Abstract
Children with the severe phenotype of the genetic immunodeficiency disease leukocyte adhesion deficiency or LAD experience life-threatening bacterial infections because of molecular defects in the leukocyte integrin CD18 molecule and the resultant failure to express the CD11/CD18 adhesion molecules on the leukocyte surface. Hematopoietic stem cell transplantation remains the only definitive therapy for LAD; however, the degree of donor chimerism and particularly the number of CD18+ donor-derived neutrophils required to reverse the disease phenotype are not known. We performed nonmyeloablative hematopoietic stem cell transplantations from healthy matched littermates in 9 dogs with the canine form of LAD known as CLAD and demonstrate that in the 3 dogs with the lowest level of donor chimerism, less than 500 CD18+ donor-derived neutrophils/μL in the peripheral blood of the CLAD recipients resulted in reversal of the CLAD disease phenotype. These results demonstrate the value of a disease-specific, large-animal model for identifying the lowest therapeutic level required for successful cellular and gene therapy. (Blood. 2004;103:3582-3589)
Introduction
Children with the severe phenotype of the genetic immunodeficiency disease leukocyte adhesion deficiency or LAD type I (LAD-I) suffer recurrent, life-threatening bacterial infections because of the inability of their neutrophils to adhere to blood vessel walls and migrate to the site of infection.1 The hallmark of LAD-I is a deficiency in surface expression of the CD11/CD18 leukocyte integrin complex because of heterogeneous genetic mutations in the leukocyte integrin CD18 gene. These mutations result in a CD18 protein which is unable to form a heterodimer with the leukocyte integrin CD11 subunits, leading to a subsequent failure to express the CD11/CD18 complexes on the leukocyte cell surface.2,3 Two other forms of LAD, types II and III, have also been characterized. LAD-II was shown to involve a defect in sialyl-Lewis X, a carbohydrate ligand for selectins,4 whereas LAD-III was recently characterized as a defect in the ability of the integrins to undergo stimulation by G-protein-coupled receptors.5
Currently, myeloablative hematopoietic stem cell transplantation remains the only definitive therapy for LAD-I. A clinical gene therapy trial for LAD-I involving 2 patients did not result in reversal of the disease phenotype.6 With hematopoietic stem cell transplantation, 100% donor chimerism may not be required for correction of the clinical phenotype in LAD-I. Analysis of a small number of LAD-I patients who developed mixed hematopoietic chimerism after a myeloablative conditioning regimen suggested that this mixed chimeric state resulted in a reversal of the LAD-I phenotype.7 However, in that study the levels of donor chimerism varied widely over time. Thus, the level of donor chimerism, including the number of CD18+ donor-derived neutrophils required to reverse or prevent the disease phenotype, remained unclear. The number of CD18+ neutrophils required to correct the phenotype in LAD-I is a critical question for potential gene transfer studies in this disease because gene transfer into hematopoietic cells has been very low in almost all clinical trials to date.
The analogous disease to LAD-I in humans has been identified in dogs and has been termed canine leukocyte adhesion deficiency or CLAD.8,9 CLAD was first described in the mid-1970s in Irish Setter dogs in which it was labeled “canine granulocytopathy syndrome.”10 CLAD is characterized by recurrent, life-threatening bacterial infections, which typically lead to death by 6 months of age.9,11 All CLAD dogs are homozygous at the same genetic mutation in the CD18 gene and lack CD18 expression on their leukocytes.12
We recently established a mixed-breed CLAD colony for the purpose of developing new therapeutic strategies for LAD-I.13 In the current study we describe the reversal of the CLAD phenotype in 3 dogs with CLAD with the establishment of a low number (eg, < 500 polymorphonuclear leukocyte [PMN]/μL) of circulating CD18+ neutrophils following a nonmyeloablative, matched littermate hematopoietic stem cell transplantation.
Materials and methods
Dogs
Dogs were housed in National Institutes of Health (NIH) facilities in Bethesda, MD, and Poolesville, MD, in accordance to NIH guidelines. These facilities are approved by the American Association for Accreditation of Laboratory Animal Care (AAALAC). Animal study protocols were approved by the Institutional Animal Care and Use Committees of the National Cancer Institute and National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD. The studies were performed in accordance with the principles outlined in the Guide for Laboratory Animals Facilities and Care of the National Academy of Sciences, National Research Council. All CLAD dogs were treated empirically with broad-spectrum antibiotics and nonsteroidal anti-inflammatory drugs (NSAIDs) for episodes of fever, illness, or injury.
Dog histocompatibility testing
Transplantation of hematopoietic stem cells
Recipient CLAD dogs received total body irradiation (TBI) at a nonmyeloablative dose of 200 cGy delivered from a 60Co source on the day of transplantation (day 0). TBI was administered 2 to 4 hours prior to the infusion of donor hematopoietic cells.
The hematopoietic stem cell source differed in the first 2 dogs, with the first CLAD dog (Scupper, designated D102) receiving bone marrow cells and the second CLAD dog (Diglett, designated D106) receiving peripheral blood stem cells. Bone marrow cells for the first CLAD dog were isolated according to procedures previously described.15 In the second CLAD dog, peripheral blood stem cells were collected from the matched littermate donor by apheresis. Peripheral blood cells were mobilized by using recombinant human granulocyte colony-stimulating factor (G-CSF) at 20 μg/kg per day administered subcutaneously for 5 days. On the fifth day, apheresis was performed for approximately 2 hours with a CS3000 Plus Blood Cell Separator (Fenwal Division, Baxter Healthcare, Deerfield, IL) by using a modification of a previously described procedure.16
The third CLAD dog (Herb, designated D114) received CD34+-selected bone marrow cells. In this dog, bone marrow cells were collected as noted for CLAD dog D102; however, the bone marrow cells were then diluted with saline, layered over NycoPrep 1.077A (Griener Bio-Onc, Longwood, FL), and centrifuged at 800g for 15 minutes at room temperature. After centrifugation, cells were washed with phosphate-buffered saline (PBS) with 1% bovine serum albumin (BSA) and 2 U/mL heparin, recentrifuged, and resuspended in acid-citrate dextrose A (ACD-A) buffer (1 × PBS [without Ca or Mg], 0.5% BSA, 0.6% ACD-A). Cells were incubated with anticanine CD34 antibody 1H6 (Fred Hutchinson Cancer Research Center [FHCRC], Seattle, WA) at 40 μg antibody per 108 cells for 20 minutes at 4°C with rocking. After incubation, the cells were washed with ACD-A buffer and incubated with immunoglobulin G1 (IgG1) microbeads (at 1 mL beads per 5 × 108 cells) (Miltenyi Biotec, Auburn, CA) for 20 minutes at 4°C with rocking. After microbead incubation, the cells were washed with ACD-A buffer, filtered through a 50-μm preseparation filter, and sorted once using the Miltenyi Biotec AutoMACS in the positive selection-sensitive mode. Both CD34+ and CD34- fractions were collected.
In all 3 transplantations, aliquots of the bone marrow harvest, the peripheral blood stem cell collection, and CD34 bone marrow-sorted cells were analyzed to determine the nucleated cell number in the donor sample, as well as assessment of the CD34+ cell number. The bone marrow hematopoietic stem cells, as well as the apheresis collection of peripheral blood stem cells, were infused intravenously into their respective recipient CLAD dog without manipulation. The CD34+-sorted fraction was directly infused into its recipient CLAD dog, along with an infusion of a portion of the CD34- sort fraction that contained 1 × 106 CD3+ cells/kg to facilitate allogeneic engraftment.17
Laboratory studies
White blood cell counts and differentials were performed on peripheral blood samples at a commercial laboratory (Antech Diagnostics, Lake Success, NY) or locally at the NIH Clinical Center. Baseline white blood cell (WBC) counts were measured prior to transplantation and after transplantation at selected time points. Routine serum chemistries were performed monthly.
Saliva collection
Saliva was collected from exercised dogs by 2 methods: (1) rinsing of the mouth with saline solution and aspiration with a plastic transfer pipet in which approximately 1 to 3 mL was collected or (2) rinsing the mouth with 50 to 150 mL saline and collection of the rinse into a clean container. The collected mouth rinses were washed with PBS or saline and were centrifuged for 15 minutes at 400g to form a cell pellet.
Flow cytometry
The percentage and absolute number of canine CD34+ cells in the bone marrow, apheresis collection, and CD34-sorted bone marrow fractions were determined by flow cytometry. Whole bone marrow and peripheral blood stem cells were incubated in ammonium chloride potassium (ACK) lysis buffer (Biosource International, Los Angeles, CA) to remove the red blood cells. Bone marrow cells for CD34 sorting that were centrifuged on a NycoPrep layer, and postsorted CD34+ bone marrow cells did not undergo red blood cell (RBC) lysis. All cells were washed with PBS + 1% BSA (ICN Biomedicals, Aurora, OH) and stained with a phycoerythrin (PE)-conjugated anticanine CD34 antibody 1H6 (Pharmingen, San Diego, CA).19 Differentiated CD34+ cells were determined by costaining with fluorescein isothiocyanate (FITC)-conjugated antihuman CD14 antibody (Dako, Carpinteria, CA) and were excluded from the CD34 analysis.
To assess chimerism, peripheral blood leukocytes were collected from the recipient and analyzed for expression of surface CD18 by flow cytometry at selected time points. Red blood cells were removed by lysis, and the leukocytes were stained with an FITC-labeled mouse antihuman CD18 monoclonal antibody (MHM23; Dako) that cross-reacts with the canine CD18 molecule.20
Chimerism in peripheral blood leukocyte subsets was determined by costaining with the relevant subset antibody as previously described.15 B cells were identified by staining with a mouse anticanine B-cell monoclonal antibody (CA2.1D6; Serotec, Raleigh, NC). To facilitate analysis, unconjugated antibodies were labeled in some cases with a Zenon R-PE or allophycocyanin (APC) mouse IgG1 labeling kit (Molecular Probes, Eugene, OR) according to the manufacturer's directions. Isotype controls were included for each labeled antibody isotype when appropriate. For dead cell discrimination, cells were incubated in PBS buffer containing 1% wt/vol BSA plus 1 μg/mL 7-amino actinomycin D (Sigma-Aldrich, St Louis, MO). Cells were then analyzed by flow cytometry on a Becton Dickinson FACSCalibur or FACSCan instrument (San Jose, CA) by using Cellquest software. Additional software analysis of the data was performed by using the program FlowJo (Tree Star, San Carlos, CA). In some cases, side scatter values were increased in gain for clarity of cell populations. In one case, FL1 linear scale data were converted to log scale.
DNA chimerism analysis using PCR
The percentage of donor/host chimerism in peripheral blood leukocytes or saliva was also determined by using DNA microsatellite repeat markers that distinguished donor and host DNA contribution. Informative markers were identified for each CLAD dog (Canine MapPairs, Microsatellite Markers; Resgen, Huntsville, AL): marker set FH2132 for dog D102, marker set FH2263 for dog D106, and marker set FH2383 for dog D114.21 Genomic DNA was isolated from the peripheral blood leukocytes or mouth rinses with a Wizard Genomic DNA Purification Kit (Promega, Madison, WI) and subjected to polymerase chain reaction (PCR) amplification in a reaction containing PCR primers to each respective marker set (a 6-Fam-endlabeled forward primer [(Synthegen LLC, Houston, TX] and an unlabeled reverse primer), 1 × PCR reaction buffer, and Platinum Taq polymerase (Life Technologies, Gaithersburg, MD). Samples were subjected to PCR amplification by using a GeneAMP PCR System 9700 (ABI, Foster City, CA) by denaturing for 94°C for 1 minute 30 seconds, followed by 30 cycles of 94°C for 30 seconds, 58°C for 30 seconds, 74°C for 45 seconds, followed by a final extension of 72°C for 5 minutes. Products were analyzed on an ABI Prism 310 or 3100 Genetic Analyzer. The percentage of donor/host chimerism was calculated as the summed areas and/or heights of the donor DNA PCR peaks divided by the summed areas and/or heights of the donor plus host DNA PCR peak(s). The PCR peaks were adjusted for amplification bias in some cases of widely separated peaks by comparison to PCR peaks from a 1:1 donor/recipient DNA mix or by comparison to PCR peaks obtained from siblings.
Results
Hematopoietic stem cell transplantation
Matched littermate, histocompatible donors were identified for a total of 9 CLAD pups by using a set of microsatellite repeats that are tightly linked to the class I and class II major histocompatibility loci,14 and these 9 animals all received hematopoietic stem cell transplants from their matched littermate donors by using bone marrow, mobilized peripheral blood stem cells, or bone marrow CD34+ cells as the stem cell source. All 9 animals received transplants at 2 to 4 months of age, and all 9 dogs are alive and well at 1 year follow-up after transplantation with levels of donor PMN chimerism ranging from 2.3% to 71.4%. There was no correlation between level of donor chimerism and stem cell dose, source of donor stem cells, age at which transplantation was performed, or sexes of donor and recipient22 (T.R.B. et al, manuscript in preparation). The results from the 3 CLAD dogs with the lowest level of donor chimerism are the subject of this report.
The 3 CLAD dogs with the lowest levels of donor chimerism received hematopoietic stem cell transplants from their matched littermate donors at 9.6 weeks (D102), 13.4 weeks (D106), and 13.6 weeks (D114) of age, respectively (Table 1). The age range (9-14 weeks) facilitated marrow harvest without killing the donor. CLAD dog D102 received bone marrow cells, CLAD dog D106 received human G-CSF-mobilized peripheral blood stem cells, and CLAD dog D114 received CD34-sorted bone marrow cells. All 3 dogs were sex mismatched with regard to their donors (D102, male donor to female recipient; D106 and D114, female donor to male recipient). The transplanted cell doses that each dog received are shown (Table 1). Dog D114, which received CD34+-sorted cells, also received a fraction of the CD34- bone marrow sort that contained 1 × 106 CD3+ cells/kg to facilitate allogeneic donor engraftment. Thus, CLAD dogs D102 and D114 received transplants with nearly a 100-fold higher dose of donor CD34+ cells, compared with CLAD dog D106, which received human G-CSF-mobilized peripheral blood cells.
Prior to the infusion of donor cells, each CLAD recipient received a nonmyeloablative dose of 200 cGy of total body irradiation. All dogs received standard posttransplantation immunosuppression, consisting of CSP and MMF as previously described.18
Phenotype of CLAD-affected dogs prior to transplantation
All 3 CLAD dogs exhibited typical signs of CLAD during the months prior to transplantation and began receiving daily antibiotic prophylaxis with cefazolin subcutaneously, or amoxicillin and clavulanate (Clavamox) orally, once the diagnosis of CLAD was established by flow cytometry at approximately 10 days postpartum. This therapy likely contributed to the relatively milder symptoms as compared with CLAD dogs raised in less controlled environments that invariably died or were euthanized by 6 months of age because of infectious complications.23 In our CLAD colony, 2 littermate CLAD dogs that did not receive bone marrow transplants because of lack of a matched donor were euthanized at 2 and 6 months of age because of intractable infection despite appropriate antibiotic therapy. The dog euthanized at 2 months of age had a severe episode of fever, lethargy, and hypertrophic osteodystrophy (HOD), leading to progressive pain and necessitating death. The second CLAD dog that did not receive a transplant had frequent fevers, submandibular lymphadenopathy, HOD, and severe skin rashes with pyoderma, ulcers, and demodicosis, finally necessitating euthanasia at 6 months of age. Prior to transplantation, the clinical signs of infection in the CLAD dogs that subsequently received transplants were similar to the clinical signs in the control dogs not receiving transplants. The clinical course of the 3 CLAD dogs before and after transplantation is listed (Table 2). All dogs, including the littermates not receiving transplants, displayed the hallmark laboratory sign of CLAD, a severe leukocytosis with counts reaching 45 000 to 60 000 cells/μL.24
Flow cytometric analysis of donor hematopoietic CD18+ chimerism after transplantation
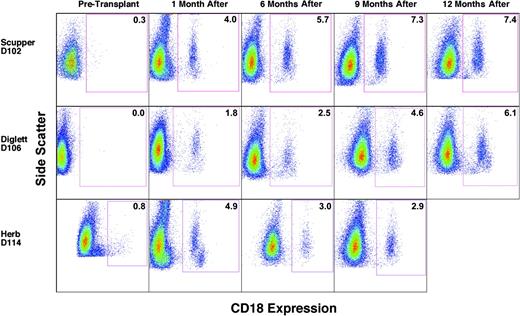
To assess donor/host chimerism after transplantation, peripheral blood leukocytes were analyzed by flow cytometry to determine the number of CD18+, donor-derived myeloid cells, primarily neutrophils, in each CLAD dog. Flow cytometric analysis performed prior to transplantation indicated the absence of CD18+ cells in the peripheral blood of the CLAD dogs (Figure 1, before transplantation). The data for dog D114 with a small number of CD18+ cells before transplantation are an artifact of linear to log scale conversion. By the 1-month time point following the infusion of allogeneic donor stem cells, CD18+ myeloid cells were present at low levels in the peripheral blood of all 3 dogs (Figure 1, 1 month). The percentage of total peripheral blood myeloid cells that were CD18+, and thus donor-derived, increased with each time point for dogs D102 and D106. In dog D114, the CD18+ myeloid cell population has remained stable since 4 months after transplantation, with the latest time point (9 months) depicted (Figure 1).
Flow cytometric analysis of CD18+ myeloid cells in the peripheral blood prior to and following matched littermate transplantation. Peripheral blood leukocytes were isolated, stained with anti-CD18 FITC antibody, and examined by flow cytometry. Myeloid cell populations were gated on forward scatter and side scatter profiles and are identified by side scatter (y-axis) and FL1 fluorescence intensity (x-axis) in the dot plots. The times indicate before transplantation and after transplantation times of 1 month (4 weeks), 6 months, 9 months, and 12 months, respectively. CD18+ myeloid cell percentages are indicated within the box. The few cells appearing in the boxes of dogs D102 and D114 at the pretransplantation time points are not statistically different from cells stained with isotype controls. Side scatter was adjusted in some cases to clarify myeloid from lymphoid populations. For dog D114, pretransplantation FL1 intensity was changed from linear scale to log scale.
Flow cytometric analysis of CD18+ myeloid cells in the peripheral blood prior to and following matched littermate transplantation. Peripheral blood leukocytes were isolated, stained with anti-CD18 FITC antibody, and examined by flow cytometry. Myeloid cell populations were gated on forward scatter and side scatter profiles and are identified by side scatter (y-axis) and FL1 fluorescence intensity (x-axis) in the dot plots. The times indicate before transplantation and after transplantation times of 1 month (4 weeks), 6 months, 9 months, and 12 months, respectively. CD18+ myeloid cell percentages are indicated within the box. The few cells appearing in the boxes of dogs D102 and D114 at the pretransplantation time points are not statistically different from cells stained with isotype controls. Side scatter was adjusted in some cases to clarify myeloid from lymphoid populations. For dog D114, pretransplantation FL1 intensity was changed from linear scale to log scale.
Phenotype of CLAD dogs after transplantation
The CLAD phenotype resolved after transplantation in all 3 CLAD animals in proportion to the severity of their clinical signs prior to transplantation. CLAD dog D102 had a mild course before transplantation on prophylactic antibiotics and had no infectious episodes after transplantation (Table 2). CLAD dog D106 experienced several episodes of severe infection prior to the time of transplantation and had 2 brief episodes of fever immediately following transplantation. An episode of HOD was noted around the time of transplantation but resolved on engraftment. Other than a slight fever at 90 days after transplantation, the dog has been free of all CLAD-related disease with no antibiotic therapy. CLAD dog D114 had multiple bouts of severe infection and abscesses before transplantation that did not resolve completely until 2 months following transplantation (Table 2). This dog also had 2 episodes of otitis externa, which is not uncommon in large dog breeds. The otitis externa was treated topically. Since treatment for a gum infection that was exacerbated by cyclosporine administration, the dog has been free of all CLAD disease and has not received antibiotics.
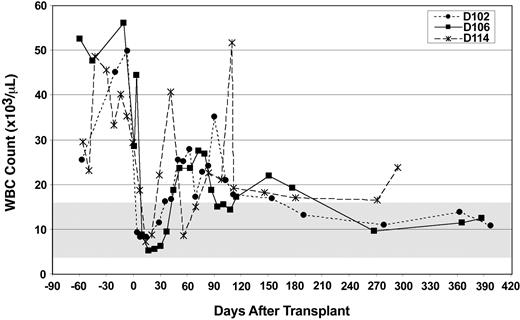
Two weeks after the infusion of donor stem cells, the WBC count in all 3 CLAD dogs reached a nadir of 5000 to 10 000 cells/μL, a drop most likely because of the 200 cGy of total body irradiation (Figure 2). Following the nadir, the WBC counts increased to the 25 000 to 40 000 cells/μL level between 1 month to 3.5 months after transplantation but then decreased to nearly normal levels by 16 weeks after transplantation (except for the leukocytosis caused by a spider bite in dog D114). By 6 months after transplantation, all WBC counts have been below 25 000 cells/μL, with 2 of the dogs (D102 and D106) returning to the normal range (4.0-15.5 × 103 cells/μL) by 9 months after transplantation (Figure 2).
Total WBC counts prior to and following matched littermate transplantation. The total peripheral WBC count (y-axis) was measured at the designated time intervals (x-axis). The time of histocompatible littermate bone marrow transplantation was at day 0. Light dashed line and filled circles represent dog D102 (Scupper), solid line and filled squares represent dog D106 (Diglett), and dark dashed line and asterisks represent dog D114 (Herb).
Total WBC counts prior to and following matched littermate transplantation. The total peripheral WBC count (y-axis) was measured at the designated time intervals (x-axis). The time of histocompatible littermate bone marrow transplantation was at day 0. Light dashed line and filled circles represent dog D102 (Scupper), solid line and filled squares represent dog D106 (Diglett), and dark dashed line and asterisks represent dog D114 (Herb).
Flow cytometric analysis of CD18+ peripheral blood leukocyte subsets
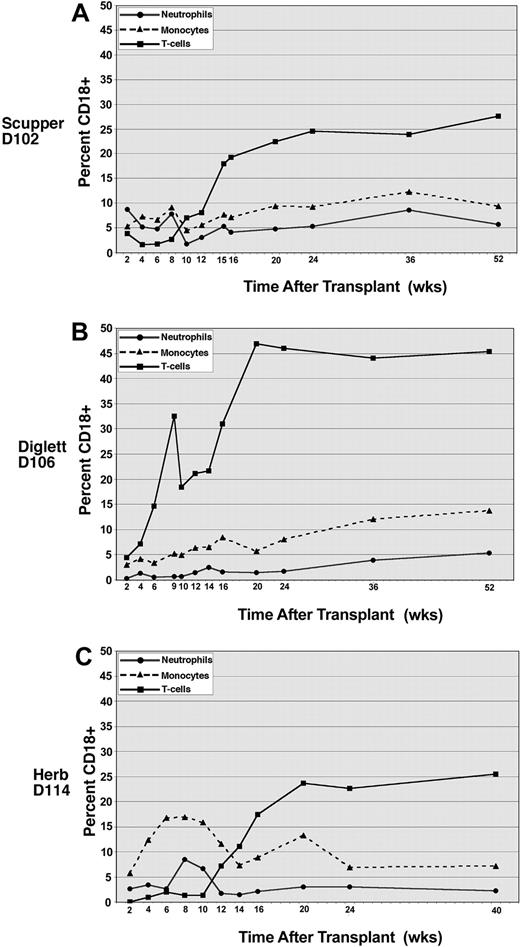
To determine the relative contribution of each leukocyte subset to the total donor leukocyte chimerism after transplantation, CD18+ peripheral blood leukocytes were costained with antibodies to identify neutrophils (antineutrophil antibody), monocytes (anti-CD14), and T lymphocytes (anti-CD3), respectively. The percentage of cells staining for CD18 in the individual leukocyte subsets is shown for all 3 dogs (Figure 3). In CLAD dog D102 the level of CD18+ neutrophils varied between 2% and 8% over the ensuing 12 months following transplantation (Figure 3A). The CD18+ monocyte contribution similarly ranged between 5% and 12% over the period of follow-up in dog D102 (Figure 3A) and has paralleled the neutrophil population closely. Surprisingly, the CD18+ T-lymphocyte population in dog D102 began increasing in percentage after the withdrawal of cyclosporine approximately 8 weeks after transplantation and has reached a level of 28% by 1 year following transplantation (Figure 3A). In contrast, the CD18+ B-cell population represented only 8% of the total B-cell population in the peripheral blood at the 1-year time point in dog D102, consistent with a selective increase in the number of CD18+ T cells, rather than an expansion of the lymphocyte compartment in general (data not shown).
Peripheral blood leukocyte subset chimerism in CLAD dogs receiving transplants. The contribution of each subset to chimerism in dog D102 (A), dog D106 (B), and dog D114 (C) was assessed by flow cytometric analysis of CD18 expression on neutrophils (•), monocytes (▴, dashed lines), and CD3 T cells (▪). Each subset was analyzed using a specific subset antibody (described in “Materials and methods”).
Peripheral blood leukocyte subset chimerism in CLAD dogs receiving transplants. The contribution of each subset to chimerism in dog D102 (A), dog D106 (B), and dog D114 (C) was assessed by flow cytometric analysis of CD18 expression on neutrophils (•), monocytes (▴, dashed lines), and CD3 T cells (▪). Each subset was analyzed using a specific subset antibody (described in “Materials and methods”).
The results in CLAD dog D106 that received a peripheral blood stem cell (PBSC) transplant are similar to the results in dog D102. Initially, CLAD dog D106 displayed a low percentage of donor-derived, CD18+ neutrophils, with the percentage of CD18+ neutrophils representing less than 3% of the total neutrophils in circulation at the 6-month time point but increasing to 5% by 1 year after transplantation (Figure 3B). Monocytes bearing CD18 similarly comprised less than 10% of total monocytes 6 months following transplantation for dog D106 but rose to 13% by 1 year (Figure 3B). In dog D106, the CD18+ T-lymphocyte compartment rapidly expanded, whereby nearly 50% of the CD3+ cells were CD18+ by 5 months following transplantation. This percentage of CD18+ T cells remained at this level at 1 year (Figure 3B). The initial rapid increase, and subsequent high percentage of CD18+ T cells in circulation, most likely reflects the large number of T cells present in the initial PBSC graft.
In the third dog (D114) that received a transplant, the results parallel most closely those of dog D102, in which the CD18+ T-cell population has risen slowly with time, reaching a level of 25% by 10 months after transplantation. The neutrophil CD18+ population also initially rose to peak at 8% at 8 weeks after transplantation and then decreased to the 2% to 4% range at 4 months after transplantation where it has remained. The more rapid increase, then decrease, in the CD18+ monocyte and neutrophil populations likely reflects the use of CD34+ cells as the transplant source. The progenitor populations contribute to the early rise in myeloid cells, with the more primitive stem cells contributing later in the CD18+ engrafting populations.
DNA analysis of hematopoietic chimerism
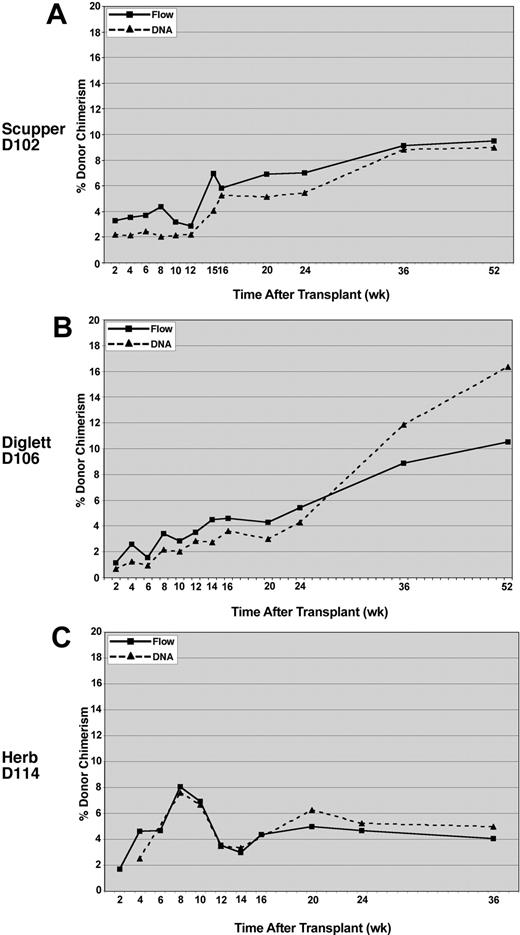
To provide an independent verification of the flow cytometric results, donor/host chimerism was assessed for peripheral blood leukocyte engraftment by using microsatellite markers that distinguished between the donor and recipient. The DNA analysis, performed on DNA extracted from total peripheral blood leukocytes, correlated closely with the donor chimerism measured by flow cytometry for all 3 recipients (Figure 4).
Comparison of donor chimerism by DNA versus flow cytometry analysis. Peripheral blood leukocytes were analyzed for CLAD dog D102 (A), CLAD dog D106 (B), and CLAD dog D114 (C). Flow cytometry with an anti-CD18 monoclonal antibody was used to detect the percentage of CD18+ leukocytes (solid lines, ▪) in the peripheral blood of the CLAD dogs. Chimerism in the peripheral blood leukocytes was also determined by PCR amplification of donor microsatellite repeats at the selected time points (dashed lines, ▴). DNA chimerism data were subjected to a correction value on the basis of peak height and area differences between donor and recipient peaks (described in “Methods and materials”).
Comparison of donor chimerism by DNA versus flow cytometry analysis. Peripheral blood leukocytes were analyzed for CLAD dog D102 (A), CLAD dog D106 (B), and CLAD dog D114 (C). Flow cytometry with an anti-CD18 monoclonal antibody was used to detect the percentage of CD18+ leukocytes (solid lines, ▪) in the peripheral blood of the CLAD dogs. Chimerism in the peripheral blood leukocytes was also determined by PCR amplification of donor microsatellite repeats at the selected time points (dashed lines, ▴). DNA chimerism data were subjected to a correction value on the basis of peak height and area differences between donor and recipient peaks (described in “Methods and materials”).
Peripheral blood absolute neutrophil counts
Because defective neutrophils are the major problem in LAD and CLAD, we determined the absolute number of donor-derived CD18+ neutrophils in the peripheral blood of each CLAD dog that received a transplant, with the CD18+ neutrophil percentage obtained by flow cytometry multiplied by the total neutrophil count in the peripheral blood (Table 3). CLAD dog D102 displayed CD18+ ANC counts lower than 500, a level of significance in determining severe neutropenia in humans, on several time points, whereas CLAD dog D106 displayed values less than 200 CD18+ neutrophils/μL at a number of time points (Table 3). Although not displaying values as low as D106, dog D114 has had CD18+ neutrophil counts in the 200 to 400 range and has been consistently free of overt CLAD symptoms. It should be noted that for these calculations neutrophils in the peripheral blood were measured.
DNA analysis of extravasated leukocytes in saliva
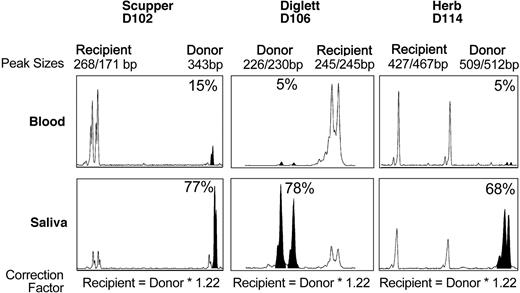
To determine whether the very low level of CD18+, donor-derived neutrophils in the peripheral blood might be due to selective extravasation of the CD18+ donor leukocytes into the tissues, donor/host chimerism was assessed by using cells isolated from the saliva after a mouth rinse. This site was selected because the oral mucosa is an important site of neutrophil turnover,25 and the lack of neutrophils plays an important role in the appearance of gingivitis in CLAD dogs and patients with LAD-I.3,23 Saliva and peripheral blood were collected from dog D102 at approximately 13 months after transplantation, from dog D106 at 5 months after transplantation, and from dog D114 at 9 months after transplantation. Too few cells were collected from the saliva to perform a flow cytometric analysis; therefore, a DNA analysis was performed by using microsatellite markers in the DNA extracted from cells in the saliva and blood from each dog. The results on the DNA extracted from the cells in the mouth rinse of all 3 dogs are shown (Figure 5). In contrast to a very low percentage (≤ 15%) of donor leukocytes in the peripheral blood, the majority of the cells in the saliva appeared to be of donor origin (> 65%) in all 3 dogs, consistent with the hypothesis that neutrophils expressing CD18 selectively extravasated from blood vessels into the oral mucosa.
DNA analysis of donor chimerism in dog saliva. Genomic DNA was isolated from peripheral blood leukocytes or cells in the saliva of each CLAD dog at 5 to 13 months after transplantation. PCR amplification of microsatellite repeats is shown. Peaks corresponding to the donor microsatellite repeats are indicated by filled-in peaks. The percentage of donor chimerism is indicated.
DNA analysis of donor chimerism in dog saliva. Genomic DNA was isolated from peripheral blood leukocytes or cells in the saliva of each CLAD dog at 5 to 13 months after transplantation. PCR amplification of microsatellite repeats is shown. Peaks corresponding to the donor microsatellite repeats are indicated by filled-in peaks. The percentage of donor chimerism is indicated.
Discussion
These results demonstrate that a very low level of CD18+ donor-derived neutrophils (eg, < 500 PMN/μL) in the peripheral blood following nonmyeloablative, matched littermate hematopoietic stem cell transplantation correlates with reversal of the severe disease phenotype in 3 dogs with CLAD. This level of CD18+ neutrophils was observed in 3 dogs that received transplants with unmanipulated bone marrow, G-CSF-mobilized peripheral blood, or CD34-sorted bone marrow cells as the source of the CD18+ donor hematopoietic stem cells. This low level of CD18+ donor-derived neutrophils not only reversed the CLAD clinical phenotype of severe, recurrent infections but also reversed the peripheral blood leukocytosis that is a hallmark of CLAD. This low level of CD18+ neutrophils represents a therapeutic goal for future gene transfer studies.
The observation that a very low number of donor-derived CD18+ neutrophils results in resolution of the clinical phenotype of CLAD relates to the number of CD18+ neutrophils required for normal host defense. Considerable empiric evidence from patients with neutropenia secondary to hematologic malignancy (or secondary to treatment of hematologic malignancy) indicates that the risk of sepsis and infection increases proportionately as the absolute number of neutrophils decreases to less than 500/μL.26,27 Similarly, patients with severe chronic neutropenia also typically have an absolute neutrophil count (ANC) of less than 500 cells/μL.28
Children with LAD-I and dogs with CLAD experience the same type of infectious complications seen in individuals with severe neutropenia, namely infection with common bacteria. In LAD-I, however, sepsis is much less frequent because of CD18-independent mechanisms, whereby neutrophils can clear the bacteria that are circulating in the blood. However, LAD-I is very similar to neutropenia in defective clearance of bacterial infections on external surfaces (skin, mouth, lung, and gut), whereby the lack of neutrophil emigration leads to a paucity of functional neutrophils.
In LAD-I, although the number of circulating CD18+ neutrophils necessary to reverse the disease phenotype has not been firmly established, the results from marrow transplantation indicate that less than full donor chimerism, and thus less than the normal number of CD18+ neutrophils, may be sufficient to reverse the disease phenotype. In studies by Thomas et al,7 4 patients with LAD-I who had mixed donor/host hematopoietic chimerism following attempted myeloablative marrow stem cell transplantations showed reversal of the LAD-I disease phenotype. Moreover, in that study only one patient with LAD-I had a low level of stable donor chimerism (2%-16% CD18+ leukocytes) following transplantation, and that patient displayed symptoms of mild gingivitis.7 In our studies, a neutrophil graft of as low as 56 CD18+ circulating neutrophils/μL, or 0.9% of normal (considering a midrange normal canine ANC of 6300 cells/μL), prevented clinical signs in one CLAD dog that received a transplant, with stable levels of 3% to 4% CD18+ circulating neutrophils sufficient to prevent overt clinical disease with more than a year of follow-up.
In all 3 CLAD dogs that received transplants, the markedly increased total WBC counts observed before transplantation returned to levels that were nearly normal by 6 months after transplantation, and within normal levels for 2 dogs by 9 months after transplantation. Although all 3 CLAD dogs showed a short duration of elevated WBC counts (approximately 2-4 months after transplantation), none of the dogs demonstrated overt clinical symptoms of CLAD during those times.
The percentage of donor chimerism that resulted in the clinical correction of overt disease in all 3 CLAD dogs was also notable for a difference between the lymphoid and the myeloid compartments in the peripheral blood. Peripheral blood CD18+/CD3+ T-lymphocyte percentages were consistently higher in all dogs by 14 weeks after transplantation, with values higher than 15% of the total T-lymphocyte population from 16 weeks after transplantation. In contrast, the neutrophil CD18+ percentages were less than 10% at all measured time points. In addition, all dogs demonstrated a rapid rise in CD18+ T-cell engraftment, especially after cessation of immunosuppression 2 months following transplantation.
The split lymphoid/myeloid chimerism, rather than necessarily reflecting a difference in engraftment between the lymphoid and myeloid compartments, may reflect the selective egress and short, terminal residence of the CD18+ neutrophils and monocytes/macrophages in the tissues, perhaps in conjunction with the recirculative ability and long lifetime of lymphocytes. The high level of chimerism of cells in the dog saliva supports an egress hypothesis, with more than 65% donor chimerism in the saliva of all 3 dogs. It is likely that nearly all leukocytes in the oral mucosa are of donor origin, although cells sloughing off from the epithelium of the host oral cavity likely “contaminate” the collected cells and thereby reduce the donor contribution.
The successful induction of low-level and stable mixed hematopoietic chimerism in the 3 CLAD dogs in this report, and the subsequent reversal of the disease phenotype, demonstrates the utility of this canine model in understanding leukocyte physiology and in establishing a goal level of correction required for cell or gene therapy.
Prepublished online as Blood First Edition Paper, January 8, 2004; DOI 10.1182/blood-2003-11-4008.
Presented in abstract form at the 45th annual meeting of the American Society of Hematology, San Diego, CA, December 6-9, 2003.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank William Telford and Veena Kapoor, Flow Cytometry Lab, Experimental Transplant and Immunology Branch, National Cancer Institute, for assistance with fluorescent antibodies; Mitchell Horwitz and Harry Malech, Laboratory of Host Diseases, National Institute of Allergy and Infectious Diseases, for use of the ABI Prism 310 Genetic Analyzer; and Herb Cullis, American Fluoroseal Corporation, for assistance with the CS3000 Plus Blood Cell Separator.