Abstract
Polymorphonuclear leukocytes (PMNs) migrate from the blood into areas of inflammation by binding to the endothelial cells of blood vessels via adhesion molecules. Vascular adhesion protein-1 (VAP-1) is one of the molecules mediating leukocyte-endothelial cell interactions. It is also an endothelial cell-surface enzyme (amine oxidase) that produces reactive oxygen species during the catalytic reaction. To study the role of the enzymatic activity of VAP-1 in PMN extravasation, we used an enzymatically inactive VAP-1 mutant, specific amine oxidase inhibitors (including a novel small molecule compound), and anti-VAP-1 antibodies in several flow-dependent models. The enzyme inhibitors diminished PMN rolling on and transmigration through human endothelial cells under conditions of laminar shear stress in vitro. Notably, the enzyme inactivating point mutation abolished the capacity of VAP-1 to mediate transmigration. Moreover, the new VAP-1 inhibitor effectively prevented the extravasation of PMNs in an animal model of inflammation. These data show that the oxidase activity of VAP-1 controls PMN exit from the blood during the relatively poorly understood transmigration step. (Blood. 2004;103:3388-3395)
Introduction
White blood cells of the polymorphonuclear series (polymorphonuclear leukocytes [PMNs] or granulocytes) are practically absent from healthy tissues. Upon inflammation, however, rapid influx of PMNs from the blood into the affected tissue takes place. PMNs leave the blood mainly via postcapillary venules and they do not recirculate to any significant extent. Lymphocytes, on the other hand, not only exit at the sites of inflammation but also continuously recirculate between the blood and lymphoid tissues. The physiologic recirculation of lymphocytes is supported by specialized high endothelial venules in lymphoid tissues, and this leukocyte type returns back to the blood via the lymphatic vasculature. Monocytes, the third major leukocyte type, normally leave the blood at low levels in various tissues under normal conditions to replenish the tissue macrophage pools, and at high levels during the later phase of inflammation. The extravasation process of all leukocyte classes consists of a series of carefully controlled steps.1-3 First, the leukocyte makes initial tethers with the luminal surface of the blood vessel and starts to roll along the endothelial lining. If it receives appropriate activation signals, it can adhere in a shear-resistant manner to the endothelium. Finally, the leukocyte transmigrates through the vascular wall and continues its odyssey toward the chemotactic inflammation-induced signals within the tissue. Multiple adhesion and signaling molecules act in concert to execute the emigration cascade. One of the endothelial molecules involved in lymphocyte trafficking is vascular adhesion protein-1 (VAP-1).4 VAP-1 is a cell-surface enzyme belonging to a specific group of amine oxidases (semicarbazide-sensitive amine oxidases [SSAOs] Enzyme Commission 1.4.3.6) that catalyze oxidative deamination of primary amines.5,6
Here we questioned what the possible mechanistic connection is between the adhesive and enzymatic activity of VAP-1. By using adenoviral constructs encoding native or mutated VAP-1, chemical inhibitors of SSAO activity, and function-blocking anti-VAP-1 monoclonal antibodies (mAbs) in in vitro flow chamber assays we were able to study the role of VAP-1 during PMN rolling, firm adhesion, and transmigration. Interestingly, VAP-1 primarily supported the transmigration step during the PMN extravasation process. Experiments with the SSAO inhibitors and VAP-1 mutants showed that the enzymatic activity of VAP-1 is a prerequisite for the adhesive function of this molecule. Finally, we showed in a rat model of acute inflammation that the new, specific, and potent SSAO inhibitor also blocks the recruitment of PMNs to affected tissue in vivo. These data show that VAP-1 functions in leukocyte adhesion in a step-wise fashion via separate antibody epitope-dependent and oxidation-dependent steps. These experiments are the first to show that an oxidative enzymatic reaction on the luminal surface of endothelial cells regulates PMN emigration in vitro and in vivo.
Patients, materials, and methods
Cells, DNA constructs, and adenoviral transfections
PMNs were isolated from healthy donors by dextran sedimentation and Percoll gradient centrifugation, as described.7 The cells were kept in Hanks balanced salt solution (HBSS) without Ca2+ and Mg2+ at +4°C and diluted into RPMI1640 containing 0.1% bovine serum albumin (BSA) just before the flow assay. Human umbilical vein endothelial cells (HUVECs) were enzymatically isolated from umbilical veins.8
A full-length VAP-1 cDNA has been described.5 To generate enzymatically inactive VAP-1, a single amino acid change was introduced at position 471 by using an in vitro mutagenesis kit. This conserved substitution changes a tyrosine residue, which is the precursor of the topa-quinone modification of SSAO, into phenylalanine. Since phenylalanine cannot be processed into topa-quinone, and since the modification is absolutely necessary for the reductive half-reaction of SSAOs, this mutant should be enzymatically dead,9 but structurally almost identical to native VAP-1. VAP-1 and VAP-1Y471F were then subcloned into pADENOGal plasmid under β-actin promoter and cytomegalovirus enhancer using a modification of an earlier described procedure.10 Replication-deficient human clinical grade adenoviruses were produced in HEK293 cells and purified using 2 CsCl gradient centrifugations and dialysis. Viruses were analyzed to be free of microbial contaminants, mycoplasma, and lipopolysaccharide. Control lacZ adenovirus was prepared similarly as described.11
Confluent HUVECs were infected in 10% fetal calf serum (FCS)-containing medium with the viruses at a multiplicity of infection 200, which led to more than 95% VAP-1 positivity. The next day, the cells were analyzed for VAP-1 surface expression using immunofluorescence stainings and fluorescence-activated cell-sorter (FACS) analyses and plated into gelatin-coated glass capillaries. The confluent monolayers were used the next morning for the flow assays. Collection of HUVECs and PMNs was approved by institutional committees and informed consent was obtained from the volunteers.
Antibodies, SSAO inhibitors, and the enzyme assays
Anti-VAP-1 mAb TK8-14 has been described.12 As a binding control mAb, HB116 against major histocompatibility complex (MHC) class 1 was used. Semicarbazide and hydroxylamine were purchased from Sigma (St Louis, MO). BTT-2027 compound was obtained from Biotie Therapies, and will be described elsewhere in detail (F. Fülöp et al, submitted manuscript; M.P., manuscript in preparation, February 2004). The dissociation constant values for inhibition (Ki) of the SSAO activity of VAP-1 with inhibitors were determined using recombinant VAP-1 expressed in Chinese-hamsterovary (CHO) cells and 1 mM benzylamine as substrate as described.13 Ki determinations for total monoamine oxidase (MAO) inhibition were done using rat liver homogenates and 0.5 mM tyramine as substrate.13 Michaelis-Menten constant (Km) values for VAP-1 (Km: 90 ± 5 μM) and for MAO (Km: 62 ± 4 μM) were calculated using a nonlinear curve fitting program based on the Michaelis-Menten equation (GraphPad Prism 3.0; GraphPad Software, San Diego, CA).
A radiochemical assay9 was used to analyze the SSAO activity in HUVECs. In brief, 14C-labeled benzylamine (a model substrate for SSAO) was used as the substrate. The assay was performed at 37°C for 90 minutes in a final volume of 400 μL 0.1-mM Krebs-Ringer phosphate glucose buffer (pH 7.35) containing detached HUVECs from 2 × 35 mm wells (the number of cells was counted in each sample and the cells were then used for 5 separate reactions) and 10 μM benzylamine with tracer 14C benzylamine (40 000 disintegrations per minute [dpm]) in the presence of 0.75 mM clorgyline (an MAO inhibitor). In the inhibitory studies, the samples were preincubated with the cells for 30 minutes. BTT-2027 was used at 40 μM, semicarbazide at 1 mM, hydroxylamine at 5 μM, and the antibodies at 10 μg/mL. Catalytic reaction was stopped by 100 μL 2-M citric acid, and the aldehyde reaction products were extracted from the analyzed mixture into toluene containing 0.35 g/L diphenyloxazole. The amount of 14C-labeled benzaldehyde was quantified by scintillation counting on β-counter Wallac-1409 (Wallac, Turku, Finland), and the activity of the enzyme was expressed as picomoles of benzaldehyde formed by 1 × 106 cells per hour.
In vitro flow chamber assay
A capillary laminar shear assay was adapted from Cooke et al.14 Briefly, transfected HUVECs were plated into gelatin-precoated perpendicular glass capillaries and grown into confluence. Thereafter, the other end of the capillary was connected via tubing and a 2-way valve to a reservoir of cells. The other end was connected into a computer controlled-syringe pump (Model 22, Harvard Apparatus, Holliston, MA). The PMN suspension (1 × 106 cells/mL in RPMI1640 containing 0.1% BSA) was drawn over the endothelial monolayer at a defined laminar shear of 1.0 dyn/cm2. The wall shear stress in this flow cell with parallel plate geometry was calculated using the momentum balance for a Newtonian fluid using 0.01 poise as the coefficient of the viscosity of the medium. All assays were done at the room temperature.
For the rolling and adhesion assays, transfected HUVECs were stimulated with 5 U/mL tumor necrosis factor α (TNF-α) for 4 hours. The freshly isolated PMNs were allowed to interact with endothelial cells under shear for one minute, and then 15 fields (each 0.3072 mm2) were recorded for 15 seconds each via an inverted microscope (Olympus IX70, Olympus Optical, Hamburg, Germany) using × 100 magnification (HMC10 Plan UIS objective, NA 0.25, Modulation Optics, Greenvale, NY) equipped with a Hoffman modulator (Hoffman modulation contrast and Model G3 NA 0.6 condenser, Modulation Optics) and a CCD camera (C5405-01, Hamamatsu Photonics, Hamamatsu, Japan) connected to a digital video recorder (Panasonic NV-DV10000, Matsushita Electrical Industrial, Osaka, Japan). The number of tethering (transient stick and go behavior) and rolling (interacting cells slowly moving into the direction of flow) cells on endothelial cells (leukocyte interactions with already bound leukocytes, ie, secondary tethers and secondary rolling were excluded) were grouped together due to relative low cell numbers in each class. Cells stably bound for the whole 15-second observation period were scored as firmly adherent.
For the transmigration assay, transfected HUVECs were stimulated with 100 U/mL TNF-α for 4 hours to induce transmigratory activity. The PMNs were perfused over the monolayer for 5 minutes. Thereafter, the capillary was constantly perfused with the RPMI1640 buffer containing 0.1% BSA and the adherent cells were allowed to transmigrate for 10 minutes (total time for transmigration is thus 15 minutes) under steady flow (1.0 dyn/cm2). At the end of the period, the fields were observed via a phase-contrast optics (U PlanF1, × 10, NA 0.3 objective and IX-LWUCD NA 0.55 condenser, Olympus Optical), and the number of all interacting (phase-bright and phase-dark) cells and transmigrated (phase-dark) cells was recorded.
The endothelial cells were preincubated for 30 minutes with the enzyme inhibitors (1 mM semicarbazide + 5 μM hydroxylamine, 40 μM BTT-2027, or vehicle as a control) or mAbs (10 μg/mL HB116, 10 μg/mL TK8-14) as indicated in the figures.
All analyses were done off-line by manual counting using Analysis-program. Independent assays were done using different HUVECs (only passage 1 and 2 cells were used) and separate adenoviral infections. In the rolling/adhesion assays the mean number of analyzed rolling cells per one experiment was 133 (corresponding to 29 cells/mm2), and that of firmly adherent 924 (corresponding to 201 cells/mm2) in the control (vehicle-treated) capillaries plated with VAP-1-transfected HUVECs (n = 7). In the transmigration assays, on average 1514 adherent and 136 transmigrated cells (corresponding to 329 and 30 cells/mm2, respectively) were scored in each control (vehicle) capillary with VAP-1-transfected cells (n = 8; ie, on average 9% of interacting cells transmigrated in the control capillaries). The number of interacting cells in HB116-treated capillaries was not markedly different from that in vehicle-treated capillaries.
For the micrographs in Figure 3A and 5A the original frames were captured from the videos with the Analysis-program, and the representative fields were cut using Corel Photopaint 7 (Corel, Dallas, TX). The arrows, numbers, and bars were added using CorelDraw 7, but no other image processing procedures were used.
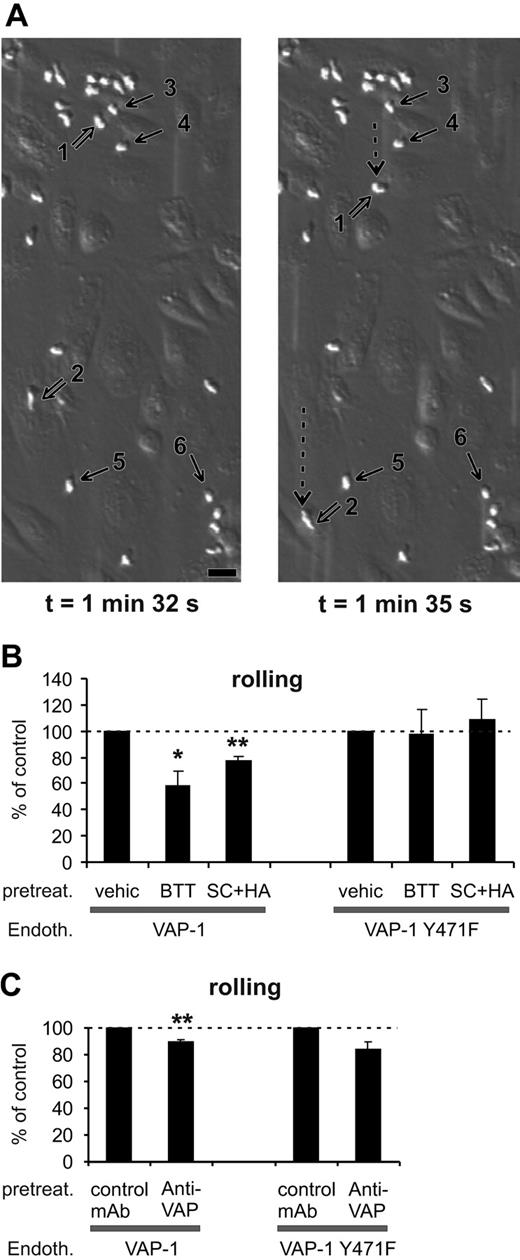
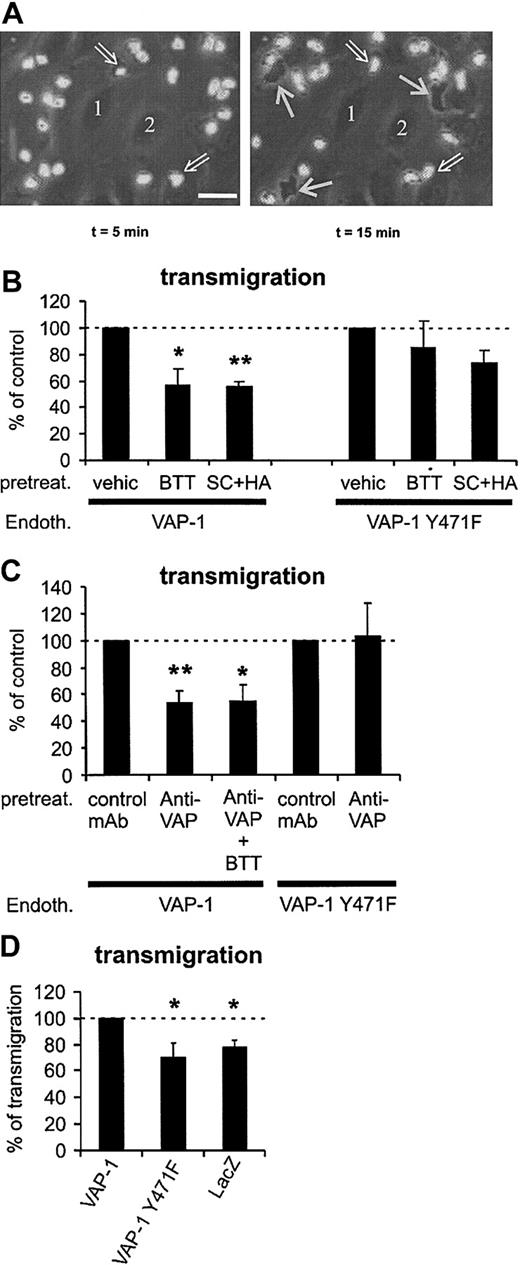
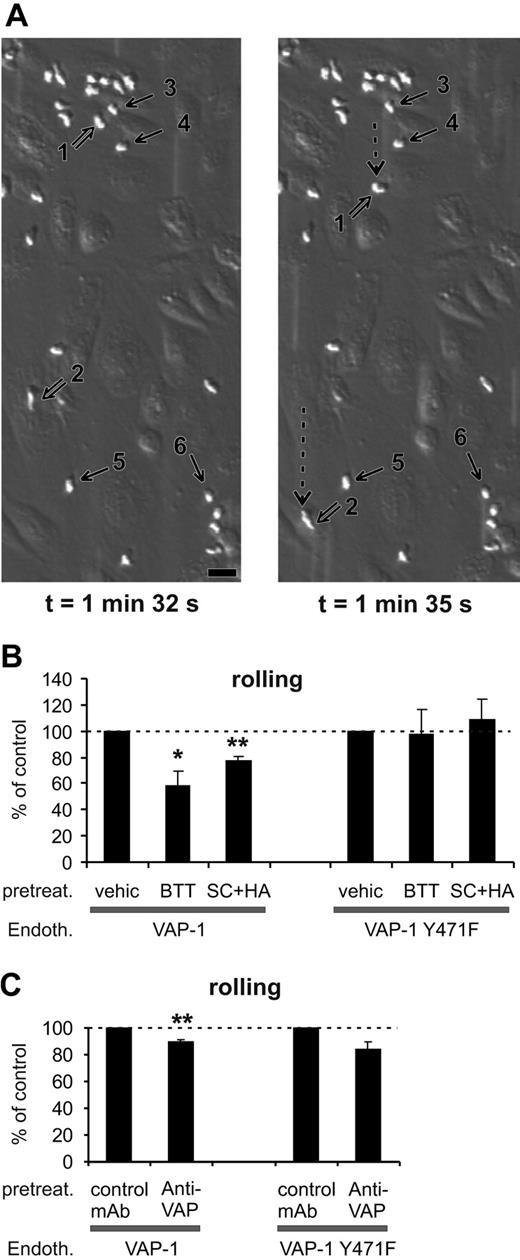
SSAO activity is important for VAP-1-dependent rolling of PMNs on HUVECs under shear. (A) Two video frames taken 3 seconds apart showing rolling and stably adherent PMNs on VAP-1-transfected HUVECs (t = 0 is by definition the moment when infused leukocytes appear on the endothelial cells in the microscopic field). The open arrows point to 2 rolling cells (nos. 1 and 2; their rolling path during the 3-second interval is indicated by the dotted arrow), and some of the firmly adherent cells are pointed out by thin black arrows (nos. 3-6). The shear was 1.0 dyn/cm2. Bar, 20 μm. (B) The SSAO inhibitors (BTT = BTT-2027, SC + HA = semicarbazide + hydroxylamine) block rolling of PMNs on VAP-1-transfected HUVECs, but have no effect on rolling on VAP-1Y471F-transfected cells. The number of rolling PMNs was counted after each treatment and compared with that seen in the vehicle-treated capillaries. The results are mean ± SEM from 3 to 5 independent experiments using HUVECs and PMNs isolated from different individuals. (C) The effect of anti-VAP-1 mAb on PMNs rolling on VAP-1- and VAP-1Y471F-transfected HUVECs was analyzed as described in panel B in the presence of control (HB116) and anti-VAP-1 (TK8-14) mAbs. The results are mean ± SEM (n = 3-4). *P < .05; **P < .01. Endoth. indicates endothelial cells; pretreat., pretreated; and vehic, vehicle.
SSAO activity is important for VAP-1-dependent rolling of PMNs on HUVECs under shear. (A) Two video frames taken 3 seconds apart showing rolling and stably adherent PMNs on VAP-1-transfected HUVECs (t = 0 is by definition the moment when infused leukocytes appear on the endothelial cells in the microscopic field). The open arrows point to 2 rolling cells (nos. 1 and 2; their rolling path during the 3-second interval is indicated by the dotted arrow), and some of the firmly adherent cells are pointed out by thin black arrows (nos. 3-6). The shear was 1.0 dyn/cm2. Bar, 20 μm. (B) The SSAO inhibitors (BTT = BTT-2027, SC + HA = semicarbazide + hydroxylamine) block rolling of PMNs on VAP-1-transfected HUVECs, but have no effect on rolling on VAP-1Y471F-transfected cells. The number of rolling PMNs was counted after each treatment and compared with that seen in the vehicle-treated capillaries. The results are mean ± SEM from 3 to 5 independent experiments using HUVECs and PMNs isolated from different individuals. (C) The effect of anti-VAP-1 mAb on PMNs rolling on VAP-1- and VAP-1Y471F-transfected HUVECs was analyzed as described in panel B in the presence of control (HB116) and anti-VAP-1 (TK8-14) mAbs. The results are mean ± SEM (n = 3-4). *P < .05; **P < .01. Endoth. indicates endothelial cells; pretreat., pretreated; and vehic, vehicle.
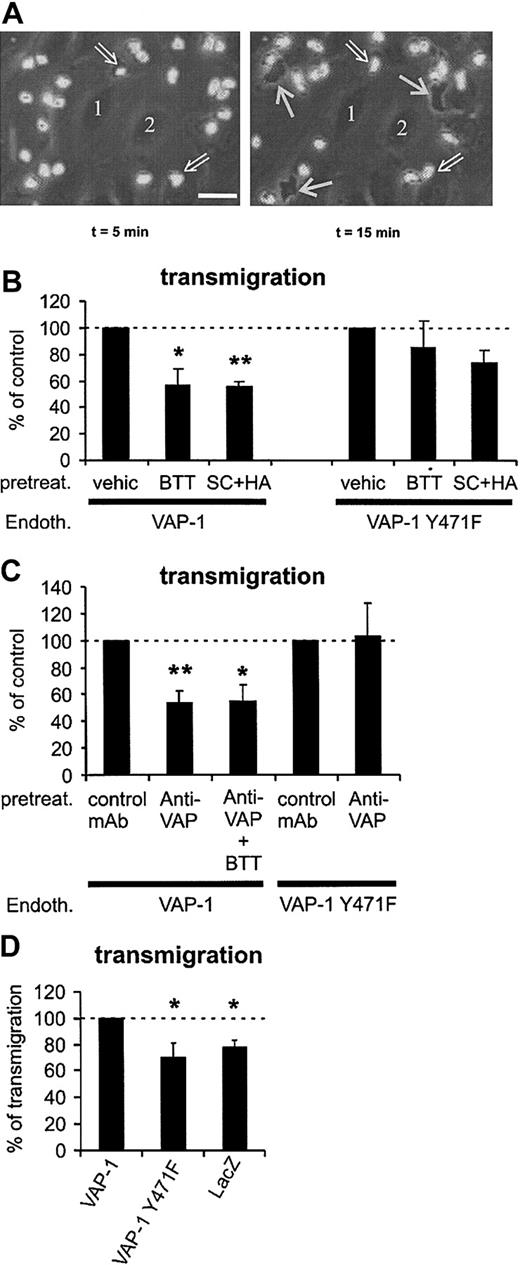
VAP-1 supports PMN transmigration under flow conditions. (A) Phase-contrast micrographs showing surface-adherent (phase-bright; 2 pointed out by open arrows) and transmigrated (phase-dark; 3 indicated by thin white arrows) leukocytes. Numbers 1 and 2 indicate 2 representative endothelial cells at the bottom of the capillary. The first frame is taken 5 minutes after the perfused leukocytes appeared in the microscopic field, and the second one was captured 10 minutes later. The area of the microscopic picture is 0.02 mm2 (ie, it is 1/15th of a single microscopic field and 15 separate microscopic fields were analyzed for each treatment in each individual experiment). The actual transmigration process can be seen in the Supplemental Video online at the Blood website. Note that the surface-bound cells do not remain stationary during the assay, since they also actively migrate on the HUVEC surface in search of junctions. Bar, 20 μm. (B) The number of transmigrated cells was determined after pretreating VAP-1- or VAP-1Y471F-transfected HUVECs by vehicle and SSAO inhibitors (BTT = BTT-2027, SC + HA = semicarbazide + hydroxylamine). (C) The effect of anti-VAP-1 mAb alone or in combination with SSAO inhibitors on the transmigration was determined as in panel B. All results are mean ± SEM of 4 to 7 independent experiments using PMNs and HUVECs from different individuals. (D) VAP-1 supports PMN transmigration. The number of PMNs transmigrating through HUVECs transfected with VAP-1, enzymatically inactive VAP-1, and lacZ was determined. The results are mean ± SEM of 4 to 5 independent experiments using HUVECs and PMNs from different individuals. The number of PMNs transmigrating through VAP-1 transfectants is defined as 100%, and thus HUVECs transfected with VAP-1 support statistically significantly more transmigration than HUVECs transfected with enzymatically inactive VAP-1 or with lacZ. *P < .05; **P < .01. Endoth. indicates endothelial cells; pretreat., pretreated; and vehic, vehicle.
VAP-1 supports PMN transmigration under flow conditions. (A) Phase-contrast micrographs showing surface-adherent (phase-bright; 2 pointed out by open arrows) and transmigrated (phase-dark; 3 indicated by thin white arrows) leukocytes. Numbers 1 and 2 indicate 2 representative endothelial cells at the bottom of the capillary. The first frame is taken 5 minutes after the perfused leukocytes appeared in the microscopic field, and the second one was captured 10 minutes later. The area of the microscopic picture is 0.02 mm2 (ie, it is 1/15th of a single microscopic field and 15 separate microscopic fields were analyzed for each treatment in each individual experiment). The actual transmigration process can be seen in the Supplemental Video online at the Blood website. Note that the surface-bound cells do not remain stationary during the assay, since they also actively migrate on the HUVEC surface in search of junctions. Bar, 20 μm. (B) The number of transmigrated cells was determined after pretreating VAP-1- or VAP-1Y471F-transfected HUVECs by vehicle and SSAO inhibitors (BTT = BTT-2027, SC + HA = semicarbazide + hydroxylamine). (C) The effect of anti-VAP-1 mAb alone or in combination with SSAO inhibitors on the transmigration was determined as in panel B. All results are mean ± SEM of 4 to 7 independent experiments using PMNs and HUVECs from different individuals. (D) VAP-1 supports PMN transmigration. The number of PMNs transmigrating through HUVECs transfected with VAP-1, enzymatically inactive VAP-1, and lacZ was determined. The results are mean ± SEM of 4 to 5 independent experiments using HUVECs and PMNs from different individuals. The number of PMNs transmigrating through VAP-1 transfectants is defined as 100%, and thus HUVECs transfected with VAP-1 support statistically significantly more transmigration than HUVECs transfected with enzymatically inactive VAP-1 or with lacZ. *P < .05; **P < .01. Endoth. indicates endothelial cells; pretreat., pretreated; and vehic, vehicle.
In all experiments the statistical significances were evaluated using Student paired t test. All P values less than .05 are shown in the graphs.
Inflamed air-pouch model in the rat
Male Sprague-Dawley rats (Harlan, Leicestershire, United Kingdom) weighing 241 ± 14 g (mean ± SD) were used as described.15 While under isoflurane anesthesia, the animals were injected under the dorsal skin with 20 mL sterile-filtered (0.22 μm) air. To maintain the air pouch, 2 additional injections of 10 mL at 3-day intervals were given. A day after the third injection, the SSAO inhibitor or vehicle was injected intraperitoneally, which was followed after 15 minutes by a carrageenan λ (2 mL, 6 mg/mL) injection into the air pouch. After 6 hours the animals were killed and the air pouch was lavaged. The number of extravasated leukocytes was counted and their subtype was determined after hematoxylin-thiazine staining from cytospins. P values were determined by one-way analysis of variance. The protocol was approved by a local review board for animal experiments.
Results
Adenoviral transduction of native and enzymatically inactive VAP-1 in primary endothelial cells
VAP-1 is the only molecular species in humans that has been shown to possess SSAO activity.6 Inhibition of SSAO activity by commercially available SSAO inhibitors semicarbazide and hydroxylamine diminishes lymphocyte adhesion to endothelial cells in vitro,16 but their effect on PMN binding is unknown. Moreover, with endogenous VAP-1 it has not been possible to dissect the molecular interdependency of the adhesive and enzymatic function of VAP-1 during the adhesion cascade.
Since VAP-1 is absent from the surface of normal or inflamed HUVECs,17 we used adenoviral transduction to express VAP-1 in the context of other endothelial adhesion molecules. We also introduced a single amino acid mutation into VAP-1 at residue 471 (VAP-1Y471F), which results in a replacement of a tyrosine with a phenylalanine. Tyrosine 471 is the precursor for the topa-quinone modification in SSAO/VAP-1, and thus it is required for the oxidative reaction.5,6 Mutation of the tyrosine residue to phenylalanine prevents the topa-quinone modification, but, based on structural studies of SSAO crystals, does not alter the overall 3-dimensional structure of the molecule at all.18 Transfections of HUVECs with native VAP-1 resulted in bright expression of VAP-1 on the cell surface of HUVECs. Concurrently, strong SSAO enzyme activity was observed in the transfected HUVECs when measured by oxidation of benzylamine that is a prototypic model substrate for SSAO.6 SSAO activity was absent from native or lacZ control-transfected HUVECs (Figures 1, 2). The VAP-1Y471F-transfected cells were also surface-positive for VAP-1 when stained with mAb TK 8-14 (Figure 1) and by several other anti-VAP-1 mAbs (data not shown), confirming the overall structural conservation of this mutated VAP-1 harboring a single conservative amino acid change. On transfected HUVECs, VAP-1Y471F was expressed at the same level as native VAP. Notably, VAP-1Y471F-transduced cells were completely devoid of any SSAO enzyme activity (Figure 2).
Expression of VAP-1 in adenovirally transfected HUVECs. Nontransfected HUVECs (native) and cells transfected with the pADENO-lacZ, pADENO-VAP-1, or pADENO-VAP-1Y471F (enzymatically inactive point mutant) were stained with 3G6 (negative control [neg. co.]), HB116 (against HLA class I), and TK8-14 (against VAP-1) and analyzed using FACS. The x-axis is the fluorescence intensity in a log-scale, and the y-axis is the relative number of cells.
Expression of VAP-1 in adenovirally transfected HUVECs. Nontransfected HUVECs (native) and cells transfected with the pADENO-lacZ, pADENO-VAP-1, or pADENO-VAP-1Y471F (enzymatically inactive point mutant) were stained with 3G6 (negative control [neg. co.]), HB116 (against HLA class I), and TK8-14 (against VAP-1) and analyzed using FACS. The x-axis is the fluorescence intensity in a log-scale, and the y-axis is the relative number of cells.
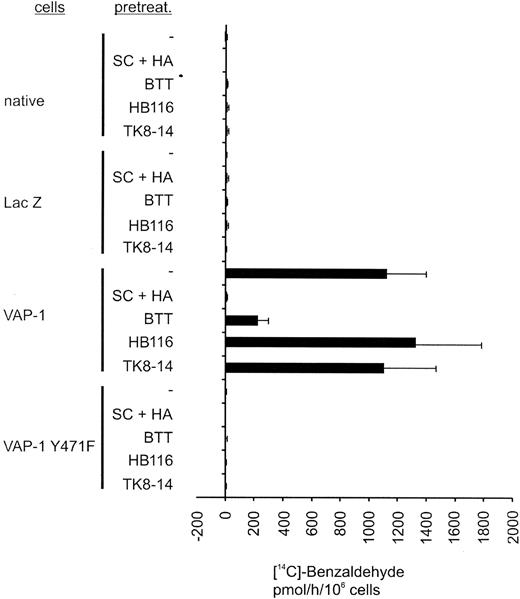
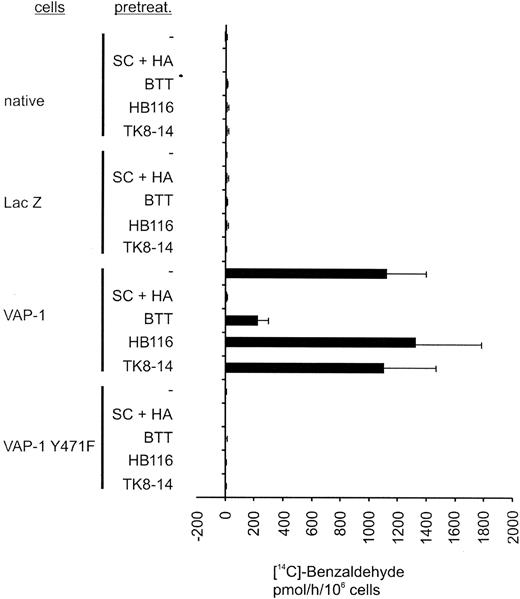
Inhibition of SSAO activity by small molecular SSAO inhibitors and by VAP-1Y471F mutation. Uninfected (native), pADENO-lacZ-, pADENO-VAP-1-, and pADENO-VAP-1Y471F-infected HUVECs were treated with the vehicle (-) or with SSAO inhibitors (SC + HA = semicarbazide + hydroxylamine, BTT = BTT-2027 compound) and antibodies (HB116 against HLA class I, TK8-14 against VAP-1), and the enzymatic activity was determined using the radiochemical method. The specific activities (mean ± SEM from 3 independent assays) are shown. The SSAO-dependent H2O2 production in HA + SC-treated native HUVECs is 0 by definition. Note that the VAP-1Y471F mutant is devoid of any SSAO activity and that the anti-VAP-1 mAb does not interfere with the SSAO activity.
Inhibition of SSAO activity by small molecular SSAO inhibitors and by VAP-1Y471F mutation. Uninfected (native), pADENO-lacZ-, pADENO-VAP-1-, and pADENO-VAP-1Y471F-infected HUVECs were treated with the vehicle (-) or with SSAO inhibitors (SC + HA = semicarbazide + hydroxylamine, BTT = BTT-2027 compound) and antibodies (HB116 against HLA class I, TK8-14 against VAP-1), and the enzymatic activity was determined using the radiochemical method. The specific activities (mean ± SEM from 3 independent assays) are shown. The SSAO-dependent H2O2 production in HA + SC-treated native HUVECs is 0 by definition. Note that the VAP-1Y471F mutant is devoid of any SSAO activity and that the anti-VAP-1 mAb does not interfere with the SSAO activity.
BTT-2027 compound is a potent, novel SSAO inhibitor
To elucidate the role of SSAO activity in PMN extravasation in vitro and in vivo, we made use of a new SSAO inhibitor with improved characteristics compared with the standard SSAO inhibitors hydroxylamine and semicarbazide. BTT-2027 was derived from an SSAO inhibitor identified in an in vitro screen of a chemical library and is the result of a chemical synthesis optimization program. It is a water-soluble carbocyclic-hydrazine compound with a molecular weight of 294 and has suitable chemical, physical, and pharmacokinetic characteristics for in vivo use (F. Fülöp et al, submitted manuscript; M.P. et al, manuscript in preparation, February 2004). It is a potent and specific inhibitor of SSAO activity and has only low inhibitory activity against the MAO-A and MAO-B amine oxidases (Table 1). The selectivity of BTT-2027 has been tested and no significant inhibitory effect or binding was found in a screening panel of 20 targets, including acetylcholine esterase, carbonic anhydrase, protein kinases A and C, as well as adrenergic, dopaminergic, histaminergic, gabaergic, and serotonergic receptor families (M.P. et al, submitted manuscript, February 2004). BTT-2027 inhibits more than 80% of the SSAO activity in VAP-1-transfected HUVECs (Figure 2). In a notable contrast, the anti-VAP-1 mAb TK8-14 (or the binding control antibody HB116 against HLAclass I) does not interfere with the SSAO activity in VAP-1-positive HUVECs.
PMN rolling on endothelial cells is diminished by SSAO inhibitors and by anti-VAP-1 mAbs under flow conditions
To mimic the physiologic flow-dependent adhesion cascade in vitro, we used a capillary flow assay.14 In this setup, PMNs can be perfused with a desired laminar shear stress over a monolayer of endothelial cells, and the cellular interactions can be visualized in real time using videomicroscopy. Moreover, this approach enables pretreatment of the endothelial cells with mAbs or other compounds. Our preliminary experiments showed that the anti-VAP-1 mAbs or SSAO inhibitors have no effect on leukocyte-endothelial interactions in this system unless HUVECs are transfected with VAP-1.
PMNs tethered, rolled, and adhered firmly to VAP-1-transfected HUVECs when the cells were stimulated with the inflammatory mediator TNF-α (Figure 3A). When the VAP-1-expressing HUVECs were pretreated with the SSAO inhibitor BTT-2027, the rolling of PMNs was significantly reduced (Figure 3B). The same result was seen when the prototype SSAO inhibitors (semicarbazide + hydroxylamine) were used instead of the BTT compound (Figure 3B). These data show that the enzymatic activity of VAP-1 is important for its adhesive properties. In marked contrast, when rolling was analyzed on VAP-1Y471F-transfected cells, neither BTT-2027 compound nor semicarbazide + hydroxylamine had any inhibitory effect (Figure 3B). Hence, the enzyme activity contributes to the adhesive properties of this molecule. Moreover, these results show that the effects of the SSAO inhibitors are specific for VAP-1, since these compounds do not cause any nonspecific effects when used with SSAO-negative cells.
Anti-VAP-1 mAb treatment had a very small but constant inhibitory effect on the number of cells rolling on VAP-1-transfected HUVECs (Figure 3C). Since the anti-VAP-1 mAb does not inhibit the enzyme activity of VAP-1 (Figure 2), we can conclude that 2 separate steps consisting of a mAb-dependent epitope and the enzyme activity are involved in the adhesive function of VAP-1 during rolling. It should be emphasized that a class-matched binding antibody (against HLA class I) was used as a control. It binds to the endothelial cells to a similar extent as anti-VAP-1 mAb (Figure 1), but it does not inhibit SSAO activity (Figure 2) or leukocyte-endothelial cell interactions.19
VAP-1 does not mediate firm adhesion of PMNs to HUVECs under flow
When the number of stably adherent PMNs (Figure 3A) was analyzed on VAP-1-transfected HUVECs under continuous shear stress no effect was seen by either BTT-2027 or semicarbazide + hydroxylamine treatments (Figure 4A). The anti-VAP-1 mAb did not diminish this form of interaction either (Figure 4B). PMN adherence to VAP-1Y471F-transfected HUVECs was also not affected by the SSAO inhibitor treatments or anti-VAP-1 mAbs (Figure 4A-B).
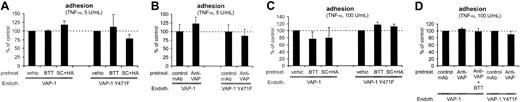
VAP-1 is not involved in stable adhesion between PMNs and HUVECs. (A) The SSAO inhibitors and (B) anti-VAP-1 mAbs do not have any effects on firm binding of PMNs to VAP-1-transfected cells stimulated with 5 U/mL TNF-α (data from the rolling assay protocol). (C-D) Stronger inflammatory stimulus increases the number of adherent cells, but does not make the binding VAP-1-dependent (data from the transmigration assay protocol). HUVECs transfected with VAP-1 or VAP-1Y471F were pretreated with the various compounds as indicated in the figure, and the numbers (mean ± SEM; n = 3-7) of firmly adherent cells were determined. None of the effects was statistically significant. Endoth. indicates endothelial cells; pretreat., pretreated; and vehic, vehicle.
VAP-1 is not involved in stable adhesion between PMNs and HUVECs. (A) The SSAO inhibitors and (B) anti-VAP-1 mAbs do not have any effects on firm binding of PMNs to VAP-1-transfected cells stimulated with 5 U/mL TNF-α (data from the rolling assay protocol). (C-D) Stronger inflammatory stimulus increases the number of adherent cells, but does not make the binding VAP-1-dependent (data from the transmigration assay protocol). HUVECs transfected with VAP-1 or VAP-1Y471F were pretreated with the various compounds as indicated in the figure, and the numbers (mean ± SEM; n = 3-7) of firmly adherent cells were determined. None of the effects was statistically significant. Endoth. indicates endothelial cells; pretreat., pretreated; and vehic, vehicle.
We also used HUVECs stimulated with a higher concentration of TNF-α to induce more stably adherent PMNs than observed in the rolling assays. However, both the SSAO inhibitors and the anti-VAP mAb were also without any effects in this model (Figure 4C-D). Moreover, combined use of BTT2027 SSAO inhibitor and anti-VAP-1 mAb had no effect. Therefore we conclude that VAP-1 is not important for firm adhesion of PMNs to HUVECs in this flow-based model.
Transmigration of PMNs through the endothelial monolayer is SSAO/VAP-1-dependent
The role of VAP-1 in the transmigration phase of the multistep adhesion cascade was studied next. Transmigrating PMNs can be readily detected in the flow-chamber assays using phase-contrast optics: the surface-adherent PMNs on top of the endothelial cells appear phase-bright, whereas the transmigrated ones underneath the endothelium become phase-dark (Figure 5A; to see the video available on the Blood website, use the Supplemental Video link at the top of the online article). Although there was not any evidence for the effects of the different treatments on the number of stably adherent cells (Figure 4), the percentage of transmigrating cells was always counted from all interacting cells (ie, surface adherent plus transmigrated). Pretreatment of the VAP-1-transfected HUVECs with the BTT-2027 compound prevented about 50% of the transmigration when compared with vehicle-treated cells (Figure 5B). Standard SSAO inhibitors semicarbazide and hydroxylamine also inhibited PMN transmigration to the same extent. The transmigration step was also blocked more than 45% by an anti-VAP-1 mAb (Figure 5C). Thus, VAP-1 is important for the transmigration step, which is poorly understood at the molecular level.
When enzymatically inactive VAP-1 was transfected into HUVECs, PMN transmigration was not affected by the SSAO inhibitor BTT-2027 (Figure 5B). Semicarbazide + hydroxylamine pretreatment showed a trend of inhibition; however, it was not statistically significant. These data show that the enzyme inhibitors (especially the BTT-2027 compound) do not significantly affect transmigration when there is no enzymatically active VAP-1 on HUVECs, and thus confirms the specificity of their function.
The use of the enzyme mutant of VAP-1 allowed us to answer the critical question of whether the enzymatic and adhesive functions of VAP-1 are interrelated during the transmigration process. Intriguingly, the anti-VAP-1 mAb pretreatment, which diminishes 50% of transmigration through HUVECs expressing native VAP-1, had absolutely no effect on transmigration through VAP-1Y471F-transfected HUVECs (Figure 5C). These data show that the enzymatic activity of VAP-1 and the antibody-targeted epitope of VAP-1 are both required for the transmigration of PMNs through the monolayer of endothelial cells under flow conditions.
These results suggest a 2-step model of VAP-1 function during transmigration in which separate engagements of antibody-targeted epitope and an oxidation-dependent step take place. To test this hypothesis directly, we treated the VAP-1 transfectants with both the enzyme inhibitors and anti-VAP-1 mAbs. The combined treatment had no additional or synergistic effect on PMN transmigration (Figure 5C). These results are fully compatible with the idea that targeting of either of the VAP-1-dependent steps is sufficient to interfere with the transmigration.
To directly test the role of VAP-1 in PMN-endothelial cell interactions we compared PMN transmigration through HUVECs transduced with VAP-1, VAP-1Y471F, and lacZ. Both VAP-1 constructs led to the similar level of VAP-1 expression on the surface of HUVECs (specific mean fluorescence intensity of VAP-1 was 210 ± 49 on VAP-1 transfectants and 241 ± 52 [mean ± SEM; n = 10] on VAP-1 mutant transfectants). LacZ transfection caused a strong enzymatic activity (as measured by X-Gal stainings) in more than 90% of transfected cells. The same PMNs were then assayed for their transmigratory capacity through these cells. The results showed that VAP-1 transfectants supported significantly more transmigration than cells transfected with the enzymatically inactive form of VAP-1. Moreover, the number of transmigrating cells was practically the same in lacZ-transfected cells and in VAP-1Y471-transfected cells (Figure 5D). These data provide crucial support for the importance of enzymatic activity of VAP-1 in the transmigration process. Use of the same model showed that VAP-1-, VAP-1Y471F-, and lacZ-transfected cells supported PMN adhesion to the same extent (100%, 98 ± 7%, and 99 ± 4%, respectively; n = 5, mean ± SEM; number of adherent PMNs on VAP-1 transfectants defines 100% binding). Finally, analyses of the rolling cells showed that VAP-1 transfectants support slightly more rolling than VAP-1Y471F transfectants (100% and 87 ± 8%, respectively; n = 5, mean ± SEM, number of rolling PMNs on VAP-1 transfectants defines 100% binding). Thus these data give essentially the same results as treatment of different transfectants with anti-VAP-1 mAbs and SSAO inhibitors in showing that VAP-1 contributes to the PMN transmigration and rolling, but not to firm adhesion, and that the enzyme activity of VAP-1 is essential for its adhesive function.
SSAO activity regulates inflammatory reaction in vivo
Finally we wanted to analyze whether the biocompatible SSAO inhibitor BTT-2027 would be effective in reducing PMN extravasation under in vivo conditions. To that end, the well-characterized model of the inflamed rat air pouch was used. In this model, a subcutaneous air pouch is induced on the back of the rat and an inflammatory reaction is elicited by injecting carrageenan into the pouch.15 The emigrated leukocytes can then be collected for analyses from the air pouch.
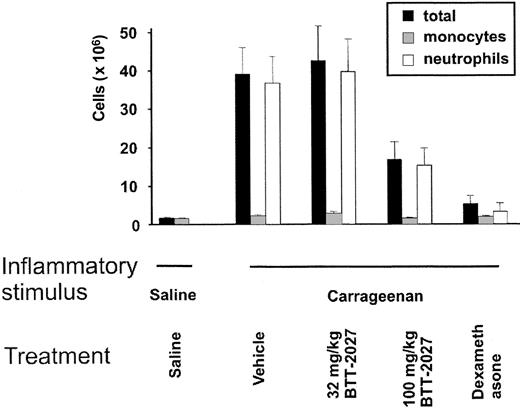
We showed that when saline as a control was injected into the air pouch, no inflammatory response was elicited. In contrast, when carrageenan was used as an inflammatory stimulus, a marked influx of leukocytes from the blood was seen. Approximately 81% to 98% of the infiltrating cells were PMNs. Notably BTT-2027 compound administered intraperitoneally significantly blunted the inflammatory response (Figure 6). This is the first time that SSAO activity has been shown to regulate the development of an inflammatory reaction in vivo. We conclude that the small molecule SSAO inhibitor BTT-2027 is effective in blocking the development of a PMN-dependent inflammatory reaction in vivo.
The SSAO inhibitor BTT-2027 blocks inflammation in vivo. Rats' air pouches were inflamed (carrageenan) or left untreated (saline), and the number of extravasated leukocytes was determined. The animals were treated with the indicated doses of SSAO inhibitors, vehicle (negative control), or dexamethasone (positive control). The numbers and subclasses of leukocytes lavaged from the air pouches are shown. The results are mean ± SEM (n = 8 in each group). The inhibitory effects of BTT-2027 on total leukocyte (P < .05) and on neutrophil (P < .05) extravasation were significant.
The SSAO inhibitor BTT-2027 blocks inflammation in vivo. Rats' air pouches were inflamed (carrageenan) or left untreated (saline), and the number of extravasated leukocytes was determined. The animals were treated with the indicated doses of SSAO inhibitors, vehicle (negative control), or dexamethasone (positive control). The numbers and subclasses of leukocytes lavaged from the air pouches are shown. The results are mean ± SEM (n = 8 in each group). The inhibitory effects of BTT-2027 on total leukocyte (P < .05) and on neutrophil (P < .05) extravasation were significant.
Discussion
We have shown here that VAP-1 mediates PMN-endothelial cell interactions in vitro and in vivo. Moreover, the adhesive function of VAP-1 was found to be dependent on its enzymatic activity during the rolling and transmigration steps of the extravasation cascade under physiologically relevant shear conditions. Blocking the SSAO activity by enzyme inhibitors effectively ameliorates the development of an inflammatory reaction in vivo. These results show that the oxidative activity of VAP-1 plays a significant role during the poorly understood transmigration step of the PMN extravasation cascade.
VAP-1 is a dual function adhesion molecule.20 The anti-VAP-1 mAbs recognize function-blocking epitopes on the extracellular domain, and prevent lymphocyte-endothelial cell interactions in several settings in vitro.16,19,21 The extracellular domain of VAP-1 also harbors the catalytically active site.6,22 The SSAO enzyme activity oxidatively deaminates primary amines into the corresponding aldehyde, ammonium, and hydrogen peroxide,6 which can modulate inflammatory reactions and leukocyte adhesion per se. We have earlier shown that a model peptide with a suitable free NH2 group can modulate the enzymatic activity of VAP-1.16 This suggests that VAP-1 counter-receptor on the leukocyte (molecularly still uncharacterized) contains a free amino-group (eg, N-terminus of a protein, free NH2 group in the side chain of certain amino acids or in aminosugars) that extends through the substrate channel into the catalytically active site of VAP-1. During the multistep oxidative reaction catalyzed by SSAO, a covalent but transient Schiff-base could therefore be formed between the enzyme (VAP-1 on the endothelial cell) and the substrate (the ligand on the leukocyte), which could physically result in an interaction between these 2 cell types.20
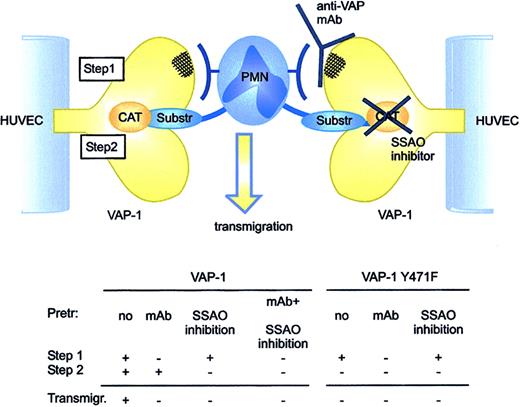
The enzymatically inactive VAP-1Y471F mutant now allowed us to study the role of antibody-dependent adhesive epitope and the enzymatic activity of VAP-1 in isolation. There are 2 independent lines of evidence indicating that the oxidative activity of VAP-1 is required for its adhesive function. (1) Blocking VAP-1 enzyme activity by small molecule SSAO inhibitors inhibits PMN-endothelial cell rolling and transmigration. The same results are seen by using 2 molecularly different SSAO inhibitors (semicarbazide + hydroxylamine and BTT-2027). (2) A single amino acid substitution abolishing the oxidase activity of VAP-1 completely prevents VAP-1-dependent transmigration of PMNs through HUVECs. Since the SSAO inhibitors had no effect on PMN interactions with VAP-1Y471F-transfected cells, their ability to inhibit PMN transmigration through HUVECs transfected with native VAP-1 was specific and VAP-1-dependent. Moreover, anti-VAP-1 mAb significantly inhibited transmigration of PMNs through the VAP-1-transfected HUVEC monolayer, but it had absolutely no effect when used with the enzymatically inactive VAP-1. It is crucial to note that the SSAO activity can be efficiently blocked by specific SSAO inhibitors on VAP-1-transfected cells, including BTT-2027, but it is not affected by the anti-VAP-1 mAbs. Furthermore, the anti-VAP-1 mAbs do not affect interaction between the (presumably larger than the model substrates) leukocyte surface ligand and VAP-1, since they do not inhibit the amount of SSAO-dependent hydrogen peroxide produced during leukocyte-endothelial interactions.16 Collectively these data indicate that VAP-1 functions normally via 2 separate but interrelated processes. First, the leukocyte binds to an antibody-dependent epitope and then the enzymatic reaction follows (Figure 7). If either of these processes is blocked, the whole transmigratory event is aborted. The sequential model of action is supported by the fact that combined use of anti-VAP-1 mAbs and SSAO inhibitors has no additive or synergistic effects on PMN interactions with VAP-1-transfected HUVECs. We believe that the antibody-dependent binding step comes first, since it is difficult to envision how in freely flowing cells a SSAO substrate expressed on the leukocyte surface could rapidly gain access through the narrow substrate channel into the catalytically active site buried within the VAP-1 ectodomain. However, we are aware of the fact that we do not have formal experimental evidence of the order of these 2 separate steps. Moreover, in VAP-1-mediated rolling there may be an enzyme-activity-independent role for VAP-1 as well, since the anti-VAP-1 mAb appears to cause a trend of decreased rolling also on VAP-1 mutant-transfected cells and since VAP-1-transfected HUVECs support only slightly more PMN rolling when compared with VAP-1Y471F-transfected cells.
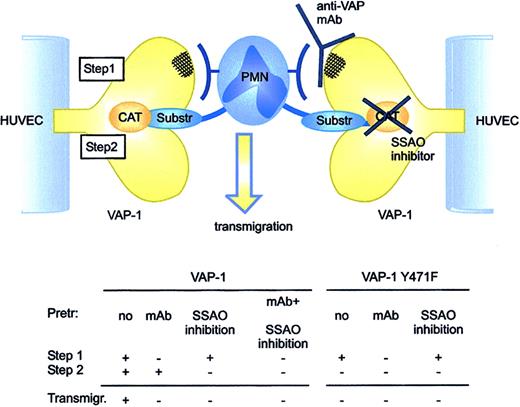
VAP-1 functions via 2 steps during PMN transmigration. First, an antibody-dependent epitope is engaged to allow initial contacts between the PMNs and VAP-1. Then, an oxidative reaction takes place that ensures transmigration of the PMNs. If either step is blocked, the transmigration is halted. The PMN counter-receptor for the antibody-dependent epitope of VAP-1 and the leukocytic substrate for VAP-1 may be the same molecule or 2 separate molecules. Anti-VAP-1 mAbs do not interfere with entry of small model substrates or substrates expressed on the leukocyte surface into the enzymatically active site of VAP-1. Substr indicates substrate; Pretr, pretreated; and Transmigr, transmigration.
VAP-1 functions via 2 steps during PMN transmigration. First, an antibody-dependent epitope is engaged to allow initial contacts between the PMNs and VAP-1. Then, an oxidative reaction takes place that ensures transmigration of the PMNs. If either step is blocked, the transmigration is halted. The PMN counter-receptor for the antibody-dependent epitope of VAP-1 and the leukocytic substrate for VAP-1 may be the same molecule or 2 separate molecules. Anti-VAP-1 mAbs do not interfere with entry of small model substrates or substrates expressed on the leukocyte surface into the enzymatically active site of VAP-1. Substr indicates substrate; Pretr, pretreated; and Transmigr, transmigration.
VAP-1 is involved in the rolling and transmigration steps of the PMN-HUVEC interactions under shear. It is quite remarkable that blocking of VAP-1 caused a 50% inhibition of granulocyte transmigration, since all the other endothelial adhesion molecules (intercellular adhesion molecule 1 [ICAM-1] and ICAM-2, vascular cell adhesion molecule 1 [VCAM-1], E- and P-selectin, etc) expressed constitutively or induced by the TNF-α treatment on the HUVECs remain completely intact in this model. In particular CD31,23 CD99,24 and junctional adhesion molecule-125 and -226 have been reported to contribute to the transmigration process of at least lymphocytes and monocytes. We thus conclude that VAP-1 also supports the transmigration step of the PMN emigration cascade.
Transfection of VAP-1, but not that of enzymatically inactive VAP-1 (at about the same expression levels), into HUVECs resulted in increased transmigration (and rolling) of PMNs through HUVECs. These data suggest that VAP-1 is an important molecule in the context of an intact adhesion cascade. Since VAP-1 expressed on nonendothelial transfectants is not sufficient to support leukocyte binding,27 it can be envisioned that ligation of VAP-1 via an antibody (mimicking the physiologic ligand) or the oxidative function of VAP-1 may ultimately result in the modification of some other (adhesion) molecule(s) crucial for the extravasation process. At least hydrogen peroxide, the potent reactive oxygen species produced via VAP-1-catalyzed amine oxidation on the endothelial cell surface, is an emerging signaling molecule, which has multiple effects on both leukocytes and endothelial cells.28-31 The report that exogenously added H2O2 can induce leukocyte rolling on endothelium32 favors this idea, and VAP-1 is an attractive source of endogenously produced H2O2 on the vascular endothelium. Of course, H2O2 can also have long-term effects via induction of the protein synthesis of other adhesion-related molecules.
We have earlier shown that an anti-VAP-1 mAb blocks extravasation of PMNs into inflamed peritoneum in rabbits33 and in vitro binding of human PMNs to myocardial vessels in frozen sections of human hearts with a reperfusion injury.7 Here we have, for the first time, been able to analyze the role of SSAO activity in vivo. Our results indicate that VAP-1 and its enzymatic activity are important for PMN recruitment to the inflamed air pouch in rats. Combined with the flow chamber data, our results indicate that VAP-1 plays a significant role at the transmigration step of the adhesion cascade. This finding is supported by earlier in vitro studies, in which VAP-1 was shown to be involved in the diapedesis of human peripheral blood lymphocytes (PBLs) through the monolayer of specialized sinusoidal liver endothelial cells.34
The BTT-2027 compound reported here provides opportunities for studies on the in vivo inhibition of SSAO activity. It was shown to be a specific SSAO inhibitor displaying only very low activity toward the distantly related MAO-A and MAO-B enzymes. We believe that the anti-inflammatory effects of this compound are mediated via VAP-1 (also known as amine oxidase, copper containing-3 or AOC3), since in humans the only other SSAO (or AOC) genes described are reported to be either tissue-specific in expression (AOC2 in retina), diamine oxidases (AOC1), or pseudogenes.35,36 In vitro, we could show that this inhibitor has no effect on PMN rolling or transmigration, when an enzymatically inactive VAP-1 was expressed on HUVECs. These data directly show that the effects of BTT-2027 are specifically mediated via VAP-1. In fact, our results show that it may be superior to semicarbazide and hydroxylamine in its specificity for VAP-1, since the latter caused a tendency of inhibition of PMN transmigration also with the VAP-1Y471F-transfected cells. Moreover, since luminal VAP-1 is detected only at the scene of inflammation in vivo in dogs, pigs,37 and humans (D.J.S., unpublished data, February 2004), the functional effects of VAP-1 blockade should be targeted quite selectively to sites of inflammation.
In conclusion, we have for the first time shown that VAP-1 is involved in transmigration of human PMNs through the endothelium. The enzymatic SSAO activity of VAP-1 is critical for this function. Use of the new SSAO inhibitor BTT-2027 gives the first evidence that an inflammatory reaction can be treated in vivo by blocking the enzymatic activity of VAP-1. The possibility of successfully combating an untoward inflammatory reaction via these small molecule compounds should open new avenues for developing useful antiadhesive therapeutics.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-09-3275.
Supported by the Finnish Academy, the Sigrid Juselius Foundation, the Technology Development Centre of Finland, the Finnish Cultural Foundation, and the European Union (QLG1-CT-1999-00295).
One of the authors (S.J.) has declared a financial interest in a company whose potential product was studied in this work. Several of the authors (P.J.V., D.J.S., and M.P.) are employed by a company (Biotie Therapies Corp) whose potential product was studied in the present work.
The online version of the article contains a data supplement.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We acknowledge the expert technical assistance from Ms R. Sjöroos, L. Reunanen, and P. Heinilä, and the secretarial help from Ms A. Sovikoski-Georgieva. Dr G. Yegutkin is thanked for introducing the enzyme assays and Dr S. Tohka for discussions on flow assays.

![Figure 1. Expression of VAP-1 in adenovirally transfected HUVECs. Nontransfected HUVECs (native) and cells transfected with the pADENO-lacZ, pADENO-VAP-1, or pADENO-VAP-1Y471F (enzymatically inactive point mutant) were stained with 3G6 (negative control [neg. co.]), HB116 (against HLA class I), and TK8-14 (against VAP-1) and analyzed using FACS. The x-axis is the fluorescence intensity in a log-scale, and the y-axis is the relative number of cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/9/10.1182_blood-2003-09-3275/6/m_zh80090460730001.jpeg?Expires=1769089447&Signature=S9cOTfe2aC7ZOIB7PKufQNiH9q9lc~rMUBT6hDErMq7UBK7jqPa3QEc787vuBqJ0FuWEh1E0EiHjDOA1-ov5SrmiAh3tniEuyrJ4y4DZ0wUD1NWqpR5HGL4XmWMXGxZm1tki~5jSdxemvemW-7IJwHRfoE~hHQJl14TnK7ajY4HW~0ohSjw9lCq9sflV5W5edMzIEkiiN3Gsm81xBR3WMtU9WRYAf0X5IvqK32EqFV0xC6B21Alpx8f5jhezpQ3NxuG12rUwVBlmuWT0ShMXaMVuMZIOfSZ9RsWehxCL1NFTsDsVExwUPKwB6dtusgoig~wi2HWcW8ifNPO~kxBTqQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 1. Expression of VAP-1 in adenovirally transfected HUVECs. Nontransfected HUVECs (native) and cells transfected with the pADENO-lacZ, pADENO-VAP-1, or pADENO-VAP-1Y471F (enzymatically inactive point mutant) were stained with 3G6 (negative control [neg. co.]), HB116 (against HLA class I), and TK8-14 (against VAP-1) and analyzed using FACS. The x-axis is the fluorescence intensity in a log-scale, and the y-axis is the relative number of cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/9/10.1182_blood-2003-09-3275/6/m_zh80090460730001.jpeg?Expires=1769506893&Signature=m48iPBPZgbMwGP5GaUK6LI~IRMYtEBFBOI5VmktO45oAIOAGeOdoDCRLJHt09yzdcWdN4C63ouc2MHRVhqonvFLPxd9IfUf3K3-M88oKuMNfObSpFzFbswGNWcLKXviNICIDaoQD0r3RE8PArgSGqVw9OZgO5A3tnRqOckXQuwdXIQBE-M3X9IE~3KPtVJcJSKHlrCcKdhx7l8txHbfkj~XG9Tjf-avn9gWI1CU9~-mgdZ7z3d26B5ypGi~yz-P7ykUfmD2u7ORb0ryMveaFg3P1-SX4VgzjJXeKHOCMRepTYCe9g0KFJdFg9MQE5HqUrxQAU16fqciBNO~nZ6QSdQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)