Abstract
Pretargeted radioimmunotherapy (PRIT) has the potential to increase the dose of radionuclide delivered to tumors while limiting radiation to normal tissues. The purpose of this phase 1 trial is to assess safety of this multistep approach using a novel tetrameric single-chain anti-CD20–streptavidin fusion protein (B9E9FP) as the targeting moiety in patients with B-cell non-Hodgkin lymphoma (NHL), and to characterize its pharmacokinetics and immunogenicity. All patients received B9E9FP (160 mg/m2 or 320 mg/m2); either 48 or 72 hours later, a synthetic clearing agent (sCA) was administered (45 mg/m2) to remove circulating unbound B9E9FP. 90Yttrium (90Y; 15 mCi/m2)/111In (5 mCi)–DOTA-biotin was injected 24 hours later. There were 15 patients enrolled in the study. B9E9FP had a mean plasma half-life (T½) of 25 ± 6 hours with a reduction in plasma level of more than 95% within 6 hours of sCA administration. 90Y/111In-DOTA-biotin infusion resulted in rapid tumor localization and urinary excretion. The ratio of average tumor to whole-body radiation dose was 49:1. No significant hematologic toxicities were noted in 12 patients. There were 2 patients who had hematologic toxicity related to progressive disease. There were 2 complete remissions (90 and 325 days) and one partial response (297 days). B9E9FP performs well as the targeting component of PRIT with encouraging dosimetry, safety, and efficacy. A dose escalation trial of 90Y-DOTA-biotin in this format is warranted.
Introduction
Early clinical trials using monoclonal antibodies against B-cell antigens to treat B-cell malignancies began more than 2 decades ago.1,2 Rituximab (Rituxan and MabThera; IDEC Pharmaceuticals, San Diego, CA, and Genentech, South San Francisco, CA)3 was the first agent from this class to receive Food and Drug Administration (FDA) approval in 2000. Rituximab has been shown to produce objective responses in 40% to 50% of patients with relapsed or refractory low-grade or transformed follicular non-Hodgkin lymphomas (NHLs) with low toxicity and with a median time to disease progression of 13.2 months.4 In an effort to improve efficacy, radionuclides have been conjugated to monoclonal antibodies or antibody-based targeting molecules to selectively deliver therapeutic radionuclides to tumor sites in animal models and cancer patients. This strategy has been termed radioimmunotherapy (RIT).
Successful treatment regimens using RIT for the treatment of chemotherapy-resistant, low-grade, or transformed B-cell NHL have been developed (90Yttrium [90Y]–2B8 or Zevalin [FDA approved5 ], IDEC Pharmaceuticals; 131Iodine [131I]–B1 or Bexxar [FDA approved6 ], Corixa, Seattle, WA). The substantial efficacy of targeted radiation associated with these agents, with objective response rates of 60% to 80% (complete remissions 20%-30%), reflects the radiation sensitivity of this neoplasm, the specificity of anti-CD20 monoclonal antibodies for B-lymphocytes, and multiple other possible mechanisms of action of RIT.5-9 In these nonmyeloablative trials using conventional RIT, tumor radiation doses have been limited in part due to the extended time required for tumor localization of the antibody, thereby resulting in loss of tumoricidal potential of the radionuclide prior to localization (isotopic decay). Additionally, the prolonged circulation of intact antibodies until maximal tumor localization results in prolonged exposure of radiosensitive tissues, particularly bone marrow.10,11 Escalation of the dose of the radiolabeled anti-CD20 to myeloablative levels with subsequent rescue using stem cells has improved the complete response rates (75%-80%), and the durability of the response with some patients remaining in complete remission (CR) for up to 12 years.12
Currently, methods to improve RIT delivery of therapeutic radionuclides in order to enhance the efficacy of this treatment strategy in lymphoma and other tumor types are ongoing. One such methodology, PRIT (Pretarget [NeoRx, Seattle, WA] radioimmunotherapy), was originally proposed by Goodwin et al.13 This multistep (2 or 3 steps) strategy separates the administration of the targeting molecule from that of a low-molecular-weight radionuclide ligand. This antigen-specific system allows efficient localization to antigen-bearing sites of high doses of radioactivity, with excess radiation cleared rapidly from the whole body via renal elimination. This procedure can utilize bifunctional monoclonal antibodies with dual specificity for tumor-associated antigens and radionuclide carriers or biotinylated antibodies14-16 or streptavidin-conjugated monoclonal antibodies that have high affinity for biotin-conjugated radionuclides. One well-studied pretargeting strategy has utilized a multistep procedure that allows the streptavidin-conjugated antibody to bind to tumor sites over 48 to 72 hours, followed by a human serum albumin “clearing agent” that binds circulating streptavidin molecules and mediates their hepatic clearance from the circulation. This is followed by administration of 90Y-DOTA(1,4,7,10-tetraazacyclododecane-tetraacetic acid)–biotin.17 This pretargeting approach has produced striking efficacy in animal models, curing established tumors with little or no toxicity,18 and has been studied in phase 1 and 2 clinical trials.19-22
A pilot trial in patients with NHL23 using streptavidin chemically conjugated to anti-CD20 (C2B8-Rituxan) as the targeting agent, a synthetic clearing agent and 30 mCi/m2 to 50 mCi/m2 of 90Y-DOTA-biotin resulted in objective responses in 4 of 7 patients with modest toxicity (transient grade III hematologic toxicity). The trial also demonstrated a substantial tumor to whole-body radiation dose ratio of 38:1. However, chemical conjugates of streptavidin to monoclonal antibodies are difficult to manufacture and thus present substantial limitations to commercial development and widespread availability.
A second-generation streptavidin targeting agent has been developed using recombinant DNA technology.24 A genetic construct of a single-chain variable region of B9E9 monoclonal antibody (anti-CD20) linked to a monomer of streptavidin (normally a tetrameric molecule) produced a fusion protein in Escherichia coli (E coli). The fusion protein is secreted into the periplasmic space where it spontaneously forms a stable tetramer composed of 4 identical subunits, each containing a streptavidin monomer genetically fused to a single-chain anti-CD20 B9E9 antibody fragment (B9E9FP). This molecule performed well in vivo as the targeting molecule in the pretargeting RIT format with high tumor to whole-body radiation ratios and high efficacy in animal models.24,25
This report describes the first experience in patients with administration of such a novel fusion protein and examines its performance as the targeting molecule in the Pretarget RIT format. Results are presented from a phase 1 pilot trial in B-cell NHL designed to study the toxicity, pharmacokinetics, and immunogenicity of the fusion protein, as well as to examine its ability to mediate high tumor to whole-body radiation dose ratios similar to antibody-streptavidin chemical conjugates. A brief report of the initial patient treated on this pilot trial has been previously published.26
Patients, materials, and methods
Patient population
Patients with histologically confirmed relapsed or refractory B-cell non-Hodgkin lymphomas (stages II, IIE, III, IV by Ann Arbor Staging Criteria) were included in this trial provided they met the following criteria: bidimensionally measurable disease, a monoclonal lymphoma cell population that is CD20+, a bone marrow specimen demonstrating less than 25% involvement by non-Hodgkin lymphoma with at least 50% of normal cellularity for the age of the patient, cumulative external beam radiation to no more than 20% of marrow volume, negative HIV test, and a pre-study Karnofsky performance status of more than 60%. In addition, patients had to be at least 18 years old, not pregnant or lactating, using accepted birth control methods, and had to have a life expectancy of at least 3 months. Within 2 weeks prior to initial treatment, patients were required to have acceptable hematologic status (platelets ≥ 100 000/mm3 and absolute neutrophil count ≥ 2000/mm3), hepatic function (total bilirubin level ≤ 2.0 mg/dL; aspartate aminotransferase (AST) and amino alanine transferase (ALT) < 2 × upper limit of normal), and renal function (creatinine ≤ 1.6 mg/dL). Patients with a history of positive antibody response to B9E9Fusion (HAFA) and/or positive human anti–streptavidin antibody (HASA) titer (if previously exposed to streptavidin-conjugated monoclonal antibody), prior radioimmunotherapy, abnormal left ventricular ejection fraction (≤ 50%), or central nervous system involvement were excluded. Patients were required to have failed at least 1 chemotherapy regimen, but there was no limitation on the number of prior therapies/relapses. All prior chemotherapy regimens (including corticosteroids) had to have been completed 4 weeks or more before study treatment. The institutional review board at each study site approved the study, and written informed consent was obtained from all patients.
Study design
The open-label, multicenter, phase 1 trial was designed to evaluate the safety and performance of the B9E9FP when used as the targeting moiety with subsequent administration of synthetic clearing agent and radiolabeled DOTA-biotin. The study was conducted at 4 institutions including the University of Alabama at Birmingham Comprehensive Cancer Center, Birmingham, AL; Stanford University Medical Center, Stanford, CA; University of Nebraska Medical Center, Omaha, NE; and Virginia Mason Medical Center, Seattle WA.
Pretarget regimen
The protocol utilized doses and schedule of administration found to be optimal in prior studies of PRIT18-24 except that the B9E9FP was used instead of an antibody streptavidin chemical conjugate as outlined in Table 1.
Cohorts in the trial
Cohort . | N . | B9E9FP, mg/m2 . | Interval 1, h . | sCA, mg/m2 . | Interval 2, h . | 111In, mCi . | 90Y, mCi/m2 . | DOTA-biotin, mg/m2 . |
|---|---|---|---|---|---|---|---|---|
| A | 3-5 | 160 ± 186Re 30 mCi | 72 | 45 | 24 | 5 | 15 | 1.3 |
| B | 3-5 | 160 | 48 | 45 | 24 | 5 | 15 | 1.3 |
| C | 3-5 | 320 ± 186Re 30 mCi | 48 | 45 | 24 | 5 | 15 | 1.3 |
| D | 3-5 | 160 | 48 | 45 | 24 | 5 | 15 | 0.65 |
Cohort . | N . | B9E9FP, mg/m2 . | Interval 1, h . | sCA, mg/m2 . | Interval 2, h . | 111In, mCi . | 90Y, mCi/m2 . | DOTA-biotin, mg/m2 . |
|---|---|---|---|---|---|---|---|---|
| A | 3-5 | 160 ± 186Re 30 mCi | 72 | 45 | 24 | 5 | 15 | 1.3 |
| B | 3-5 | 160 | 48 | 45 | 24 | 5 | 15 | 1.3 |
| C | 3-5 | 320 ± 186Re 30 mCi | 48 | 45 | 24 | 5 | 15 | 1.3 |
| D | 3-5 | 160 | 48 | 45 | 24 | 5 | 15 | 0.65 |
sCA indicates synthetic clearing agent.
Cohort A utilized a 72-hour first interval between administration of the B9E9FP (160 mg/m2) and the synthetic clearing agent. Cohort B utilized a 48-hour first interval with the remaining components identical to cohort A. Cohort C utilized a 100% increase in dose of the B9E9FP (320 mg/m2) with the remaining components identical to cohort B. Cohort D utilized half the dose of DOTA-biotin (0.65 mg/m2) which doubled the specific activity of the compound with all other components identical to cohort B. All patients received the same amounts of 111In (5 mCi) and 90Y (15 mCi/m2) bound to DOTA-biotin. This trial was designed to characterize the use of the B9E9FP as the targeting moiety and did not include escalation of the dosage of 90Y-DOTA-biotin. In addition, cohorts A and C included a 186Rhenium (186Re) trace labeling (30 mCi) to aid in analysis of B9E9FP biodistribution, pharmacokinetics, and the efficiency of synthetic clearing agent at B9E9FP doses of 160 mg/m2 and 320 mg/m2.
The B9E9Fusion and 186Re-B9E9Fusion were administered intravenously in less than or equal to 100 mL normal saline over a period of 15 to 30 minutes. The sCA was administered in 150 mL saline over 30 minutes. The 111In/90Y-DOTA-biotin was administered intravenously (bolus injection) after assays were performed to demonstrate adequate clearance of B9E9FP by the sCA. There were 3 to 5 patients enrolled in each cohort (the trial was designed to enroll 3 patients in each cohort but allowed the enrollment of up to 2 additional patients). Patient safety was assessed by clinical observations, vital signs, laboratory parameters, and monitoring of adverse events. Toxicity was graded according to the National Cancer Institute (NCI) Common Toxicity Grading Scale. Computed tomography (CT) imaging for response assessment was done at 6 weeks, 12 weeks, and then at 12-week intervals. Response was defined as the best response after treatment while the patient was on study using the lymphoma response criteria as described by Cheson et al.27
Investigational products
NeoRx Corporation supplied all agents to the clinical sites. At the clinical sites, agents were kept at 2° C to 8° C until injection or radiolabeling. The agents included in the study are described in the paragraphs that follow.
B9E9FP. The B9E9FP has been described previously.24 In brief, it is a tetramer of 4 single-chain variable regions (scFv) of the murine anti-CD20 antibody B9E9 fused to the genomic single-chain of streptavidin (SA) of Streptomyces avidinii. The B9E9 scFv gene consists of the variable regions of the kappa and gamma chains separated by a DNA linker sequence. The SA single-chain coding sequence is joined to the 3′ terminus of the B9E9 scFv gene, and the 2 genes are separated in frame by a second DNA linker sequence. The signal sequence from the SA gene is fused at the 5′ terminus of the B9E9Fusion gene under control of the lac promoter. The fusion protein is expressed in the periplasm of E coli. The protein spontaneously forms a stable soluble tetramer with a molecular weight of 173 688 Da. The B9E9FP was formulated at a concentration of 5 mg/mL in phosphate-buffered saline containing 5% sorbitol. It was supplied in 10 mL type I borosilicate glass vials as a sterile, clear, colorless solution for injection.
Rhenium-labeled fusion protein was supplied by NeoRx Corporation as an aseptically filled clear, sterile, solution for injection in a 100 mL glass vial containing approximately 20 mL phosphate buffer saline (PBS)/ascorbic acid mixture with 5% sorbitol. The total radioactivity per vial was approximately 40 mCi at calibration time.
Synthetic clearing agent (sCA). The sCA has been described previously25 and used in a prior phase 1 trial.23 It is a synthetic mono-biotin poly-N-acetyl-galactosamine compound. The biotin moiety binds to the streptavidin moiety in the B9E9FP and the carbohydrate interacts with a high-affinity asialoglycoprotein receptor on hepatocytes, resulting in rapid internalization and catabolism of the fusion protein/sCA complex. Thus, circulating B9E9Fusion bound to sCA is rapidly endocytosed into the liver cells, thereby eliminating its ability to bind to the subsequently administered biotin-radioisotope. The sCA was supplied as an aseptically filled sterile solution for injection. It was supplied in 10 mL type I borosilicate glass vials as a clear, colorless solution at a fill volume of 4.3 mL containing 12.7 mg sCA/mL. The sCA was stored for 4 weeks or less at 2° Cto8° C.
Radiolabeled DOTA-biotin. This reagent has been described previously28 and used in Pretarget trials.19-23 It is a synthetic molecule that consists of biotin linked to a macrocyclic amino benzyl DOTA chelate through an N-methyl glycine moiety, having a molecular weight of about 807 kDa. This small size allows it to distribute quickly throughout the body. This biotin ligand is captured by the B9E9FP prelocalized to CD20-expressing cells through binding to the streptavidin moiety. DOTA-biotin was provided as a sterile solution in water. It was supplied in 2 mL type I borosilicate glass vials at a fill volume of 0.3 mL and a concentration of 12 mg/mL and used for preparation of the radiolabeled material.
DOTA-biotin was radiolabeled with 90Y (a high-energy pure beta emitter) as the therapeutic agent and with 111In (gamma emitter) for imaging and organ dosimetry. The Yttrium-labeled DOTA-biotin was a terminally sterilized, clear solution approximately 30 mCi to 40 mCi at calibration time in 10 mL PBS with 55 mg/mL ascorbic acid in a 20 mL glass vial. The Indium-labeled DOTA-biotin contained approximately 6 mCi at calibration time, in 10 mL PBS with 55 mg/mL ascorbic acid. 111In-DOTA-biotin (5 mCi) was mixed with 15 mCi/m2 of 90Y-DOTA-biotin just prior to administration.
Pharmacokinetics
A streptavidin-specific enzyme-linked immunosorbent assay (ELISA) was developed for quantitation of B9E9FP in serum. B9E9FP concentration data measured by ELISA were reported in units of μg/mL. 186Re-B9E9FP concentration data were measured by gamma counting. Serum concentrations of B9E9FP based on radioactivity were corrected for decay of the radioisotope from the time of drug administration to the time of measurement by cocounting samples with a standard of the injected preparation. Analyses of 111In and 90Y-labeled DOTA-biotin concentrations in serum and urine were performed by gamma counting and liquid scintillation counting (LSC), respectively, and were reported in units of percent injected dose per milliliter. Reported 111In/90Y-DOTA-biotin serum concentrations were corrected for radioactive decay from the time of blood sampling until the time of measurement by cocounting samples with a standard of the injected preparation. Blood samples were obtained before and after the administration of the B9E9FP and the clearing agent. Blood and urine samples were collected before and after the administration of the radiolabeled DOTA-biotin.
Imaging/dosimetry
Planar quantitative gamma camera imaging was used to derive residence times in source organs. Gamma camera images were acquired immediately after 186Re-B9E9FP infusion, prior to the infusion of the clearing agent, and 24 hours later immediately prior to the infusion of the radiolabeled DOTA-biotin. Following DOTA-biotin infusion, images were acquired immediately, at 3 hours, and then daily for 3 days. 111In-DOTA-biotin was used as a surrogate imaging tracer for 90Y-DOTA-biotin.
Counts from the whole body and regions of interest over source organs were recorded. Source organs included the whole body, kidneys, liver, spleen, tumors, and other organs if activity was visible above body background, after the blood pool had cleared (eg, lungs and testes). Body background was subtracted. The fraction of injected dose in the whole body, all source organs, and plasma was derived to create time activity curves to estimate radiation-absorbed dose. The dose to marrow was estimated from the exposure of the marrow to circulating radioactivity, and doses from specific targeting of disease in the marrow was not considered. The 111In count data were used to predict the 90Y radiation absorbed dose. Dose estimates to the target organs were derived using MIRDOSE3 software using Windows 98.29 Dosimetry calculations were performed centrally at NeoRx Corporation and Pacific Northwest Laboratories, Richland, WA.
Immune response
Human anti–streptavidin antibody (HASA) titers were determined using a sandwich ELISA previously described.19 Relative reactivity was expressed as a multiple of the titer in a normal human serum pool from untreated individuals (normal human serum [NHS] units). Additionally, human anti–fusion antibody (HAFA) and human anti–murine antibody (HAMA) titers were determined using a double antigen capture ELISA.23
Results
Patient demographics
As shown in Table 2, 15 patients aged 35 to 80 years (median 59 years) were enrolled in the study; 3 in cohort A, 5 in cohort B, 4 in cohort C, and 3 in cohort D (Table 1 shows the doses of the different cohorts). One patient (no. 7 in cohort B) did not receive 90Y-DOTA-biotin due to transient renal insufficiency thought to be due to a CT contrast reaction that occurred after baseline studies and a large lymphoma tumor mass (7 × 8 cm) in the left kidney. The elevated creatinine (2.6 mg/dL) was noted before B9E9FP infusion and 90Y-DOTA-biotin was not administered (this patient was included for all safety evaluations, but excluded from efficacy analyses). There were 7 patients with bulky disease (masses ≥ 7 cm). All 15 patients had received prior chemotherapy with a median of 3 prior regimens (range, 1-7), and 2 patients had been previously treated with autologous bone marrow transplantation. No patients had received prior radioimmunotherapy. There were 5 patients (33%) who received prior conventional external beam radiation therapy (to < 20% of the active bone marrow regions).
Patient demographics and therapy response
Patient . | Cohort . | Age . | Sex . | Stage at enrollment . | Histology . | Prior RT . | Prior therapy . | Lymphoma involvement of BM . | Response . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | A | 59 | F | III | Mantle cell | No | CHOP, BMT (Auto) | No | CR |
| 2 | A | 61 | M | IV | Mantle cell | Yes | CHOP, FDR, FDR/MTX, ESHAP, MINE, RTX, ICE | Yes | PD |
| 3 | A | 63 | F | III | Follicular | No | CHOP, FDR, RTX | No | PR |
| 4 | B | 57 | F | IV | Mantle cell | No | CHOP, RTX, FDR, ESHAP | Yes | SD |
| 5 | B | 72 | M | IV | Mantle cell | Yes | CHOP, RTX, MINE, RTX, DHAP | Yes | PD |
| 6 | B | 61 | M | III | Mantle cell | No | FDR/MTX, RTX, FDR | No | SD |
| 7 | B | 68 | F | III | DLCL | No | CHOP/RTX, ICE | Yes | NE |
| 8 | B | 52 | M | III | DLCL | Yes | CHOP, RTX, DHAP, ICE | No | PD |
| 9 | C | 50 | M | IV | Mantle cell | No | CHOP | Yes | PD |
| 10 | C | 77 | M | IV | Follicular | Yes | CHOP/RTX, VP-16/MTX/PRD, FDR | Yes | PD |
| 11 | C | 35 | M | IV | DLCL | Yes | CHOP, RTX, ICE, BMT (Auto) | No | PD |
| 12 | C | 53 | M | IV | SLL | No | CHOP/RTX | No | CR |
| 13 | D | 38 | F | II | Follicular | No | FDR/MTX | No | SD |
| 14 | D | 53 | M | III | Follicular | No | CVP, FDR/MTX/DCD | No | PD |
| 15 | D | 80 | F | IV | Mantle cell | No | CP (×4), CHOP/R | No | PD |
Patient . | Cohort . | Age . | Sex . | Stage at enrollment . | Histology . | Prior RT . | Prior therapy . | Lymphoma involvement of BM . | Response . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | A | 59 | F | III | Mantle cell | No | CHOP, BMT (Auto) | No | CR |
| 2 | A | 61 | M | IV | Mantle cell | Yes | CHOP, FDR, FDR/MTX, ESHAP, MINE, RTX, ICE | Yes | PD |
| 3 | A | 63 | F | III | Follicular | No | CHOP, FDR, RTX | No | PR |
| 4 | B | 57 | F | IV | Mantle cell | No | CHOP, RTX, FDR, ESHAP | Yes | SD |
| 5 | B | 72 | M | IV | Mantle cell | Yes | CHOP, RTX, MINE, RTX, DHAP | Yes | PD |
| 6 | B | 61 | M | III | Mantle cell | No | FDR/MTX, RTX, FDR | No | SD |
| 7 | B | 68 | F | III | DLCL | No | CHOP/RTX, ICE | Yes | NE |
| 8 | B | 52 | M | III | DLCL | Yes | CHOP, RTX, DHAP, ICE | No | PD |
| 9 | C | 50 | M | IV | Mantle cell | No | CHOP | Yes | PD |
| 10 | C | 77 | M | IV | Follicular | Yes | CHOP/RTX, VP-16/MTX/PRD, FDR | Yes | PD |
| 11 | C | 35 | M | IV | DLCL | Yes | CHOP, RTX, ICE, BMT (Auto) | No | PD |
| 12 | C | 53 | M | IV | SLL | No | CHOP/RTX | No | CR |
| 13 | D | 38 | F | II | Follicular | No | FDR/MTX | No | SD |
| 14 | D | 53 | M | III | Follicular | No | CVP, FDR/MTX/DCD | No | PD |
| 15 | D | 80 | F | IV | Mantle cell | No | CP (×4), CHOP/R | No | PD |
CR indicates complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; BMT, bone marrow transplantation; FDR, fludarabine; MTX, mitoxantrone; ESHAP, etoposide, methylprednisolone, cytarabine, cisplatin; MINE, methyl-gag, ifosfamide, methotrexate, and etoposide; RTX, rituxan; ICE, ifosfamide, cytarabine, etoposide; DHAP, cisplatin, cytarabine, dexamethasone; CP, chlorambucil, prednisone.
Safety
All 15 patients were eligible for treatment with the Pretargeted radioimmunotherapy regimen based on the eligibility criteria specified in the protocol, and all of them were included in the safety evaluation.
As can be seen in Table 3, 11 of the 14 patients that received 90Y-DOTA-biotin had no hematologic toxicity. One of 14 had readily reversible grade II neutropenia (patient no. 1) and 2 patients (patients no. 5 and no. 11) had grades III and IV hematologic toxicity likely reflecting confounding factors. Patient no. 5 had received multiple chemotherapy regimens3 and 2 courses of radiation therapy for aggressive mantle cell lymphoma with marrow involvement. Although his platelet count was 141 000/mm3 15 days prior to treatment (baseline value) it had fallen to 98 000/mm3 on the first day of treatment, with a value of 49 000/mm3 at week 5 (nadir); the thrombocytopenia persisted for 62 days. His bone marrow at week 6 was extensively replaced with lymphoma cells (75% involvement of the marrow), and he was categorized as having progressive disease. His neutrophil blood counts followed a similar pattern of grade III toxicity more likely secondary to progressive disease than to the PRIT. Patient no. 11 had received 2 prior chemotherapy regimens, external beam radiation therapy, and most recently, high-dose chemotherapy with stem cell transplant. His baseline platelet count was 116 000/mm3; it decreased to 71 000/mm3 the week of therapy, and reached a nadir at week 3 (grade IV). This time course is quite atypical for RIT, where nadir hematologic counts are normally seen in weeks 5 to 7. Progressive disease was documented at week 6. Subsequently, this patient had grade IV neutropenia after palliative radiation therapy. Both patients had continued cytopenias and received palliative radiation therapy that started 88 and 90 days after treatment with the regimen.
Hematologic toxicity
. | . | Grades . | . | . | ||
|---|---|---|---|---|---|---|
| Cohort . | Patient no. . | Absolute neutrophil count . | Platelets . | Hemoglobin . | ||
| A | 1 | II (7/4)* | 0 | I | ||
| A | 2 | 0 | 0 | 0 | ||
| A | 3 | 0 | 0 | 0 | ||
| B | 4 | 0 | 0 | 0 | ||
| B | 5 | III (7/16)* | III (5/62)* | 0 | ||
| B | 6 | 0 | 0 | 0 | ||
| B | 7 | NA | NA | NA | ||
| B | 8 | 0 | 0 | 0 | ||
| C | 9 | 0 | 0 | 0 | ||
| C | 10 | 0 | 0 | 0 | ||
| C | 11 | IV (13/54+)* | IV (3/124+)* | IV (13/54+)* | ||
| C | 12 | 0 | 0 | 0 | ||
| D | 13 | 0 | 0 | 0 | ||
| D | 14 | 0 | 0 | 0 | ||
| D | 15 | 0 | 0 | 0 | ||
. | . | Grades . | . | . | ||
|---|---|---|---|---|---|---|
| Cohort . | Patient no. . | Absolute neutrophil count . | Platelets . | Hemoglobin . | ||
| A | 1 | II (7/4)* | 0 | I | ||
| A | 2 | 0 | 0 | 0 | ||
| A | 3 | 0 | 0 | 0 | ||
| B | 4 | 0 | 0 | 0 | ||
| B | 5 | III (7/16)* | III (5/62)* | 0 | ||
| B | 6 | 0 | 0 | 0 | ||
| B | 7 | NA | NA | NA | ||
| B | 8 | 0 | 0 | 0 | ||
| C | 9 | 0 | 0 | 0 | ||
| C | 10 | 0 | 0 | 0 | ||
| C | 11 | IV (13/54+)* | IV (3/124+)* | IV (13/54+)* | ||
| C | 12 | 0 | 0 | 0 | ||
| D | 13 | 0 | 0 | 0 | ||
| D | 14 | 0 | 0 | 0 | ||
| D | 15 | 0 | 0 | 0 | ||
NA indicates not applicable.
Nadir of the cytopenias in weeks/duration of the cytopenias in days.
Nonhematologic toxicities were generally transient and only grade I or II in severity (Table 4). These included hematuria with no alteration of the serum creatinine level in 4 patients (all grade I, microscopic), skin rash in 5 patients (all grades I and II), asthenia in 6 patients (5 grade I, and 1 grade III), fever in 2 patients (grade I), and arthralgia (grade III) with rash within 2 weeks of the study drugs in one patient, suggestive of a serum-sicknesslike syndrome (negative HAFA and anti-id titers and minimal elevated HASA titer at week 4). Although 6 patients experienced gastrointestinal symptoms grades I and II (abdominal pain associated with cholelithiasis, dyspepsia, nausea, vomiting, diarrhea) only in one patient was the event (grade III) thought to be possibly related to the pretargeted regimen (nausea, vomiting, diarrhea, and dehydration). The 111In-DOTA-biotin imaging studies in this latter patient did not show any gastrointestinal excretion or localization. No hepatic toxicity was noted in these patients.
Nonhematologic toxicity
. | Cohort A . | Cohort B . | Cohort C . | Cohort D . |
|---|---|---|---|---|
| Hematuria | ||||
| Grade I | 2 | 2 | 0 | 0 |
| Grade II | 0 | 0 | 0 | 0 |
| Grade III | 0 | 0 | 0 | 0 |
| Skin rash | ||||
| Grade I | 0 | 2 | 1 | 1 |
| Grade II | 1 | 0 | 0 | 1 |
| Grade III | 0 | 0 | 0 | 0 |
| GI symptoms (nausea, vomiting) | ||||
| Grade I | 0 | 1 | 0 | 0 |
| Grade II | 0 | 0 | 0 | 0 |
| Grade III | 0 | 0 | 1 | 0 |
| Asthenia | ||||
| Grade I | 1 | 2 | 2 | 0 |
| Grade II | 0 | 0 | 0 | 0 |
| Grade III | 0 | 0 | 0 | 1 |
. | Cohort A . | Cohort B . | Cohort C . | Cohort D . |
|---|---|---|---|---|
| Hematuria | ||||
| Grade I | 2 | 2 | 0 | 0 |
| Grade II | 0 | 0 | 0 | 0 |
| Grade III | 0 | 0 | 0 | 0 |
| Skin rash | ||||
| Grade I | 0 | 2 | 1 | 1 |
| Grade II | 1 | 0 | 0 | 1 |
| Grade III | 0 | 0 | 0 | 0 |
| GI symptoms (nausea, vomiting) | ||||
| Grade I | 0 | 1 | 0 | 0 |
| Grade II | 0 | 0 | 0 | 0 |
| Grade III | 0 | 0 | 1 | 0 |
| Asthenia | ||||
| Grade I | 1 | 2 | 2 | 0 |
| Grade II | 0 | 0 | 0 | 0 |
| Grade III | 0 | 0 | 0 | 1 |
GI indicates gastrointestinal.
Data show number of patients having toxicity.
As of June 2003, a total of 8 patients had died. None of the patients died while on study. There were 7 patients who died due to disease progression (8, 9, 20, 21, 28, 30, and 65 weeks after therapy with the pretargeted regimen), and one died in week 45 with multisystem organ failure after high-dose chemotherapy with autologous peripheral stem cell rescue (PBSCT).
Efficacy
There were 14 patients evaluable for response (one patient did not receive 90Y and was not included in efficacy analyses). As can be seen in Table 2, 2 patients achieved CR with a duration of 91 and 325 days, respectively, and one patient had a partial remission (PR) lasting 297 days. Thus, the overall objective response rate was 21% (3/14). In addition, 3 patients (21%) had stable disease. Median survival for the 14 patients was 316 days and median time to progression was 46 days.
Immunogenicity
There were 14 patients followed for antibody response (mean follow-up 24 weeks; range, 7-55 weeks; Table 5). Six patients have remained negative for all 3 specificities evaluated in the study (human anti–fusion protein antibody [HAFA], human anti–streptavidin antibody [HASA], and human anti–murine antibody [HAMA]). Three patients developed a strong antibody response to all 3 B9E9FP motifs with rapid onset (3 to 7 weeks) and persistence for 22+, 29+, and 39+ weeks. Five patients developed modest antibody responses to only one or 2 of the assay target molecules of variable duration and the responses were often delayed (13-28 weeks). Of the 5 patients who received the higher dose of B9E9FP (320 mg/m2), all had at least a transient positive titer to at least one of the motifs. Of the 9 patients receiving the lower dose (160 mg/m2), 6 had no antibody response, 2 had a modest response, and one had a major antibody response. There was no apparent correlation between clinical adverse events and the development of an antibody response. Antibody response also was not correlated with the type of B-cell lymphoma.
Immunogenicity
. | . | Maximum titer, NHS units (weeks after injection) . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Cohort . | Patient . | HAFA . | HASA . | Anti-murine . | Follow-up, wk . | ||
| A | 1 | N | 7 (28) | N | 55 | ||
| A | 2 | N | N | N | 12 | ||
| A | 3 | N | N | N | 42 | ||
| B | 4 | 6 (13) | N | 5 (13) | 25 | ||
| B | 5 | N | N | N | 17 | ||
| B | 6 | 683 (3) | 1527 (7) | 42 (3) | 39 | ||
| B | 8 | N | N | N | 14 | ||
| C | 9 | 231 (4) | 642 (4) | 22 (4) | 22 | ||
| C | 10 | 208 (4) | 65 (6) | 102 (4) | 29 | ||
| C | 11 | 4 (4) | N | N | 15 | ||
| C | 12 | N | 11 (26) | N | 36 | ||
| D | 13 | 3 (2) | 4 (4) | N | 12 | ||
| D | 14 | N | N | N | 7 | ||
| D | 15 | N | N | N | 7 | ||
. | . | Maximum titer, NHS units (weeks after injection) . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Cohort . | Patient . | HAFA . | HASA . | Anti-murine . | Follow-up, wk . | ||
| A | 1 | N | 7 (28) | N | 55 | ||
| A | 2 | N | N | N | 12 | ||
| A | 3 | N | N | N | 42 | ||
| B | 4 | 6 (13) | N | 5 (13) | 25 | ||
| B | 5 | N | N | N | 17 | ||
| B | 6 | 683 (3) | 1527 (7) | 42 (3) | 39 | ||
| B | 8 | N | N | N | 14 | ||
| C | 9 | 231 (4) | 642 (4) | 22 (4) | 22 | ||
| C | 10 | 208 (4) | 65 (6) | 102 (4) | 29 | ||
| C | 11 | 4 (4) | N | N | 15 | ||
| C | 12 | N | 11 (26) | N | 36 | ||
| D | 13 | 3 (2) | 4 (4) | N | 12 | ||
| D | 14 | N | N | N | 7 | ||
| D | 15 | N | N | N | 7 | ||
NHS indicates normal human serum pool; positive response defined in “Patients, materials and methods.” N indicates negative.
Pharmacokinetics
B9E9FP.Table 6 provides the pharmacokinetic parameters for the B9E9FP. The fusion protein plasma disappearance curves fit a one-compartment model with a plasma T½ of 25±6 hours. The plasma T½ at the 320 mg/m2 dose (n = 5, includes patient 13 in cohort D who inadvertently received a dose of 320 mg/m2) was 26±2 hours, which was not different than the 24±2 hours plasma T½ (n = 11) at 160 mg/m2 dose. The higher dose of 320 mg/m2 produced a proportional increase in maximum concentration (Cmax) and area under the curve (AUC; 223 ± 20 μg/mL and 7529 ± 536 h/μg/mL, respectively) as compared with the values observed at the 160 mg/m2 dose (112 ± 8.5 μg/mL and 3458 ± 255 h/μg/mL, respectively). The initial plasma volume of distribution was 3034 ± 566 mL, which approximates normal plasma volume.
Pharmacokinetics of the B9E9FP
Patient . | Dose, mg/m2 . | Cmax, μg/mL . | AUC, μg*h/mL . | Vd, mL . | T1/2, h . | Clearance, mL/h . |
|---|---|---|---|---|---|---|
| Cohort A | ||||||
| 1 | 160 | 82 | 3167 | 3462 | 30 | 80 |
| 2 | 160 | 104 | 3125 | 2950 | 23 | 89 |
| 3 | 160 | 104 | 5010 | 2814 | 38 | 51 |
| Cohort average | — | 97 | 3767 | 3075 | 30 | 73 |
| SD | — | 12 | 1076 | 341 | 7.6 | 19 |
| Cohort B | ||||||
| 4 | 160 | 138 | 3308 | 3123 | 20.3 | 106 |
| 5 | 160 | 67 | 2128 | 4155 | 21.9 | 132 |
| 6 | 160 | 161 | 3052 | 2178 | 15.6 | 97 |
| 7 | 160 | 104 | 3007 | 3763 | 22.9 | 114 |
| 8 | 160 | 120 | 3798 | 2904 | 23.5 | 86 |
| Cohort average | — | 116 | 3155 | 3197 | 21.4 | 104 |
| SD | — | 37 | 635 | 747 | 3.8 | 17 |
| Cohort C | ||||||
| 9 | 320 | 292 | 6876 | 2645 | 18.5 | 99 |
| 10 | 320 | 225 | 8393 | 2826 | 28.2 | 69 |
| 11 | 320 | 165 | 5734 | 3730 | 25.6 | 101 |
| 12 | 320 | 213 | 8547 | 3475 | 32.1 | 75 |
| Cohort average | — | 224 | 7388 | 3169 | 26.1 | 86 |
| SD | — | 52 | 1336 | 517 | 5.7 | 16 |
| Cohort D | ||||||
| 13 | 320* | 224.4 | 8097 | 2892 | 25.4 | 79 |
| 14 | 160 | 122.4 | 4445 | 2579 | 27.4 | 65 |
| 15 | 160 | 125.6 | 3542 | 2152 | 19.8 | 76 |
| Cohort average | — | 157.5 | 5361 | 2541 | 24.2 | 73 |
| SD | — | 58.0 | 2412 | 371 | 4.0 | 7 |
| Patient average | — | — | — | 3034 | 25 | 87 |
| SD | — | — | — | 566 | 6.0 | 15 |
Patient . | Dose, mg/m2 . | Cmax, μg/mL . | AUC, μg*h/mL . | Vd, mL . | T1/2, h . | Clearance, mL/h . |
|---|---|---|---|---|---|---|
| Cohort A | ||||||
| 1 | 160 | 82 | 3167 | 3462 | 30 | 80 |
| 2 | 160 | 104 | 3125 | 2950 | 23 | 89 |
| 3 | 160 | 104 | 5010 | 2814 | 38 | 51 |
| Cohort average | — | 97 | 3767 | 3075 | 30 | 73 |
| SD | — | 12 | 1076 | 341 | 7.6 | 19 |
| Cohort B | ||||||
| 4 | 160 | 138 | 3308 | 3123 | 20.3 | 106 |
| 5 | 160 | 67 | 2128 | 4155 | 21.9 | 132 |
| 6 | 160 | 161 | 3052 | 2178 | 15.6 | 97 |
| 7 | 160 | 104 | 3007 | 3763 | 22.9 | 114 |
| 8 | 160 | 120 | 3798 | 2904 | 23.5 | 86 |
| Cohort average | — | 116 | 3155 | 3197 | 21.4 | 104 |
| SD | — | 37 | 635 | 747 | 3.8 | 17 |
| Cohort C | ||||||
| 9 | 320 | 292 | 6876 | 2645 | 18.5 | 99 |
| 10 | 320 | 225 | 8393 | 2826 | 28.2 | 69 |
| 11 | 320 | 165 | 5734 | 3730 | 25.6 | 101 |
| 12 | 320 | 213 | 8547 | 3475 | 32.1 | 75 |
| Cohort average | — | 224 | 7388 | 3169 | 26.1 | 86 |
| SD | — | 52 | 1336 | 517 | 5.7 | 16 |
| Cohort D | ||||||
| 13 | 320* | 224.4 | 8097 | 2892 | 25.4 | 79 |
| 14 | 160 | 122.4 | 4445 | 2579 | 27.4 | 65 |
| 15 | 160 | 125.6 | 3542 | 2152 | 19.8 | 76 |
| Cohort average | — | 157.5 | 5361 | 2541 | 24.2 | 73 |
| SD | — | 58.0 | 2412 | 371 | 4.0 | 7 |
| Patient average | — | — | — | 3034 | 25 | 87 |
| SD | — | — | — | 566 | 6.0 | 15 |
Results determined by ELISA (see “Patients, materials, and methods”).
Cmax indicates observed maximum concentration; AUC, area under the curve; Vd, volume of distribution; T1/2, half-life.
Patient 13 inadvertently received 320 mg/m2.
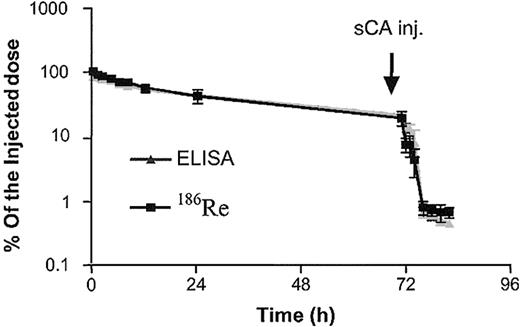
Cohorts A and C had 186R trace-labeled B9E9FP with phamacokinetic parameters similar to those calculated from the protein levels (data not shown; Figure 1).
B9E9FP serum clearance. Serum B9E9FP concentration data from patients in cohort A (n = 3) generated from 186Re counting or ELISA shown as percent of injected dose (mean ± SD).
B9E9FP serum clearance. Serum B9E9FP concentration data from patients in cohort A (n = 3) generated from 186Re counting or ELISA shown as percent of injected dose (mean ± SD).
Synthetic clearing agent (sCA). The dose of sCA, 45 mg/m2, provides significant molar excess for the amount of B9E9FP circulating at 48 and 72 hours. The B9E9FP levels were reduced 98.4%±1.6% 6 hours after sCA infusion (n = 14). A typical example of the effect of sCA on B9E9FP levels is illustrated in Figure 1 for the 3 patients in cohort A. The nadir value 6 hours after sCA was similar for patients receiving 320 mg/m2 (0.32 ± 0.09 μg/mL) as those receiving 160 mg/m2 (0.44 ± 0.22 μg/mL). Therefore, at the dose levels studied, the synthetic clearing agent was uniformly able to reduce circulating B9E9FP levels to less than 5% of preclearing agent levels.
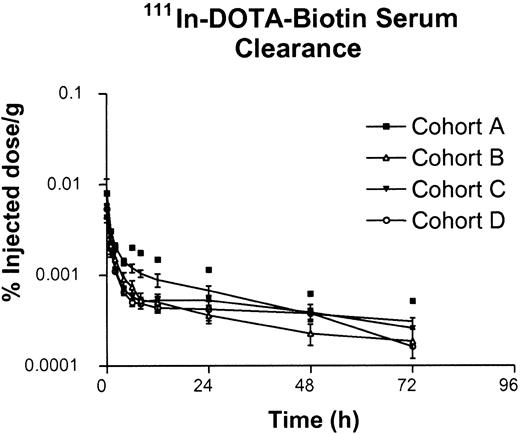
DOTA-biotin. The pharmacokinetic data for radiolabeled DOTA-biotin are provided in Table 7. The rapid whole-body distribution is reflected in the calculated high volume of distribution, as well as in the initial plasma concentration of only 25% ± 2.3% of the injected dose (n = 10) in the plasma volume (drawn 5 ± 0.3 minutes after infusion). The plasma disappearance curve fita 2-compartment model (Figure 2) with a T½ alpha of 0.62 ± 0.35 hours and a T½ beta of 24 ± 11 hours. The alpha component of the curve accounts for more than 95% of the injected dose whereas the beta component probably reflects DOTA-biotin bound to a small residual pool of B9E9FP, which had not undergone hepatic clearance. The 90Y kinetics were similar to that of 111In (Table 7).
DOTA-biotin pharmacokinetic parameters
. | 111In pharmacokinetics . | . | . | . | 90Yttrium pharmacokinetics . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients . | Vd, mL . | Clearance, mL/h . | T1/2 alpha, h . | T1/2 beta, h . | Vd, mL . | Clearance, mL/h . | T1/2 alpha, h . | T1/2 beta, h . | ||||||
| Cohort A | ||||||||||||||
| 2 | 5 361 | 1 538 | 0.45 | 18 | 5 690 | 1 883 | 0.48 | 19 | ||||||
| 3 | 4 682 | 1 146 | 0.41 | 20 | 5 545 | 1 675 | 0.52 | 20 | ||||||
| Average | 5 021 | 1 342 | 0.43 | 20 | 5 617 | 1 779 | 0.50 | 19 | ||||||
| SD | ||||||||||||||
| Cohort B | ||||||||||||||
| 4 | 3 616 | 2 435 | 0.22 | 13 | 4 019 | 3 341 | 0.22 | 10 | ||||||
| 5 | 15 300 | 1 611 | 1.56 | 30 | 14 829 | 1 871 | 1.47 | 23 | ||||||
| 6 | 13 829 | 4 583 | 0.15 | 13 | 11 631 | 3 926 | 0.17 | 14 | ||||||
| 8 | 9 293 | 1 265 | 0.90 | 43 | 9 303 | 2 083 | 0.88 | 24 | ||||||
| Average | 10 509 | 2 473 | 0.71 | 25 | 9 946 | 2 805 | 0.69 | 18 | ||||||
| SD | 5 259 | 1 490 | 0.66 | 15 | 4 554 | 989 | 0.62 | 7 | ||||||
| Cohort C | ||||||||||||||
| 9 | 18 524 | 6 150 | 0.80 | 15 | 21 773 | 6 400 | 0.72 | 18 | ||||||
| 10 | 15 603 | 1 664 | 0.69 | 46 | 14 275 | 1 306 | 0.73 | 47 | ||||||
| 11 | 10 823 | 2 435 | 0.58 | 18 | 12 932 | 3 318 | 0.68 | 18 | ||||||
| 12 | 10 506 | 1 501 | 0.55 | 35 | 10 704 | 1 780 | 0.56 | 33 | ||||||
| Average | 13 869 | 2 937 | 0.65 | 29 | 14 921 | 3 201 | 0.67 | 29 | ||||||
| SD | 3 892 | 2 180 | 0.12 | 15 | 4 799 | 2 299 | 0.08 | 14 | ||||||
| Cohort D | ||||||||||||||
| 13 | 10 080 | 2 002 | 0.51 | 30 | 9 068 | 1 871 | 0.54 | 31 | ||||||
| 14 | 9 497 | 2 089 | 0.56 | 23 | 9 081 | 1 965 | 0.55 | 28 | ||||||
| 15 | 15 802 | 4 192 | 0.67 | 13 | 12 309 | 2 834 | 0.73 | 14 | ||||||
| Average | 11 793 | 2 761 | 0.58 | 22 | 10 153 | 2 223 | 0.60 | 24 | ||||||
| SD | 3 484 | 1 240 | 0.08 | 9 | 1 867 | 531 | 0.10 | 9 | ||||||
| Overall average | 10 995 | 2 508 | 0.62 | 24 | 10 858 | 2 635 | 0.63 | 23 | ||||||
| Overall SD | 4 656 | 1 520 | 0.35 | 11 | 4 691 | 1 377 | 0.32 | 10 | ||||||
. | 111In pharmacokinetics . | . | . | . | 90Yttrium pharmacokinetics . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients . | Vd, mL . | Clearance, mL/h . | T1/2 alpha, h . | T1/2 beta, h . | Vd, mL . | Clearance, mL/h . | T1/2 alpha, h . | T1/2 beta, h . | ||||||
| Cohort A | ||||||||||||||
| 2 | 5 361 | 1 538 | 0.45 | 18 | 5 690 | 1 883 | 0.48 | 19 | ||||||
| 3 | 4 682 | 1 146 | 0.41 | 20 | 5 545 | 1 675 | 0.52 | 20 | ||||||
| Average | 5 021 | 1 342 | 0.43 | 20 | 5 617 | 1 779 | 0.50 | 19 | ||||||
| SD | ||||||||||||||
| Cohort B | ||||||||||||||
| 4 | 3 616 | 2 435 | 0.22 | 13 | 4 019 | 3 341 | 0.22 | 10 | ||||||
| 5 | 15 300 | 1 611 | 1.56 | 30 | 14 829 | 1 871 | 1.47 | 23 | ||||||
| 6 | 13 829 | 4 583 | 0.15 | 13 | 11 631 | 3 926 | 0.17 | 14 | ||||||
| 8 | 9 293 | 1 265 | 0.90 | 43 | 9 303 | 2 083 | 0.88 | 24 | ||||||
| Average | 10 509 | 2 473 | 0.71 | 25 | 9 946 | 2 805 | 0.69 | 18 | ||||||
| SD | 5 259 | 1 490 | 0.66 | 15 | 4 554 | 989 | 0.62 | 7 | ||||||
| Cohort C | ||||||||||||||
| 9 | 18 524 | 6 150 | 0.80 | 15 | 21 773 | 6 400 | 0.72 | 18 | ||||||
| 10 | 15 603 | 1 664 | 0.69 | 46 | 14 275 | 1 306 | 0.73 | 47 | ||||||
| 11 | 10 823 | 2 435 | 0.58 | 18 | 12 932 | 3 318 | 0.68 | 18 | ||||||
| 12 | 10 506 | 1 501 | 0.55 | 35 | 10 704 | 1 780 | 0.56 | 33 | ||||||
| Average | 13 869 | 2 937 | 0.65 | 29 | 14 921 | 3 201 | 0.67 | 29 | ||||||
| SD | 3 892 | 2 180 | 0.12 | 15 | 4 799 | 2 299 | 0.08 | 14 | ||||||
| Cohort D | ||||||||||||||
| 13 | 10 080 | 2 002 | 0.51 | 30 | 9 068 | 1 871 | 0.54 | 31 | ||||||
| 14 | 9 497 | 2 089 | 0.56 | 23 | 9 081 | 1 965 | 0.55 | 28 | ||||||
| 15 | 15 802 | 4 192 | 0.67 | 13 | 12 309 | 2 834 | 0.73 | 14 | ||||||
| Average | 11 793 | 2 761 | 0.58 | 22 | 10 153 | 2 223 | 0.60 | 24 | ||||||
| SD | 3 484 | 1 240 | 0.08 | 9 | 1 867 | 531 | 0.10 | 9 | ||||||
| Overall average | 10 995 | 2 508 | 0.62 | 24 | 10 858 | 2 635 | 0.63 | 23 | ||||||
| Overall SD | 4 656 | 1 520 | 0.35 | 11 | 4 691 | 1 377 | 0.32 | 10 | ||||||
Vd indicates volume of distribution; T1/2, half-life.
Elimination of DOTA-biotin. Serum 111In-DOTA-biotin concentration from cohorts A, B, C, and D generated from 111In gamma counting shown as percent of injected dose per gram (mean ± SEM).
Elimination of DOTA-biotin. Serum 111In-DOTA-biotin concentration from cohorts A, B, C, and D generated from 111In gamma counting shown as percent of injected dose per gram (mean ± SEM).
The first voided urine sample was obtained 1.1 ± 0.5 hours (range, 0.5-2 hours) after DOTA-biotin infusion and contained 40% ± 14% (mean ± SD) of the administered dose. Urinary excretion over 55 to 72 hours accounted for 83%± 18% of the administered dose. Thus, urinary excretion is the major route of DOTA-biotin egress from the body, secondary to the low molecular weight of this ligand. The amount of DOTA-biotin in the urine did not change appreciably between the 4 cohorts studied, suggesting that the dose of fusion protein administered did not significantly impact the clearance of radiolabeled DOTA-biotin, nor did the specific activity of the radiolabeled DOTA-biotin over the ranges used in this study.
Imaging and dosimetry
Imaging with 186Re.186Re-labeled B9E9FP images were acquired for patients in cohorts A and C, and were similar in both groups. The images before the administration of the sCA were typical of a directly radiolabeled monoclonal antibody with substantial activity in the blood pool (heart and large vessels). Positive tumor localization was observed in some patients prior to clearing agent. Images after the administration of the sCA showed a marked decrease in the blood pool with an increase in the liver and bowel activity due to the hepatic metabolism and biliary excretion of the 186Rhenium-B9E9Fusion clearing agent complex.
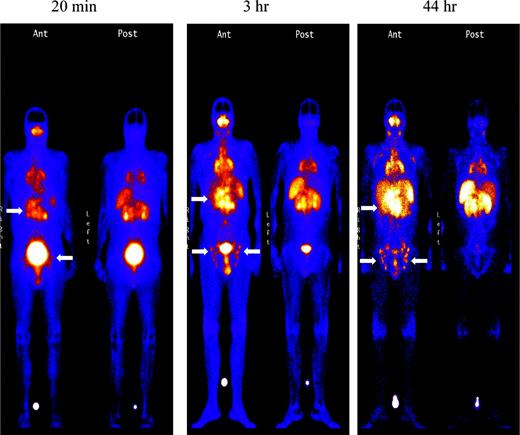
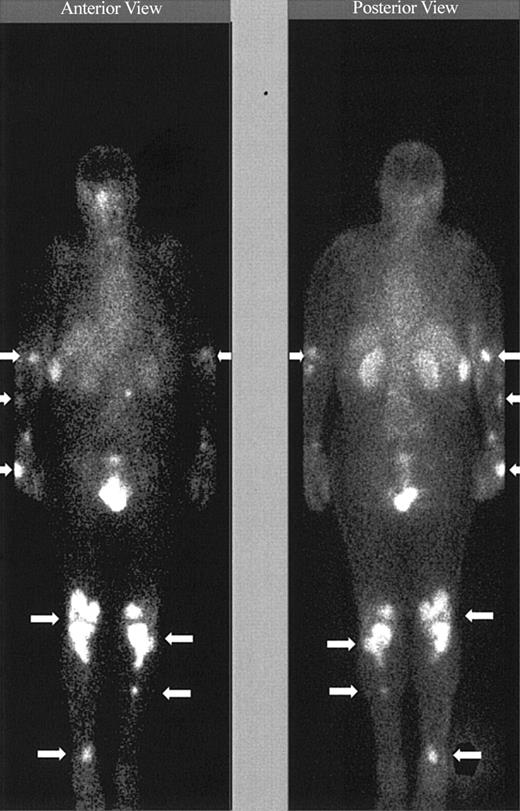
Imaging with Indium. Images obtained 20 minutes and 3 hours after infusion of the 111In-labeled DOTA-biotin demonstrated a rapid clearance of the blood pool radioactivity with early prominent kidney and bladder imaging reflecting the urinary route of excretion (Figure 3). Tumor sites were well delineated by 3 hours in all patients and were often visualized as early as 20 minutes after injection (Figure 3). Figure 4 demonstrates dramatic radiolocalization of the radionuclide in known nodal and extranodal tumor sites: subcutaneous nodules, skin, testes, and knees. The patient had serious knee pain, and magnetic resonance imaging confirmed that the unusual involvement around the knee area was consistent with non-Hodgkin lymphoma involvement of bone. Tumor localization of radioactivity persisted for the duration of the study. In 15 tumors evaluated, the estimated localization per gram of tumor was 0.01% to 0.04% of the injected dose.
Tumor targeting of known sites of disease. Images obtained 20 minutes, 3 hours, and 44 hours after the administration of 111In (5 mCi)/90Y (15 mCi/m2)–DOTA-biotin. Gamma camera wholebody scans of patient no. 1 (anterior views) obtained at 20 minutes, 3 hours, and 44 hours after infusion of 111In/90Y-DOTA-biotin. Arrows are showing radionuclide localization to known sites of tumor involvement.
Tumor targeting of known sites of disease. Images obtained 20 minutes, 3 hours, and 44 hours after the administration of 111In (5 mCi)/90Y (15 mCi/m2)–DOTA-biotin. Gamma camera wholebody scans of patient no. 1 (anterior views) obtained at 20 minutes, 3 hours, and 44 hours after infusion of 111In/90Y-DOTA-biotin. Arrows are showing radionuclide localization to known sites of tumor involvement.
Tumor targeting of known sites of disease. Image obtained 46 hours after the administration of 111In (5 mCi)/90Y (15 mCi/m2)–DOTA-biotin. Gamma camera whole-body scans of patient no. 3 (anterior view on left and posterior view on right) obtained 46 hours after infusion of 111In/90Y-DOTA-biotin. Arrows are showing radionuclide localization to known sites of tumor involvement.
Tumor targeting of known sites of disease. Image obtained 46 hours after the administration of 111In (5 mCi)/90Y (15 mCi/m2)–DOTA-biotin. Gamma camera whole-body scans of patient no. 3 (anterior view on left and posterior view on right) obtained 46 hours after infusion of 111In/90Y-DOTA-biotin. Arrows are showing radionuclide localization to known sites of tumor involvement.
Dosimetry. Dosimetry estimates for normal organs and tumor sites in 14 patients are presented in Table 8. The whole-body, liver, and bone marrow estimates were consistent and similarly low for all 4 cohorts of patients. Spleen dose estimates were quite variable, presumably reflecting varying degrees of lymphoma involvement with no significant differences among the 4 cohorts. The bladder and kidney had the highest radiation exposure with the highest values observed in cohort C, although the cohorts were not significantly different from each other given the small number of patients. Doubling the specific activity of the 90Y-DOTA-biotin (cohort D) produced very similar kidney and bladder estimates as the standard specific activity (cohort B). The tumor dose estimates varied between 2 cGy/mCi and 69 cGy/mCi for individual tumor sites with an overall mean of 26 ± 4 cGy/mCi (10 patients, 20 tumor sites, distributed across all 4 cohorts). All 4 cohorts achieved high tumor to whole-body radiation dose ratios, with an average of 49, similar to prior pretargeting RIT studies using chemical conjugates of antibody and streptavidin. There were no statistically significant differences between estimated tumor doses between cohorts, reflecting the limited number of observations and the expected variation in tumor size, localization, and other variables that influence tumor dose estimates. The dose to normal marrow, based on the serum clearance alone, was not more than 8 cGy for any patient (mean 0.25 ± 0.04 cGy/mCi).
Dosimetry (cGy/mCi) predicted from 111In data
. | 90Y cGy/mCi . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients . | Whole body . | Marrow . | Liver . | Spleen . | Kidney . | Bladder wall . | Tumor(s) . | ||||||
| Cohort A | |||||||||||||
| 1 | ND | ND | ND | ND | ND | ND | ND | ||||||
| 2 | 0.68 | 0.27 | 0.89 | 1.34 | 3.21 | 4.28 | ND | ||||||
| 3 | 0.59 | 0.36 | 1.32 | 1.92 | 6.08 | 4.47 | 45, 56 | ||||||
| Mean ± SD | 0.6 ± 0.04 | 0.3 ± 0.04 | 1.1 ± 0.2 | 1.6 ± 0.3 | 4.6 ± 1.4 | 4.4 ± 0.09 | NA | ||||||
| Cohort B | |||||||||||||
| 4 | 0.30 | 0.17 | 1.28 | 2.33 | 6.7 | 5.02 | 51 | ||||||
| 5 | 0.75 | 0.16 | 1.89 | 6.97 | 5.21 | 4.11 | 8, 2 | ||||||
| 6 | 0.49 | 0.08 | 2.14 | 3.01 | 11.2 | 9.67 | 13, 18, 20 | ||||||
| 8 | 0.36 | 0.06 | 1.15 | 0.98 | 5.5 | 10.45 | 19, 45 | ||||||
| Mean ± SD | 0.5 ± 0.09 | 0.1 ± 0.02 | 1.6 ± 0.2 | 3.3 ± 1.3 | 7.2 ± 1.4 | 7.3 ± 1.6 | NA | ||||||
| Cohort C | |||||||||||||
| 9 | 0.45 | 0.70 | 1.06 | 5.15 | 15.0 | 9.45 | 8, 30, 27 | ||||||
| 10 | 0.71 | 0.26 | 1.05 | 3.1 | 7.7 | 7.63 | 9 | ||||||
| 11 | 0.54 | 0.20 | 2.13 | 4.5 | 16.6 | 10.1 | ND | ||||||
| 12 | 0.71 | 0.23 | 0.97 | 4.82 | 5.31 | 7.99 | ND | ||||||
| Mean ± SD | 0.6 ± 0.06 | 0.3 ± 0.11 | 1.3 ± 0.3 | 4.4 ± 0.5 | 11 ± 3 | 8.8 ± 0.58 | NA | ||||||
| Cohort D | |||||||||||||
| 13 | 0.49 | 0.21 | 1.26 | 13.5 | 8.13 | 8.14 | 69, 34 | ||||||
| 14 | 0.59 | 0.17 | 1.15 | 3.87 | 9.73 | 7.75 | 13, 32, 20 | ||||||
| 15 | 0.46 | 0.16 | 0.69 | 0.69 | 6.28 | 7.34 | 6 | ||||||
| Mean ± SD | 0.5 ± 0.03 | 0.2 ± 0.01 | 1 ± 0.2 | 6 ± 3.9 | 8 ± 1 | 7.7 ± 0.23 | NA | ||||||
| Overall mean ± SD | 0.6 ± 0.05 | 0.2 ± 0.04 | 1.2 ± 0.2 | 3.8 ± 1.2 | 7.7 ± 1.7 | 7 ± 0.63 | 26 ± 4 | ||||||
. | 90Y cGy/mCi . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients . | Whole body . | Marrow . | Liver . | Spleen . | Kidney . | Bladder wall . | Tumor(s) . | ||||||
| Cohort A | |||||||||||||
| 1 | ND | ND | ND | ND | ND | ND | ND | ||||||
| 2 | 0.68 | 0.27 | 0.89 | 1.34 | 3.21 | 4.28 | ND | ||||||
| 3 | 0.59 | 0.36 | 1.32 | 1.92 | 6.08 | 4.47 | 45, 56 | ||||||
| Mean ± SD | 0.6 ± 0.04 | 0.3 ± 0.04 | 1.1 ± 0.2 | 1.6 ± 0.3 | 4.6 ± 1.4 | 4.4 ± 0.09 | NA | ||||||
| Cohort B | |||||||||||||
| 4 | 0.30 | 0.17 | 1.28 | 2.33 | 6.7 | 5.02 | 51 | ||||||
| 5 | 0.75 | 0.16 | 1.89 | 6.97 | 5.21 | 4.11 | 8, 2 | ||||||
| 6 | 0.49 | 0.08 | 2.14 | 3.01 | 11.2 | 9.67 | 13, 18, 20 | ||||||
| 8 | 0.36 | 0.06 | 1.15 | 0.98 | 5.5 | 10.45 | 19, 45 | ||||||
| Mean ± SD | 0.5 ± 0.09 | 0.1 ± 0.02 | 1.6 ± 0.2 | 3.3 ± 1.3 | 7.2 ± 1.4 | 7.3 ± 1.6 | NA | ||||||
| Cohort C | |||||||||||||
| 9 | 0.45 | 0.70 | 1.06 | 5.15 | 15.0 | 9.45 | 8, 30, 27 | ||||||
| 10 | 0.71 | 0.26 | 1.05 | 3.1 | 7.7 | 7.63 | 9 | ||||||
| 11 | 0.54 | 0.20 | 2.13 | 4.5 | 16.6 | 10.1 | ND | ||||||
| 12 | 0.71 | 0.23 | 0.97 | 4.82 | 5.31 | 7.99 | ND | ||||||
| Mean ± SD | 0.6 ± 0.06 | 0.3 ± 0.11 | 1.3 ± 0.3 | 4.4 ± 0.5 | 11 ± 3 | 8.8 ± 0.58 | NA | ||||||
| Cohort D | |||||||||||||
| 13 | 0.49 | 0.21 | 1.26 | 13.5 | 8.13 | 8.14 | 69, 34 | ||||||
| 14 | 0.59 | 0.17 | 1.15 | 3.87 | 9.73 | 7.75 | 13, 32, 20 | ||||||
| 15 | 0.46 | 0.16 | 0.69 | 0.69 | 6.28 | 7.34 | 6 | ||||||
| Mean ± SD | 0.5 ± 0.03 | 0.2 ± 0.01 | 1 ± 0.2 | 6 ± 3.9 | 8 ± 1 | 7.7 ± 0.23 | NA | ||||||
| Overall mean ± SD | 0.6 ± 0.05 | 0.2 ± 0.04 | 1.2 ± 0.2 | 3.8 ± 1.2 | 7.7 ± 1.7 | 7 ± 0.63 | 26 ± 4 | ||||||
ND indicates not determined; NA, not applicable.
Discussion
This pretargeting RIT strategy utilizing the high-affinity binding of streptavidin and biotin has had dramatic efficacy in preclinical animal models.18,24 Phase 1 and 2 trials20,21 in patients using a chemical conjugate of streptavidin with NRLU10 (anti-epCAM) demonstrated high tumor to whole-body radiation dose ratios and considerable sparing of marrow toxicity due to the rapid distribution and excretion of the small-molecular-weight radiation moiety (90Y-DOTA-biotin). However, localization of the NRLU10-streptavidin targeting component in the bowel and kidney resulted in high estimated absorbed radiation doses to these organs with dose-limiting toxicity.21 A subsequent pilot study in patients with B-cell non-Hodgkin lymphoma receiving an anti-CD20 (chimeric 2B8; Rituxan) monoclonal antibody chemically conjugated to streptavidin produced no targeting of the gastrointestinal tract and evidence of efficacy at moderate doses of 90Y-DOTA-biotin.23 This experience encouraged further development of this strategy using a second-generation targeting molecule produced by recombinant DNA technology.
In this trial, the B9E9FP had a plasma T½ of 25 plus or minus 6 hours at both doses evaluated, 160 mg/m2 and 320 mg/m2. Following infusion of the synthetic clearing agent, the B9E9FP was rapidly cleared from the circulation by hepatic clearance and metabolism. The subsequent infusion of 111In 90Y DOTA-biotin resulted in rapid radioconjugate localization to tumor sites and rapid whole-body distribution and excretion. Thus, the fusion protein performed well as the targeting moiety in this pretarget RIT format. In addition, there were no significant toxicities noted related to fusion protein infusion and/or clearance. It is interesting that this unusual molecule composed of 4 antigen combining sites (sFvs) and tetrameric streptavidin as the backbone in place of antibody Fc region behaves quite similar to antibody-streptavidin conjugates in vivo.
The radiation dosimetry estimates reflected low whole-body, marrow, and liver exposure as in prior pretargeting RIT trials.20,23 Dose estimates to the kidney (7.7 ± 1.7 cGy/mCi) and bladder (7 ± 0.6 cGy/mCi) had the highest normal organ radiation exposure but even these are relatively modest (eg, a dose of 50 mCi 90Y would generate 400 cGy on average or 830 cGy at the highest dose estimate [16.6 cGy/mCi]). Radiation doses to the kidney are usually limited to 1500 cGy or less based on external beam criteria. The dose estimates to tumors were variable, as in other RIT trials, reflecting variation in tumor size, location, and other factors with a mean of 26 plus or minus 4 cGy/mCi, which resulted in a high mean tumor to whole-body dose ratio of 49. In fact, the mean dose estimates to tumor at the fixed low dose of 90Y administered (15 mCi/m2) would result in tumor dose of 600 cGy to 800 cGy for a patient with a body surface of 1.8 m2. These values are similar to those seen with Zevalin and Bexxar administered at their maximal tolerated dose levels.5,6 Maximal doses of Zevalin and Bexxar are determined to produce between 60 cGy and 75 cGy exposure to whole body. Based on the highly consistent whole-body exposure estimates observed in this trial (0.6 ± 0.05 cGy/mCi), doses of approximately 100 mCi 90Y-DOTA-biotin should produce a similar myelotoxic profile to either a Zevalin or Bexxar dose, with a potential to increase tumor dose proportionately.
Hematologic toxicity secondary to bone marrow irradiation was generally absent or mild with 11 of 14 patients having no toxicity and one patient with transient grade II neutropenia with nadir counts occurring at 5 to 7 weeks. There were 2 patients who had rapidly progressing disease with grade III neutropenia and thrombocytopenia (patient no. 5) or grade IV pancytopenia (patient no. 11) with a pattern of marrow suppression atypical for RIT and primarily reflecting their progressive underlying disease and palliative external beam radiation given after RIT. The nonhematologic toxicities secondary to radiation were minimal. Therefore, it is likely that higher doses of 90Y-DOTA-biotin will be tolerable in a dose escalation phase 1 trial to determine maximum tolerated dose of the radiolabeled DOTA-biotin. On the basis of this study and prior experience using antibody-streptavidin chemical conjugates for targeting,19-23 we would propose to use a dose of 160 mg/m2 of B9E9Fusion, clearing agent administered at 48 hours (45 mg/m2), and the DOTA-biotin conjugate administered at a 0.65 mg/m2 dosage.
The immune response to the fusion protein was substantial in 3 patients and directed to both streptavidin and the B9E9 V-regions (anti-HAMA). Five patients had transient low antibody responses and 6 of 14 had no observed antibody response over their periods of observation (7-42 weeks). Interestingly, all 5 patients receiving 320 mg/m2 had positive antibody response to B9E9Fusion whereas 6 of 9 patients receiving 160 mg/m2 had no antibody response. The frequency of substantive immune response is lower that that reported in streptavidin-monoclonal antibody trials in solid tumor patients.21 This probably reflects the immunosuppression of non-Hodgkin lymphoma patients secondary to their disease and prior cytotoxic treatment and is consistent with the low immune response (HAMA) reported in Zevalin and Bexxar trials.5-7 In addition, the absence of murine immunoglobulin constant regions in the B9E9FP may reduce its immunogenicity compared with chemical conjugates of streptavidin with murine monoclonal antibody reagents.21,23 The immunogenicity of this regimen may limit second courses of therapy at time of disease relapse in some patients but patient benefit/efficacy is to be based on single courses of therapy. Single courses of therapy provide an 8- to 12-day period of continuous targeted radiation similar to that provided by Zevalin and Bexxar, which were FDA approved as single-course regimens.
Although this trial was a pilot phase 1 study to assess toxicity and evaluate performance of the B9E9FP, clinical efficacy was noted even at this relatively low dose of 90Y-DOTA-biotin. Three of 14 patients had an objective tumor response, including complete remission in a patient with heavily pretreated mantle cell lymphoma (patient no. 1) with a duration of 11 months; complete remission in a patient with small cell lymphocytic lymphoma (patient no. 12) with a duration of 3 months; and a partial response in a follicular lymphoma (patient no. 3) with a duration of 9+ months. This represents an encouraging experience given the nature of the patient population (heavily pretreated and unfavorable histologies) combined with the low administered dose of 90Y. Efficacy should be much enhanced at the maximal tolerated dose of this regimen.
This pretarget radioimmunotherapy regimen has the potential to deliver higher radiation doses to tumor sites than currently used radiolabeled antibody strategies. This study strongly supports proceeding to a 90Y-DOTA-biotin dose escalation phase 1 trial to establish maximum tolerated dose.
Prepublished online as Blood First Edition Paper, March 2, 2004; DOI 10.1182/blood-2003-09-3284.
Supported in part by Department of Defense (Small Business Innovation Research) grants no. 1-R44-CA85130-01 and no. 4-R44-CA85130-02 for the development of the B9E9 fusion protein, and by a National Institutes of Health (CURE Program) Career Development Award (K12-CA76937; A.F.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Sharon Garrison for manuscript preparation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal