were irradiated (30 Gy) before use. Results of proliferation, interferon-γ (IFN-γ) production, and cytotoxicity assays are expressed as the mean of duplicate samples.
Proliferation and IFN-γ production were determined by coculturing 2.5 × 104 responders with 5 × 104 stimulators. After 48 hours, supernatant was harvested for IFN-γ enzyme-linked immunosorbent assay (ELISA; Sanquin, Amsterdam, the Netherlands); the detection limit was 2 pg/mL. The remaining cultures were labeled with 1.0 μCi (0.037 MBq) 3H-thymidine for 16 hours, after which incorporation was measured using liquid scintillation counting.
Cytotoxicity was evaluated by incubating 2500 51Cr-labeled target cells with serial dilutions of effector CTLs for 4 hours; supernatants were harvested for gamma counting: % specific lysis = (experimental release–spontaneous release)/(maximal release–spontaneous release) × 100%.
Cytokine profiles were tested using the Human Th1/Th2 cytokine cytometric bead assay (BD Biosciences, San Diego, CA). Detection limits were IFN-γ, 7.1 pg/mL; tumor necrosis factor α (TNF-α), 2.8 pg/mL; IL-10, 2.8 pg/mL; IL-5, 2.4 pg/mL; IL-4, 2.6 pg/mL; IL-2, 2.6 pg/mL.
Synthetic peptides and HLA-A2/mHag complexes
Ligand immobilization on latex beads
The 5.3-μm polystyrene sulfate latex beads (Interfacial Dynamics, Portland, OR) were incubated sequentially with streptavidin-allophycocyanin (1 μg/107 beads; Molecular Probes, Leiden, The Netherlands), with recombinant human CD80/Fc-chimera and CD54 (0.5 μg and 1.5 μg/107 beads, respectively; R&D Systems, Minneapolis, MN), and with 1% human albumin (Sanquin) for 20 minutes in 0.5 mL phosphate-buffered saline (PBS) per 107 beads at 4° C. The beads were then incubated with biotinylated HLA-A2/mHag complexes (0.5 μg/107 beads) for 40 minutes in 1 mL PBS/107 beads at 4° C. After each incubation step, the beads were washed with 1 mL PBS. See the Supplementary Data on the Blood website (click on the “Data Set” link at the top of the online article) for determination of optimal ligand densities.
Results and discussion
aAPC-mediated stimulation of mHag-specific CTL clones
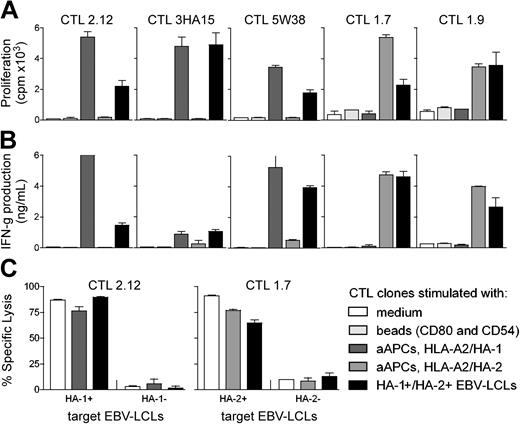
The capacity of aAPCs to stimulate mHag-specific CTLs in an efficient and ligand-specific manner was analyzed and compared with that of professional APCs. Three HA-1–specific CTL clones (2.12, 3HA15, 5W38) and 2 HA-2–specific CTL clones (1.7, 1.9) were stimulated with (1) aAPCs coated with the costimulatory molecules CD80 and CD54 only, (2) aAPCs coated with costimulatory molecules and either HLA-A2/HA-1 or HLA-A2/HA-2 complexes, or (3) with HLA-A2+ Epstein-Barr virus (EBV)–transformed B-cell lines naturally expressing HA-1 and HA-2 (HA-1+/HA-2+ EBV-LCLs; Figure 1A-B). All mHag-specific CTL clones showed significant proliferation and IFN-γ production in response to aAPCs coated with the relevant HLA-A2/mHag complexes and costimulatory molecules, comparable to the responses to HA-1+/HA-2+ EBV-LCLs. No proliferation or IFN-γ production was induced by aAPCs coated with irrelevant HLA-A2/mHag complexes or with costimulatory molecules only.
aAPC-mediated stimulation of mHag-specific CTL clones. Proliferation (A) and IFN-γ production (B) of various HA-1–specific CTL clones (2.12, 3HA15, 5W38) and HA-2–specific CTL clones (1.7, 1.9) incubated for 48 hours with medium only, with aAPCs coated with the costimulatory molecules CD80 and CD54, with aAPCs coated with costimulatory molecules and either HLA-A2/HA-1 or HLA-A2/HA-2 complexes, or with HA-1+/HA-2+ EBV-LCLs. Cytotoxic activity (C) of 2 CTL clones (2.12, 1.7) was determined after incubation for 7 days with medium only, with HA-1+/HA-2+ EBV-LCLs, or with aAPCs coated with specific ligand. Cytotoxic activity is shown for an effector-target ratio of 8:1. Data are presented as the mean percentage of lysis ± SD.
aAPC-mediated stimulation of mHag-specific CTL clones. Proliferation (A) and IFN-γ production (B) of various HA-1–specific CTL clones (2.12, 3HA15, 5W38) and HA-2–specific CTL clones (1.7, 1.9) incubated for 48 hours with medium only, with aAPCs coated with the costimulatory molecules CD80 and CD54, with aAPCs coated with costimulatory molecules and either HLA-A2/HA-1 or HLA-A2/HA-2 complexes, or with HA-1+/HA-2+ EBV-LCLs. Cytotoxic activity (C) of 2 CTL clones (2.12, 1.7) was determined after incubation for 7 days with medium only, with HA-1+/HA-2+ EBV-LCLs, or with aAPCs coated with specific ligand. Cytotoxic activity is shown for an effector-target ratio of 8:1. Data are presented as the mean percentage of lysis ± SD.
Improper or partial T-cell receptor signaling by altered peptide ligands or T-cell receptor engagement in the absence of adequate costimulatory signals can lead to changes in cytokine secretion profiles or anergy of T cells.21-23 Similarly, aAPCs may alter the phenotype and function of CTL clones. Therefore, mHag-specific CTL clones (2.12 and 1.7) were stimulated for 7 days with aAPCs or with HA-1+/HA-2+ EBV-LCLs. Subsequently, cytotoxic activity and secretion of IFN-γ, TNF-α, IL-10, IL-5, IL-4, and IL-2 were measured. The specific cytotoxicity (Figure 1C) and the cytokine profiles (data not shown) were comparable for either mode of stimulation.
aAPC-mediated enrichment of mHag-specific polyclonal CTL lines
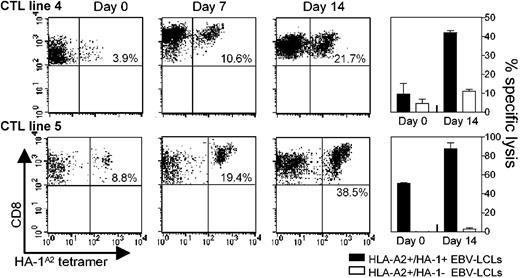
The main goal of this study was to investigate whether aAPCs can replace autologous DCs for the enrichment of polyclonal mHag-specific cultures. Hereto, 5 different polyclonal HA-1–specific CTL lines (1, 2, 3, 4, 5), originally induced and restimulated twice with HA-1 peptide-pulsed autologous DCs, were stimulated as follows. CTL lines 1, 2, 3, and 4 were restimulated for 7 days with (1) aAPCs only, (2) aAPCs and autologous PBMCs, (3) aAPCs and autologous CD4+Th cells, or (4) HA-1 peptide-pulsed autologous DCs. HA-1A2 tetramer staining and cytotoxicity assays were performed at day 0 and at day 7 (Table 1). For all CTL lines, similar or improved enrichment for HA-1–specific CTLs was observed after restimulation with aAPCs and autologous feeder cells when compared to DC-mediated enrichment. The feeder cells could either be total PBMCs or activated CD4+Th cells. These responses were driven by antigen presentation via aAPCs because none of the CTL lines showed enrichment after 7 days of incubation with PBMCs or CD4+Th cells only (data not shown). In a separate experiment, CTL lines 4 and 5 were restimulated repeatedly with aAPCs and autologous feeder cells. Both lines showed a 5-fold increase in absolute numbers of HA-1A2 tetramer–positive cells without loss of HA-1–specific cytotoxic activity after 14 days (Figure 2).
aAPC-mediated expansion of mHag-specific polyclonal CTL lines. Polyclonal CTL lines 4 and 5 were stimulated with aAPCs and either autologous CD4+Th cells (CTL line no. 4) or autologous PBMCs (CTL line no. 5) in the presence of 20 U/mL IL-2 and 10 ng/mL IL-7. Responder-stimulator ratios were as follows: CTLs/aAPCs/PBMCs, 1:1:1; CTLs/aAPCs/CD4+Th, 2:2:1. Prior to each restimulation, HA-1A2 tetramer staining of viable CD8+ T lymphocytes and cytotoxicity assays were performed. Cytotoxicity results are shown for an effector-target ratio of 10:1. The percentages in the scatterplot subpanels represent the percentage of viable CD8+ HA-A1A2 tetramer–positive lymphocytes in the total population of viable CD8+ lymphocytes.
aAPC-mediated expansion of mHag-specific polyclonal CTL lines. Polyclonal CTL lines 4 and 5 were stimulated with aAPCs and either autologous CD4+Th cells (CTL line no. 4) or autologous PBMCs (CTL line no. 5) in the presence of 20 U/mL IL-2 and 10 ng/mL IL-7. Responder-stimulator ratios were as follows: CTLs/aAPCs/PBMCs, 1:1:1; CTLs/aAPCs/CD4+Th, 2:2:1. Prior to each restimulation, HA-1A2 tetramer staining of viable CD8+ T lymphocytes and cytotoxicity assays were performed. Cytotoxicity results are shown for an effector-target ratio of 10:1. The percentages in the scatterplot subpanels represent the percentage of viable CD8+ HA-A1A2 tetramer–positive lymphocytes in the total population of viable CD8+ lymphocytes.
In summary, aAPCs coated with HLA-A2/mHag complexes, CD80, and CD54 can be used to selectively enrich mHag-specific CTLs for adoptive immunotherapy. In combination with autologous feeder cells, aAPCs stimulate CTLs as efficiently as peptide-pulsed DCs. The aAPCs can be generated in a nonlaborious way and can be easily removed from the cell culture by a density gradient. All aAPC-constituents are obtainable at clinical grade. Future studies will address the issue of whether aAPCs can be used for the primary in vitro induction of mHag-specific CTLs.
Prepublished online as Blood First Edition Paper, March 18, 2004; DOI 10.1182/blood-2003-07-2461.
Supported in part by grants from the Dutch Cancer Society (Koninging Wilhelmina Fonds), the Leukemia and Lymphoma Society, and Foundation `De Drie Lichten.'
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Prof F. Claas and Dr M. J. B. van Stipdonk for critical reading and comments.