Abstract
Identification of growth factors in neoplasias may be a target for future therapies by blocking either growth factor receptor interaction or the induced pathway. Using gene expression profiling, we identified overexpression of 2 receptors for a proliferation-inducing ligand (APRIL) and B-cell activating factor (BAFF) in malignant plasma cells compared with normal plasma cells. APRIL and BAFF are involved in a variety of tumor and autoimmune diseases, including B-cell malignancies. We confirmed the expression of BAFF and APRIL receptors (B-cell maturation antigen [BCMA], transmembrane activator and calcium modulator and cyclophilin ligand interactor [TACI], and BAFF-R) in a majority of 13 myeloma cell lines and in the purified primary myeloma cells of 11 patients. APRIL and BAFF were potent survival factors for exogenous cytokine-dependent myeloma cell lines and were autocrine growth factors for the RPMI8226 and L363 autonomously growing cell lines. These factors activated nuclear factor (NF)–κB, phosphatidylinositol-3 (PI-3) kinase/AKT, and mitogen-activated protein kinase (MAPK) kinase pathways and induced a strong up-regulation of the Mcl-1 and Bcl-2 antiapoptotic proteins in myeloma cells. BAFF or APRIL was also involved in the survival of primary myeloma cells cultured with their bone-marrow environment, and protected them from dexamethasone (DEX)–induced apoptosis. Finally, the serum levels of BAFF and APRIL were increased about 5-fold in patients with multiple myeloma (MM) as compared with healthy donors. Altogether, these data suggest that APRIL/BAFF inhibitors may be of clinical value in MM. (Blood. 2004;103:3148-3157)
Introduction
Multiple myeloma (MM) is a clonal B-cell neoplasia characterized by the accumulation of malignant plasma cells within the bone marrow, in close contact with stromal cells. Several autocrine or paracrine soluble factors can promote myeloma cell survival and proliferation.1 Interleukin 6 (IL-6), which is mainly produced by cells of the tumor microenvironment, is a major myeloma growth factor.2 Interferon alpha (IFN-α), insulin-like growth factor-1 (IGF-1), hepatocyte growth factor (HGF), and heparin-binding epidermal growth factor–like growth factor (HB-EGF) can also promote the survival or proliferation of myeloma cells.3-7 The inhibition of myeloma cell growth factors may have clinical applications, eventually in combination with other drugs. For example, anti–IL-6 monoclonal antibody (MoAb) may lead to tumor regression in some advanced myeloma patients.8,9
In order to identify new myeloma cell growth factors, we recently compared gene expression profiles of myeloma cells with those of normal plasmablasts and peripheral blood B cells.10-12 Interestingly, the TACI (transmembrane activator and calcium modulator and cyclophilin ligand interactor) and BCMA (B-cell maturation antigen) genes coding for 2 receptors of B-cell activating factor (BAFF, also called BLys)13,14 were highly expressed in malignant plasma cells.10,11 BAFF is a tumor necrosis factor (TNF) family member essentially expressed by monocytes, macrophages, dendritic cells, and some T cells.15 It is produced as both a membrane-bound and a proteolytically cleaved soluble protein.13,14 A third receptor for BAFF, called BAFF-R, was recently identified.16 The expression of BCMA and BAFF-R is B-cell–specific, whereas TACI is also found on a subset of activated T cells.15 Finally, BAFF shares significant homology with a proliferation-inducing ligand (APRIL), which is expressed at a low level by normal lymphoid and myeloid cells, and at a high level by a variety of human cancers.17,18 APRIL, which is directly secreted without cell-surface expression, binds to BCMA and TACI but not to BAFF-R.15
Several studies have indicated that BAFF is a survival factor for immature, naive, and activated B cells.15 The production of BAFF by myeloid dendritic cells in response to innate immune signals was shown to promote T-cell–independent immunoglobulin class switching and to sustain survival of extrafollicular plasmablasts.19 BAFF-transgenic mice develop mature B-cell hyperplasia with autoimmune manifestations, especially production of autoantibodies.15 Moreover, dysregulation of the BAFF pathway seems to be involved in autoimmunity in humans.15 On the contrary, BAFF- or BAFF-R–deficient mice and mice treated with TACI-Fc or BCMA-Fc display severe loss of mature B cells.16
The role of APRIL is less well characterized. Recent reports have shown that APRIL provides survival and activation signals to normal B and T cells.20-22 In addition, APRIL is highly expressed in several tumor tissues and stimulates growth of tumor cells in vitro and in vivo.17
The signal transduction pathways driven by BAFF and APRIL are not fully characterized. The activation of nuclear factor (NF)–κB by TACI, BCMA, and BAFF-R23 is consistent with the antiapoptotic role of BAFF, since NF-κB enhances the transcription of several cell survival genes.24,25 Depending on the B-cell maturation stage, BAFF was reported to induce the antiapoptotic proteins Bcl-2, A1, and Bcl-XL and to reduce the proapoptotic protein Bak.23,26,27 BAFF also activates Jun kinase (JNK), Elk-1, p38 kinase, activating protein 1 (AP-1), and NF-AT in various models.15
The striking roles of BAFF, APRIL, and their receptors in normal B-cell homeostasis and in several tumor models raise the possibility that they may be involved in the pathogenesis of B-cell malignancies. Recent studies reported the aberrant expression of BAFF and APRIL by tumor B cells isolated from a subset of patients with chronic lymphoid leukemia, suggesting the existence of an autocrine survival loop in this disease.28,29 In vitro, a BCMA-Fc fusion protein is able to enhance apoptosis of B-cell chronic lymphocytic leukemia (B-CLL) cells.29 In addition, patients with follicular non-Hodgkin lymphomas have increased levels of soluble BAFF in their serum, and BAFF seems to favor B-lymphoma cell survival.30
In this study, we show that myeloma cell lines and primary myeloma cells express BAFF, APRIL, and their receptors and that BAFF and APRIL are myeloma cell growth factors and rescue myeloma cells from apoptosis induced by dexamethasone. BAFF and APRIL activated nuclear factor (NF)–κB, phosphatidylinositol-3 (PI-3) kinase/AKT, and mitogen-activated protein kinase (MAPK) kinase pathways in myeloma cells and induced a strong up-regulation of the Mcl-1 and Bcl-2 antiapoptotic proteins. Finally, we demonstrate a 5-fold increase in the serum levels of BAFF or APRIL in patients with MM compared with age-related healthy individuals.
Materials and methods
Myeloma cell lines and primary samples
XG-1, XG-2, XG-5, XG-6, XG-7, XG-11, XG-13, XG-14, and XG-20 are IL-6–dependent human myeloma cell lines (HMCLs) obtained in our laboratory.31 Upon removal of IL-6, these cells progressively apoptose within 10 to 14 days. These HMCLs were routinely maintained in RPMI 1640 and 10% fetal calf serum (FCS; Biowittaker, Walkersville, MD), except XG-14, which was maintained in X-VIVO 20 (Biowittaker) supplemented with 3 ng/mL IL-6 (Peprotech, Rocky Hill, NJ). The human myeloma cell lines RPMI8226, U266, LP1, and L363 (ATCC, Rockville, MD) grew autonomously in RPMI–10% FCS. All cell lines were free of Mycoplasma, as assayed by an enzyme-linked immunosorbent assay (ELISA) kit (Boehringer, Mannheim, Germany). Peripheral blood B cells (PBBs) were purified using CD19 microbeads (Miltenyi Biotech, Paris, France), and dendritic cells (DCs) were generated from adherent monocytes in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-13.
Bone marrow or peripheral blood samples were collected from 5 patients with plasma cell leukemia (PCL) and 14 patients with intramedullary myeloma after informed consent was obtained. Mononuclear cells were obtained by centrifugation on Ficoll-hypaque medium. For reverse transcriptase–polymerase chain reaction (RT-PCR) analysis, myeloma cells were purified (> 95% purity) using CD138 microbeads (Miltenyi Biotech, Paris, France), whereas phenotype and apoptosis were analyzed on whole mononuclear cells. Polyclonal plasmablastic cells (PPCs) were generated from purified CD19+ PBBs in vitro.10 Briefly, PBBs were cultured in RPMI 1640 and 10% FCS in the presence of mitomycin-treated CD40L transfectant, IL-2 (20 U/mL), IL-4 (50 ng/mL), IL-10 (50 ng/mL), and IL-12 (2 ng/mL; R&D Systems, Abington, United Kingdom). After 4 days of culture, B cells were harvested and cultured without CD40 ligand (CD40L) transfectant and with IL-2, IL-10, IL-12, and IL-6 (5 ng/mL). On day 6 of culture, cells were stained with fluorescein isothiocyanate (FITC)–conjugated anti-CD20 (Beckman-Coulter, Marseilles, France) and phycoerythrin (PE)–conjugated anti-CD38 (Becton Dickinson, San Jose, CA) and CD20-CD38++ PPCs were sorted with a FACSvantage (Becton Dickinson).
Sera from 26 patients with myeloma at diagnosis, 10 patients with PCL, and 9 age-related healthy individuals were collected as described previously.32
mRNA analysis
We generated cDNA with 2 μg total RNA using the Superscript II reverse transcriptase (Life Technologies) and oligo d(T) (Amersham Pharmacia Biotech, Orsay, France). Each 25-μL PCR reaction contained 1 μL of the first-strand cDNA, 1 μM of each primer (sense and antisense), 0.2 mM each of dNTP (2′-deoxynucleoside 5′-triphosphate), 1.5 mM MgCl2, 1 × polymerase buffer, and 2 units of Taq polymerase (Life Technologies). The following primers were used: BAFF, 5′-GGA GAA GGC AAC TCC AGT CAG AAC (sense) and 5′-CAA TTC ATC CCC AAA GAC ATG GAC (antisense); APRIL, 5′-CCT TGC TAC CCC ACT CTT G (sense) and 5′-ACA CTC AGA ATA TCC CCT TGG (antisense); BCMA, 5′-TTA CTT GTC CTT CCA GGC TGT TCT (sense) and 5′-CAT AGA AAC CAA GGA AGT TTC TAC C (antisense); TACI, 5′-CAC CCT AAG CAA TGT GC (sense) and 5′-TGG GAC TCA GAG TGC C (antisense); BAFF-R, 5′-GGA GAA GGC AGG AAC CAC (sense) and 5′-AAG GCA AGC ACA CCA AA (antisense); β2-microglobulin (β2M), 5′-CCA GCA GAG AAT GGA AAG TC (sense) and 5′-GAT GCT GCT TAC ATG TCT CG (antisense). The sizes of the PCR products were as follows: BAFF, 311 bp; APRIL, 729 bp; BCMA, 806 bp; TACI, 931 bp; BAFF-R, 300 bp; and β2M, 269 bp. The amplification profile was 1 minute at 94°C, 1 minute at 62°C (BAFF), 67°C (APRIL), 58°C (BCMA), 60°C (TACI), 61°C (BAFF-R), and 60°C (β2M), 1 minute at 72°C, followed by a final extension of 10 minutes at 72°C. Reaction products were electrophoresed on a 1.5% agarose gel.
Flow cytometry analysis
The overall expression of receptors for BAFF on HMCLs was evaluated by incubating 5 × 105 cells with 10 μg/mL of a human BAFF–murine CD8 (BAFF-muCD8) biotinylated fusion protein (Ancell, Bayport, MN) in phosphate-buffered saline (PBS) containing 30% human AB serum at 4°C for 30 minutes followed by incubation with PE-conjugated streptavidin (Beckman-Coulter). For primary samples, cells were double stained with BAFF-muCD8 fusion protein and FITC-conjugated anti-CD138 (Beckman-Coulter). The expression of BAFF was evaluated using an anti-BAFF antibody (Buffy-1; Alexis Biotechnology, Lausen, Switzerland). Flow cytometry analysis was done on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA).
Study of apoptosis
IL-6–dependent HMCLs were starved of IL-6 for 3 hours and cultured in 24-well, flat-bottomed microtiter plates at 105 cells per well in RPMI 1640–10% FCS or X-VIVO 20 culture medium with or without IL-6 (3 ng/mL), BAFF (200 ng/mL; Peprotech), or APRIL (200 ng/mL; R&D Systems). After 3 days of culture, cells were washed twice in PBS and apoptosis was assayed with FITC-conjugated annexin V labeling (Boehringer). Fluorescence was analyzed on a FACScan flow cytometer. In order to study the dexamethasone (DEX)–induced apoptosis, autonomously growing HMCLs were cultured for 3 days in 24-well, flat-bottomed microtiter plates at 105 cells per well in RPMI 1640–10% FCS with or without DEX (10-6 M), IL-6 (3 ng/mL), BAFF (200 ng/mL), or APRIL (200 ng/mL) and apoptosis was assayed with annexin V labeling.
Proliferation assay
HMCLs were IL-6 starved for 3 hours and cultured for 5 days in 96-well, flat-bottomed microtiter plates at 104 cells per well in RPMI 1640–10% FCS or X-VIVO 20 with or without IL-6 (3 ng/mL), BAFF (200 ng/mL), APRIL (200 ng/mL), the B-E8 anti–IL-6 antibody (10 μg/mL) (Diaclone, Besancon, France), an inhibitor of PI-3K/AKT pathway (Ly 294002; 25 μM), an inhibitory peptide of NF-κB pathway (SN50), or the corresponding inactive peptide (100 μg/mL) (BIOMOL, Plymouth Meeting, PA), or a fusion protein of TACI and the human Fc fragment of immunoglobulin (TACI-Fc; 10 μg/mL; R&D Systems). Cells were pulsed with tritiated thymidine (Amersham Pharmacia Biotech) for the last 12 hours of culture, harvested, and counted on a liquid scintillation analyzer.
Mononuclear cell culture
Mononuclear cells from tumor samples of 8 patients with MM were cultured for 4 days at 5 × 105 cells/mL in RPMI 1640 medium, 5% FCS, 1 ng/mL IL-6, with or without 10-6 M dexamethasone (DEX), BAFF (200 ng/mL), or APRIL (200 ng/mL). In each culture group, viability and cell counts were assayed and myeloma cells were stained with an anti–CD138-PE MoAb (Immunotech).
ELISA
ELISA microplates (Nunc MaxiSorp; Nalge Nunc International, Rochester, NY) were coated overnight at 4°C with 100 μL mouse anti-human BAFF antibody (RDI, Flanders, NJ) or mouse anti-human APRIL antibody (R&D Systems) (10 μg/mL in PBS). Plates were washed 5 times with PBS, 0.1% Tween 20, and blocked with PBS, 1% BSA for 2 hours at room temperature. Patients' or healthy donors' sera were added and plates were incubated for 2.5 hours at 37°C and washed. Rabbit anti-human BAFF antibody (Upstate, Lake Placid, NY) or goat anti-human APRIL antibody (R&D Systems) (2 μg/mL in PBS, 1% BSA, 0.05% Tween 20) were added for 2 hours at room temperature and the bound antibodies were detected with goat anti-rabbit (Sigma, 1:15 000) or rabbit anti-goat (Dako, Copenhagen, Denmark; 1:1000) peroxidase-conjugated antibodies. The peroxidase reaction was developed with a tetramethylbenzidine (TMB) substrate kit (Sigma, St Louis, MO). Light absorbance was measured at 450 nm and standard curves were generated using known concentrations of recombinant human BAFF or APRIL. The sensitivity of the ELISA was 1.5 ng/mL for BAFF and 3 ng/mL for APRIL. The intra-assay variability of the ELISA was determined by measuring serum samples from 7 patients in 2 separate experiments and was less than 14% for BAFF and less than 17% for APRIL.
Western blot analysis
HMCLs were starved overnight in RPMI 1640–1% bovine serum albumin (BSA) without IL-6. Cells were lysed in 10 mM tris-HCl (pH 7.05), 50 mM NaCl, 50 mM NaF, 30 mM sodium pyrophosphate (NaPPi), 1% Triton X-100, 5 μM ZnCl2, 100 μM Na3VO4, 1 mM dithiothreitol (DTT), 20 mM β-glycerophosphate, 20 mM P-nitrophenolphosphate (PNPP), 2.5 μg/mL aprotinin, 2.5 μg/mL leupeptin, 0.5 mM phenylmethylsulphonyl fluoride (PMSF), 0.5 mM benzamidine, 5 μg/mL pepstatin, and 50 nM okadaic acid. Lysates were cleared by centrifugation at 10 000g for 10 minutes and resolved by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) before transfer to a nitrocellulose membrane (Schleicher and Schuell, Dassel, Germany). Membranes were blocked for 1 hour at room temperature in 140 mM NaCl, 3 mM KCl, 25 mM tris-HCl (pH 7.4), 0.1% Tween 20 (TBS-T), 5% BSA, then incubated for 1 hour at room temperature with primary antibodies (phospho-specific antibodies anti-ERK1/2, anti–signal transducer and activator of transcription 3 (STAT3) and anti-AKT; New England Biolabs, Beverly, MA) at a 1:1000 dilution in 1% BSA TBS-T. The primary antibodies were visualized with goat anti-rabbit (Sigma) or goat anti-mouse (Bio-Rad, Hercules, CA) peroxidase-conjugated antibodies using an enhanced chemiluminescence detection system. As a control for protein loading, we used anti-STAT3 (1:2000; Transduction Laboratories, Lexington, KY), anti-ERK1/2 (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA) and anti-AKT (New England Biolabs) antibodies. Rabbit polyclonal antibodies specific for Bcl-x and Mcl-1 were obtained from Santa Cruz Biotechnology and Bcl-2 antibody from Dako.
Blots were quantified by densitometry using acquisition into Adobe Photoshop (Apple, Cupertino, CA) and analyzing with the NIH Image software (National Institutes of Health, Bethesda, MD).
Nuclear transcription factor–κB assay
NF-κB activation was determined with a Trans-am NF-κB p50 Transcription Factor Assay Kit (Active Motif, Carlsbad, CA) according to the manufacturer's instructions. This ELISA used a 96-well plate coated with an oligonucleotide containing the NF-κB consensus binding site (5′-GGGACTTTCC-3′). Following overnight starvation, cells were seeded on a 24-well plate (106 cells/well) and were stimulated for 1 hour with IL-6 (3 ng/mL), BAFF (200 ng/mL), APRIL (200 ng/mL), or TNF-α (20 ng/mL). Cell lysates were diluted (1:10) and added to the ELISA plate. NF-κB binding to the target oligonucleotide was detected by incubation with primary antibody specific for the activated form of p50, visualized by anti-IgG horseradish peroxidase conjugate, and quantified at 450 nm. Each condition was run in triplicate.
Statistical analysis
Statistical significance was tested using a nonparametric Wilcoxon test for pairs or a Student t test for pairs.
Results
BCMA, TACI, and BAFF-R expression in malignant and normal plasma cells
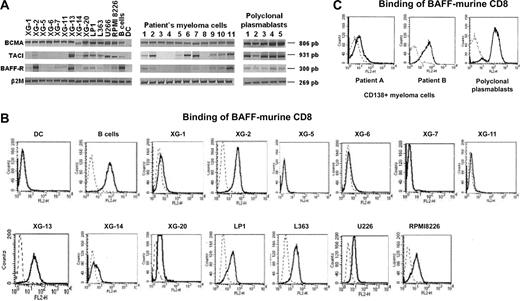
RT-PCR analysis indicated that 13 of 13 HMCLs expressed BCMA according to our microarray results (Figure 1A).10,11 The expression pattern of TACI and BAFF-R was more heterogeneous. As shown in Figure 1A, TACI and BAFF-R were expressed, respectively, by 8 of 13 and 9 of 13 HMCLs. Unlike DCs, purified B cells expressed BCMA, TACI, and BAFF-R as reported.33 We next looked for the expression in primary myeloma cells of 6 patients with intramedullary myeloma (patients 1-6) and of 5 patients with plasma cell leukemia (PCL) (patients 7-11). BCMA RNA was detected in 11 of 11 samples (Figure 1A). TACI and BAFF-R were simultaneously expressed by 8 of 11 primary myeloma samples.
Expression of BCMA, TACI, and BAFF-R in myeloma cells and normal plasmablasts. (A) Expression of BCMA, TACI, and BAFF-R mRNA was analyzed by RT-PCR in 13 HMCLs, in CD138+ purified primary myeloma cells from 6 patients with intramedullary MM (patients 1-6), and 5 patients with plasma cell leukemia (patients 7-11), and in 5 normal in vitro–generated polyclonal plasmablastic cells (PPCs). B cells and dendritic cells (DCs) were used as positive and negative controls, respectively. (B) Cell-surface expression of receptors for BAFF by HMCLs was determined by flow cytometry using a biotinylated human BAFF–murine CD8 fusion protein and phycoerythrin-conjugated streptavidin. (C) Cell-surface expression of receptors for BAFF by primary myeloma cells and PPCs was determined by flow cytometry using a biotinylated human BAFF–murine CD8 fusion protein and phycoerythrin-conjugated streptavidin. Broken lines indicate Ig control; and solid lines, BAFF-mu CD8.
Expression of BCMA, TACI, and BAFF-R in myeloma cells and normal plasmablasts. (A) Expression of BCMA, TACI, and BAFF-R mRNA was analyzed by RT-PCR in 13 HMCLs, in CD138+ purified primary myeloma cells from 6 patients with intramedullary MM (patients 1-6), and 5 patients with plasma cell leukemia (patients 7-11), and in 5 normal in vitro–generated polyclonal plasmablastic cells (PPCs). B cells and dendritic cells (DCs) were used as positive and negative controls, respectively. (B) Cell-surface expression of receptors for BAFF by HMCLs was determined by flow cytometry using a biotinylated human BAFF–murine CD8 fusion protein and phycoerythrin-conjugated streptavidin. (C) Cell-surface expression of receptors for BAFF by primary myeloma cells and PPCs was determined by flow cytometry using a biotinylated human BAFF–murine CD8 fusion protein and phycoerythrin-conjugated streptavidin. Broken lines indicate Ig control; and solid lines, BAFF-mu CD8.
Expression of BAFF and APRIL receptors was found in 5 of 5 in vitro–generated normal plasmablasts. In particular, BCMA and TACI were detected at a high level whereas BAFF-R was less expressed. These results are in agreement with our Affymetrix data10,11 and with the recent study of Avery et al34 (Figure 1A).
To confirm the membrane expression of receptors for BAFF, we used a biotinylated human BAFF–murine CD8 fusion protein, which binds to TACI, BCMA, and BAFF-R. In agreement with previous studies, this BAFF–murine CD8 did not label monocyte-derived DCs but efficiently bound purified B cells (Figure 1B). BAFF–murine CD8 fusion protein bound to 8 of 13 HMCLs. All of them expressed high levels of TACI or BAFF-R (XG-2, XG-13, XG-14, XG-20, LP1, L363, U266, and RPMI 8226). The other 5 HMCLs that were not labeled by BAFF-murine CD8 expressed BCMA alone or BCMA and a low level of BAFF-R (XG-1, XG-5, XG-6, XG-7, and XG-11; Figure 1A). In addition, we confirmed the presence of membrane receptors on primary myeloma cells and normal plasmablasts (Figure 1C).
BAFF and APRIL expression in malignant and normal plasma cells
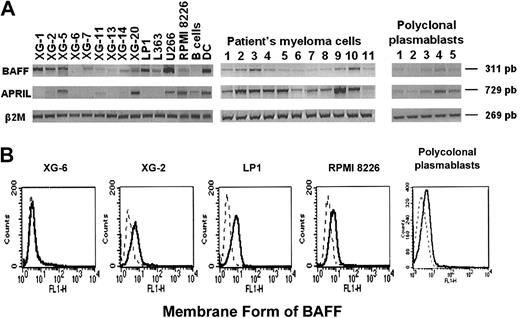
Since an autocrine production of APRIL and BAFF was previously reported in several tumor models,17,28,29 we looked for their expression in HMCLs and in primary myeloma cells. BAFF RNA was detected in 12 of 13 HMCLs and in 11 of 11 primary myeloma samples. An anti-BAFF antibody stained 10 of 12 HMCLs that expressed BAFF mRNA, showing the presence of the membrane-bound form of BAFF. Data for 4 cell lines are shown in Figure 2B. XG-6 showed no cell-surface expression of BAFF, in agreement with the absence of detectable BAFF RNA in these cells (Figure 2B). APRIL RNA was expressed in most primary samples (10 of 11) and in 6 of 13 HMCLs (Figure 2A). As APRIL is a secreted protein, we looked for APRIL protein in myeloma cell culture supernatants. Using ELISA, soluble APRIL levels were 30.9 ng/mL and 33.3 ng/mL in culture supernatants of RPMI8226 and XG-20, respectively, that expressed APRIL mRNA. APRIL was not detectable in culture supernatant of XG-6 which did not express the gene. Polyclonal plasmablasts expressed BAFF and APRIL RNA and were labeled by the anti-BAFF antibody (Figure 2A-B).
Expression of BAFF and APRIL in myeloma cells and normal plasmablasts. (A) Expression of BAFF and APRIL mRNA was analyzed by RT-PCR in 13 HMCLs, in CD138+ purified primary myeloma cells from 6 patients with intramedullary MM (patients 1-6), and 5 patients with plasma cell leukemia (patients 7-11), and in 5 normal in vitro–generated polyclonal plasmablastic cells (PPCs). B cells and dendritic cells (DCs) were used as negative and positive controls, respectively. (B) Cell-surface expression of BAFF was determined by flow cytometry using an anti-BAFF antibody. Broken lines indicate Ig control; and solid lines, anti-BAFF Ab.
Expression of BAFF and APRIL in myeloma cells and normal plasmablasts. (A) Expression of BAFF and APRIL mRNA was analyzed by RT-PCR in 13 HMCLs, in CD138+ purified primary myeloma cells from 6 patients with intramedullary MM (patients 1-6), and 5 patients with plasma cell leukemia (patients 7-11), and in 5 normal in vitro–generated polyclonal plasmablastic cells (PPCs). B cells and dendritic cells (DCs) were used as negative and positive controls, respectively. (B) Cell-surface expression of BAFF was determined by flow cytometry using an anti-BAFF antibody. Broken lines indicate Ig control; and solid lines, anti-BAFF Ab.
BAFF and APRIL rescue IL-6–dependent HMCLs from apoptosis induced by IL-6 deprivation
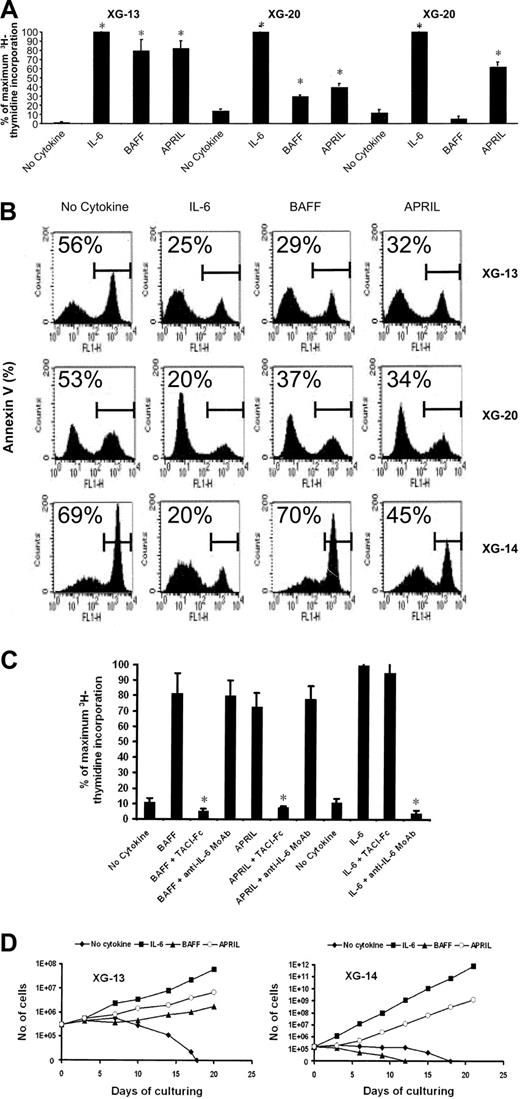
To investigate the effect of BAFF and APRIL on myeloma cell survival and proliferation, we first used 3 cell lines whose growth is dependent on addition of IL-6: XG-13 and XG-20 HMCLs that expressed TACI and BAFF-R and XG-14 that expressed mainly TACI. In the absence of exogenous cytokines, the 3 HMCLs did not proliferate and a strong proliferation was induced by recombinant IL-6 (Figure 3A).31 BAFF and APRIL were also potent proliferation factors for XG-13 and XG-20 cells, whereas XG-14 cells responded to APRIL only (Figure 3A). Using annexin V as an indicator of apoptosis, we looked for the effect of BAFF and APRIL on myeloma cell survival. BAFF and APRIL efficiently protected XG-13 cells (respectively, P = .01 and P = .001; n = 5) and XG-20 cells (respectively, P = .0003 and P = .0002; n = 5) from IL-6 deprivation–induced apoptosis. Only APRIL protected XG-14 (P = .003; n = 5) cells from apoptosis, in agreement with the above-mentioned proliferation data (Figure 3B). A TACI-Fc fusion protein abrogated specifically the myeloma cell proliferation induced by BAFF or APRIL, whereas an anti–IL-6 MoAb did not affect it (Figure 3C). Conversely, TACI-Fc had no effect on IL-6–induced proliferation that was completely inhibited by an anti–IL-6 MoAb (Figure 3C).
BAFF and APRIL protect HMCLs from IL-6 deprivation–induced apoptosis. (A) XG-13, XG-14, and XG-20 were IL-6 starved for 3 hours and cultured without cytokine, or in the presence of BAFF (200 ng/mL), APRIL (200 ng/mL), or IL-6 (3 ng/mL). Results are the mean values plus or minus standard deviation (SD) of the tritiated thymidine incorporation determined on sextuplet culture wells and are expressed as the percentage of the proliferation obtained with IL-6. Results are those of one experiment representative of 5. *Mean value is significantly different from that obtained without adding cytokine using a Student t test (P ≤ .05). (B) XG-13 and XG-14 HMCLs were cultured at 105 cells/mL without cytokine or in the presence of IL-6 (3 ng/mL), BAFF (200 ng/mL), or APRIL (200 ng/mL). Cells were recovered after 3 days of culture and apoptotic cells were detected by annexin V staining. Results are those of one experiment representative of 5. The percentage of apoptotic cells is indicated in each panel. (C) XG-13 cells were IL-6 starved for 3 hours and cultured without cytokine, or in the presence of IL-6 (3 ng/mL), BAFF (200 ng/mL), or APRIL (200 ng/mL). When indicated, TACI-Fc (10 μg/mL) or anti–IL-6 MoAb (10 μg/mL) was added. Results are the mean values plus or minus SD of the tritiated thymidine incorporation determined on sextuplet culture wells and are expressed as the percentage of the proliferation obtained with IL-6. Results are for one experiment representative of 3. * Mean value is statistically significantly different from that obtained with either BAFF, APRIL, or IL-6 using a Student t test (P ≤ .05). (D) XG-13 and XG-14 cells were cultured, respectively, at 2.5 × 105 and at 1.5 × 105 cells/mL without cytokine or in the presence of IL-6 (3 ng/mL), BAFF (200 ng/mL), or APRIL (200 ng/mL). Every 3 or 4 days, cells were counted and diluted with fresh culture medium containing the initial cytokine concentration. Results are the cumulative cell numbers obtained within 20 days of culture and are those of one experiment representative of 2.
BAFF and APRIL protect HMCLs from IL-6 deprivation–induced apoptosis. (A) XG-13, XG-14, and XG-20 were IL-6 starved for 3 hours and cultured without cytokine, or in the presence of BAFF (200 ng/mL), APRIL (200 ng/mL), or IL-6 (3 ng/mL). Results are the mean values plus or minus standard deviation (SD) of the tritiated thymidine incorporation determined on sextuplet culture wells and are expressed as the percentage of the proliferation obtained with IL-6. Results are those of one experiment representative of 5. *Mean value is significantly different from that obtained without adding cytokine using a Student t test (P ≤ .05). (B) XG-13 and XG-14 HMCLs were cultured at 105 cells/mL without cytokine or in the presence of IL-6 (3 ng/mL), BAFF (200 ng/mL), or APRIL (200 ng/mL). Cells were recovered after 3 days of culture and apoptotic cells were detected by annexin V staining. Results are those of one experiment representative of 5. The percentage of apoptotic cells is indicated in each panel. (C) XG-13 cells were IL-6 starved for 3 hours and cultured without cytokine, or in the presence of IL-6 (3 ng/mL), BAFF (200 ng/mL), or APRIL (200 ng/mL). When indicated, TACI-Fc (10 μg/mL) or anti–IL-6 MoAb (10 μg/mL) was added. Results are the mean values plus or minus SD of the tritiated thymidine incorporation determined on sextuplet culture wells and are expressed as the percentage of the proliferation obtained with IL-6. Results are for one experiment representative of 3. * Mean value is statistically significantly different from that obtained with either BAFF, APRIL, or IL-6 using a Student t test (P ≤ .05). (D) XG-13 and XG-14 cells were cultured, respectively, at 2.5 × 105 and at 1.5 × 105 cells/mL without cytokine or in the presence of IL-6 (3 ng/mL), BAFF (200 ng/mL), or APRIL (200 ng/mL). Every 3 or 4 days, cells were counted and diluted with fresh culture medium containing the initial cytokine concentration. Results are the cumulative cell numbers obtained within 20 days of culture and are those of one experiment representative of 2.
Finally, we looked for the ability of BAFF and APRIL to support the long-term growth of XG-13 and XG-14 HMCLs. As shown in Figure 3D, XG-13 and XG-14 cells died within 17 to 18 days upon removal of IL-6. IL-6 induced an exponential growth of the 2 HMCLs, with a doubling time of 48 hours for XG-13 and 20 hours for XG-14. APRIL and BAFF were both able to support the long-term growth of XG-13 cells with a doubling time, respectively, 2.2-fold and 1.75-fold higher than that obtained with IL-6. In agreement with the survival and proliferation data shown above, only APRIL supported long-term growth of XG-14 cells with a doubling time 1.5-fold higher than that obtained with IL-6.
These data indicate that BAFF and APRIL myeloma cell growth factors are able to support the long-term growth of cytokine-dependent HMCLs.
Autocrine BAFF and/or APRIL are involved in the autonomous growth of cytokine-independent HMCLs
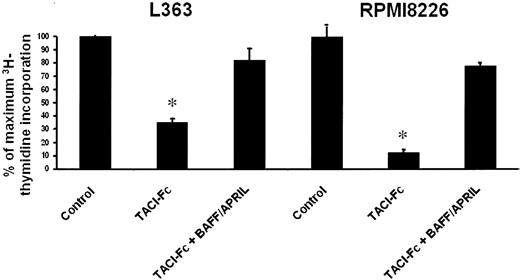
As BAFF and/or APRIL are growth factors for IL-6–dependent HMCLs and are produced by some autonomously growing HMCLs, we investigated whether BAFF/APRIL could be autocrine myeloma growth factors. We used RPMI8226 and L363 HMCLs that expressed BAFF and/or APRIL together with their receptors (Figures 1 and 2). The autonomous proliferation of L363 and RPMI8226 cells was blocked by TACI-Fc, which neutralizes both BAFF and APRIL (Figure 4). Adding an excess of recombinant BAFF and APRIL abrogated the inhibitory effect of TACI-Fc. These data indicated that a BAFF/APRIL autocrine loop is involved in the autonomous growth of some HMCLs.
Autocrine BAFF or APRIL are involved in the growth of autonomously growing HMCLs. L363 and RPMI8226 cells were starved for 3 hours and cultured without cytokine, or in the presence of TACI-Fc (10 μg/mL) or TACI-Fc (10 μg/mL) and BAFF/APRIL (200 ng/mL BAFF and 200 ng/mL APRIL). Results are the mean values plus or minus SD of the tritiated thymidine incorporation determined on sextuplet culture wells and are expressed as the percentage of the proliferation obtained without cytokine. * Mean value is statistically significantly different from that obtained without adding TACI-Fc using a Student t test (P ≤ .05).
Autocrine BAFF or APRIL are involved in the growth of autonomously growing HMCLs. L363 and RPMI8226 cells were starved for 3 hours and cultured without cytokine, or in the presence of TACI-Fc (10 μg/mL) or TACI-Fc (10 μg/mL) and BAFF/APRIL (200 ng/mL BAFF and 200 ng/mL APRIL). Results are the mean values plus or minus SD of the tritiated thymidine incorporation determined on sextuplet culture wells and are expressed as the percentage of the proliferation obtained without cytokine. * Mean value is statistically significantly different from that obtained without adding TACI-Fc using a Student t test (P ≤ .05).
BAFF and APRIL rescue myeloma cells from dexamethasone-induced apoptosis
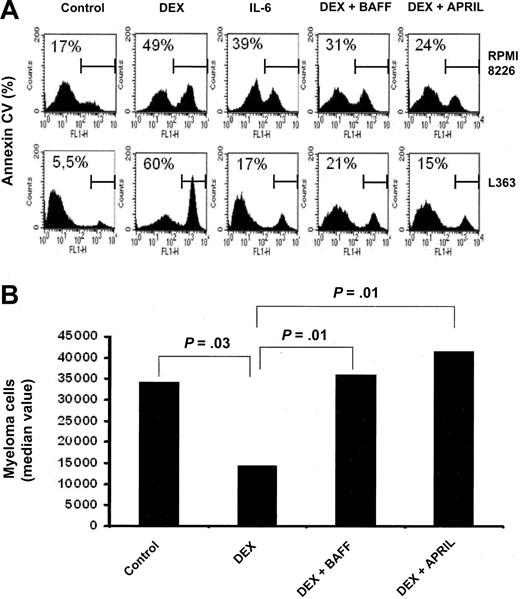
We next sought to determine whether BAFF or APRIL could protect myeloma cells from the apoptosis induced by DEX, a potent drug for MM treatment. As indicated in Figure 5A, DEX induced apoptosis in RPMI 8226 and L363 HMCLs. Both BAFF and APRIL significantly protected the RPMI 8226 HMCL from DEX-induced apoptosis (respectively, P = .001 and P = .0002; n = 5). The same results were obtained with L363 (respectively, P = .0007 and P = .001; n = 5). In fact, both BAFF and APRIL were as potent as IL-6 in protecting myeloma cells from DEX-induced apoptosis (Figure 5A).
BAFF and APRIL rescue myeloma cells from dexamethasone-induced apoptosis. (A) RPMI 8226 and L363 myeloma cells were cultured in the presence of DEX (10-6 M) with or without IL-6 (3 ng/mL), BAFF (200 ng/mL), or APRIL (200 ng/mL). Cells were recovered after 3 days of culture and apoptotic cells were detected by annexin V staining. Results are those of one experiment of 5. The percentage of apoptotic cells is indicated in each panel. (B) Mononuclear cells from 8 patients with MM were cultured for 4 days in the presence of IL-6 (1 ng/mL) with or without DEX (10-6 M), BAFF (200 ng/mL), or APRIL (200 ng/mL). At day 4 of culture, the viability and total cell counts were assessed and the percentage of CD138+ viable plasma cells was determined by flow cytometry. Results are median values of the numbers of myeloma cells in the culture wells. The values were compared with a Wilcoxon test for pairs.
BAFF and APRIL rescue myeloma cells from dexamethasone-induced apoptosis. (A) RPMI 8226 and L363 myeloma cells were cultured in the presence of DEX (10-6 M) with or without IL-6 (3 ng/mL), BAFF (200 ng/mL), or APRIL (200 ng/mL). Cells were recovered after 3 days of culture and apoptotic cells were detected by annexin V staining. Results are those of one experiment of 5. The percentage of apoptotic cells is indicated in each panel. (B) Mononuclear cells from 8 patients with MM were cultured for 4 days in the presence of IL-6 (1 ng/mL) with or without DEX (10-6 M), BAFF (200 ng/mL), or APRIL (200 ng/mL). At day 4 of culture, the viability and total cell counts were assessed and the percentage of CD138+ viable plasma cells was determined by flow cytometry. Results are median values of the numbers of myeloma cells in the culture wells. The values were compared with a Wilcoxon test for pairs.
We next investigated whether BAFF and APRIL could protect primary myeloma cells from DEX-induced apoptosis. Since purified myeloma cells are highly susceptible to spontaneous apoptosis in vitro, myeloma cells were cultured in the presence of their bone marrow environment. In addition, recombinant IL-6 was added to reduce the variability resulting from the heterogeneous endogenous IL-6 production in cultured tumor samples.35 As shown in Figure 5B, DEX reduced the median number of viable myeloma cells of 8 patients by 58% (P = .03; n = 8). BAFF and APRIL enhanced survival of myeloma cells in the presence of DEX (respectively, P = .01 and P = .01; n = 8) yielding a number of malignant plasma cells that was not statistically different between DEX and BAFF, DEX and APRIL, and the control group (Figure 5B).
BAFF/APRIL inhibitor induces apoptosis of primary myeloma cells
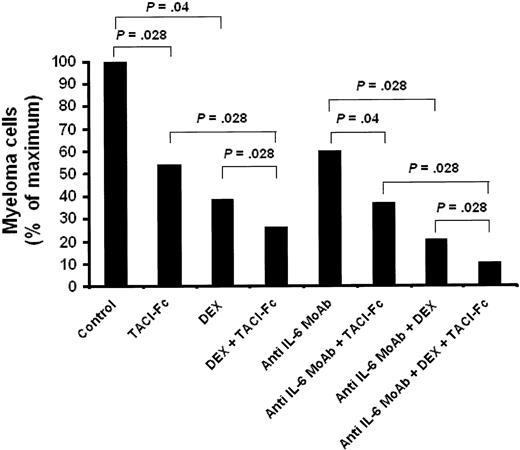
Our data showing that BAFF and APRIL are survival factors for malignant plasma cells suggest that new therapeutic agents inhibiting BAFF/APRIL may be promising for myeloma treatment. We thus investigated the effect of the TACI-Fc fusion protein, able to block BAFF and APRIL, on primary myeloma cell survival and on drug sensitization. Primary myeloma cells were cultured with their bone marrow environment and recombinant IL-6. Detailed results obtained with 6 patients are shown in Table 1. TACI-Fc significantly reduced the median number of viable myeloma cells by 48% (P = .028; n = 6). TACI-Fc also potentiated the inhibitory effect of DEX or B-E8 anti–IL-6 MoAb (respectively, P = .028, P = .046; n = 6). When the 3 inhibitors were used together, a 90% reduction of viable myeloma cells was observed within 4 days of culture (Figure 6A). Of interest, the nonmyeloma cells present in the culture wells were unaffected by these 3 inhibitors (results not shown).
TACI-Fc induces apoptosis of primary myeloma cells. Mononuclear cells from tumor samples of 6 patients with MM were cultured for 4 days in the presence of IL-6 (1 ng/mL) with or without DEX (10-6 M), TACI-Fc (10 ng/mL), or B-E8 (10 ng/mL). At day 4 of culture, the cell count and viability were determined and the percentage of CD138+ viable plasma cells was determined by flow cytometry. The power of an inhibitor is given as the percentage of reduction of the median value of viable myeloma cell count with the inhibitor compared with the median value of viable myeloma cell count without inhibitor.
TACI-Fc induces apoptosis of primary myeloma cells. Mononuclear cells from tumor samples of 6 patients with MM were cultured for 4 days in the presence of IL-6 (1 ng/mL) with or without DEX (10-6 M), TACI-Fc (10 ng/mL), or B-E8 (10 ng/mL). At day 4 of culture, the cell count and viability were determined and the percentage of CD138+ viable plasma cells was determined by flow cytometry. The power of an inhibitor is given as the percentage of reduction of the median value of viable myeloma cell count with the inhibitor compared with the median value of viable myeloma cell count without inhibitor.
Signal transduction and antiapoptotic protein regulation by BAFF or APRIL
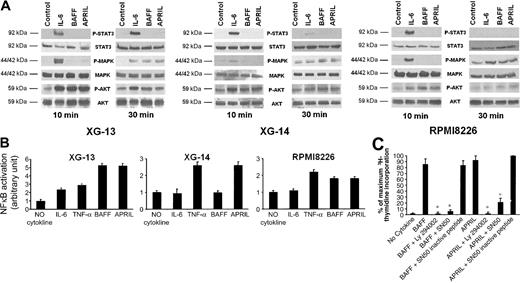
As shown in Figure 7A, BAFF and APRIL induced a rapid phosphorylation of AKT and a late phosphorylation of MAPK in 3 myeloma cell lines (XG-13, XG-14, and RPMI8226), whereas no phosphorylation of STAT3 was detected. IL-6 induced the phosphorylation of STAT3, MAPK, and AKT, in agreement with previous data.36,37
Signal transduction induced by BAFF or APRIL in myeloma cells. (A) XG-13, XG-14, and RPMI8226 cells were starved overnight and cultured without cytokine, or with either IL-6 (30 ng/mL), BAFF (800 ng/mL), or APRIL (800 ng/mL) for 10 and 30 minutes at 37°C. Cell lysates were analyzed by Western blotting with antisera against phospho-STAT3 (pSTAT3), phospho-ERK1/2 (pMAPK), and phospho-AKT (pAKT). Immunoblotting for STAT3, MAPK, and AKT confirmed equal protein loading. Western blots are of one representative experiment of 3. (B) XG-13, XG-14, and RPMI8226 cells were starved overnight and cultured without cytokine, or with either IL-6 (3 ng/mL), TNF-α (20 ng/mL), BAFF (200 ng/mL), or APRIL (200 ng/mL) for 30 minutes at 37°C. NF-κB activity was detected by ELISA according to the manufacturer's instructions. (C) XG-13 cells were IL-6 starved for 3 hours and cultured without cytokine or in the presence of BAFF (200 ng/mL) or APRIL (200 ng/mL). When indicated, Ly 294002 (25 μM), SN50 (100 μg/mL), or the SN50 inactive peptide (100 μg/mL) was added. Results are the mean values ± SD of the tritiated thymidine incorporation determined on sextuplet culture wells and are expressed as the percentage of the proliferation obtained with APRIL and SN50 inactive peptide. *Mean value is statistically significantly different from that obtained with BAFF or APRIL using a Student t test (P ≤ .05).
Signal transduction induced by BAFF or APRIL in myeloma cells. (A) XG-13, XG-14, and RPMI8226 cells were starved overnight and cultured without cytokine, or with either IL-6 (30 ng/mL), BAFF (800 ng/mL), or APRIL (800 ng/mL) for 10 and 30 minutes at 37°C. Cell lysates were analyzed by Western blotting with antisera against phospho-STAT3 (pSTAT3), phospho-ERK1/2 (pMAPK), and phospho-AKT (pAKT). Immunoblotting for STAT3, MAPK, and AKT confirmed equal protein loading. Western blots are of one representative experiment of 3. (B) XG-13, XG-14, and RPMI8226 cells were starved overnight and cultured without cytokine, or with either IL-6 (3 ng/mL), TNF-α (20 ng/mL), BAFF (200 ng/mL), or APRIL (200 ng/mL) for 30 minutes at 37°C. NF-κB activity was detected by ELISA according to the manufacturer's instructions. (C) XG-13 cells were IL-6 starved for 3 hours and cultured without cytokine or in the presence of BAFF (200 ng/mL) or APRIL (200 ng/mL). When indicated, Ly 294002 (25 μM), SN50 (100 μg/mL), or the SN50 inactive peptide (100 μg/mL) was added. Results are the mean values ± SD of the tritiated thymidine incorporation determined on sextuplet culture wells and are expressed as the percentage of the proliferation obtained with APRIL and SN50 inactive peptide. *Mean value is statistically significantly different from that obtained with BAFF or APRIL using a Student t test (P ≤ .05).
We also looked for NF-κB signaling, as there is accumulating evidence that BAFF and APRIL activate NF-κB transcription factors in B cells.33,38-40 We found that BAFF and APRIL, like TNF-α, enhanced NF-κB binding activity in XG-13 and RPMI8226 cells, whereas IL-6 induced a weak and transient activation of NF-κB, in agreement with previous studies41 (Figure 7B). For XG-14 cells that biologically responded only to APRIL, we found that APRIL, unlike BAFF, enhanced NF-κB binding activity.
Interestingly, an inhibitor of PI3K/AKT (Ly 294002) abrogated the proliferation of XG-13 cells induced by BAFF or APRIL. A peptide inhibitor of the NF-κB pathway (SN50) also inhibited BAFF- or APRIL-induced myeloma cell proliferation, unlike the corresponding inactive peptide (Figure 7C).
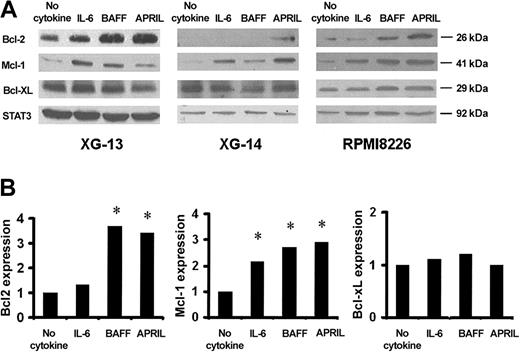
We then studied the regulation by BAFF or APRIL of 3 Bcl-2 family antiapoptotic members (Mcl-1, Bcl-2, and Bcl-xL) known to be involved in growth factor–mediated myeloma cell survival or in BAFF-mediated survival in B cells.42-47 BAFF and APRIL induced an up-regulation of Mcl-1 and Bcl-2 in XG-13 and RPMI8226 cells, whereas only APRIL increased Mcl-1 and Bcl-2 levels in XG-14 cells. In contrast, no change was noted in Bcl-xL protein expression (Figure 8A-B). IL-6 increased Mcl-1 but neither Bcl-2 nor Bcl-xL levels according to our previous studies.45
Regulation of Bcl-2 family antiapoptotic proteins by BAFF and APRIL. (A) XG-13, XG-14, and RPMI8226 cells were starved overnight before culture with no cytokine, or with IL-6 (30 ng/mL), BAFF (800 ng/mL), or APRIL (800 ng/mL) for 6 hours in RPMI–1% BSA. At the end of the culture, cells were immediately lysed and assayed for Bcl-2 family antiapoptotic protein expression using Western blot analysis. In this experiment, STAT3 expression was used as loading protein control. (B) Blots of 3 independent experiments were scanned and the values were normalized using STAT3-band intensities as internal standards. Results are the median values for the 3 main Bcl-2 family members expressed in the XG-13 HMCLs starved of IL-6 and cultured for 6 hours with no cytokine, or with IL-6 (30 ng/mL), BAFF (800 ng/mL), or APRIL (800 ng/mL). *Significant increase in expression with a Student t test for pairs (P < .05).
Regulation of Bcl-2 family antiapoptotic proteins by BAFF and APRIL. (A) XG-13, XG-14, and RPMI8226 cells were starved overnight before culture with no cytokine, or with IL-6 (30 ng/mL), BAFF (800 ng/mL), or APRIL (800 ng/mL) for 6 hours in RPMI–1% BSA. At the end of the culture, cells were immediately lysed and assayed for Bcl-2 family antiapoptotic protein expression using Western blot analysis. In this experiment, STAT3 expression was used as loading protein control. (B) Blots of 3 independent experiments were scanned and the values were normalized using STAT3-band intensities as internal standards. Results are the median values for the 3 main Bcl-2 family members expressed in the XG-13 HMCLs starved of IL-6 and cultured for 6 hours with no cytokine, or with IL-6 (30 ng/mL), BAFF (800 ng/mL), or APRIL (800 ng/mL). *Significant increase in expression with a Student t test for pairs (P < .05).
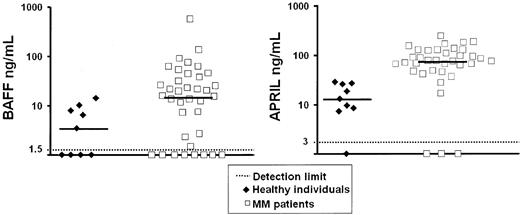
Levels of circulating BAFF and APRIL in sera of patients with MM
To further assess the biologic relevance of our data, we looked for levels of soluble BAFF and APRIL in the sera of 36 patients with MM and 9 age-related healthy individuals. Results shown in Figure 9 demonstrated that BAFF or APRIL median serum levels were increased, respectively, 4.2-fold (P = .02) and 5.9-fold in patients (P = 6.10-9) compared with healthy individuals.
Serum level of circulating BAFF and APRIL in myeloma patients. Serum levels of BAFF and APRIL were determined by ELISA in the sera from 36 patients with myeloma and 9 age-related healthy individuals.
Serum level of circulating BAFF and APRIL in myeloma patients. Serum levels of BAFF and APRIL were determined by ELISA in the sera from 36 patients with myeloma and 9 age-related healthy individuals.
Discussion
Accumulating experimental evidence supports the notion that BAFF is essential for the survival of normal immature and mature B cells15 as well as normal plasmablastic cells.34 BAFF plays a key role in the survival of B-CLL tumor cells.28,29 In addition, APRIL stimulates the growth of some human and murine tumor cell lines in vitro and in vivo.17 As BAFF/APRIL receptor genes are overexpressed in malignant plasma cells,10,11 our aim was to look for a role played by BAFF and APRIL in MM.
We demonstrate here that BAFF and APRIL are growth factors for 2 myeloma cell lines that highly expressed TACI and BAFF-R and whose survival is completely dependent on addition of exogenous growth factors. APRIL is also a growth factor for a third cell line, XG-14, which expressed only TACI, unlike BAFF-R. We also show that an autocrine loop involving BAFF, APRIL, and their receptors is involved in the autonomous growth of 2 well-known HMCLs, L363 and RPMI8226. BAFF and APRIL contribute to the survival of primary myeloma cells cultured together with their bone marrow environment and these 2 factors prevent DEX-induced apoptosis in primary myeloma cells. Since the initial submission of this manuscript, another paper also indicated that BAFF and APRIL are myeloma cell growth factors.48 Altogether, the current data and this paper extend previous reports indicating that BAFF/APRIL are involved in various B-cell neoplasias, in particular B-CLL and follicular lymphoma,28-30 and in autoimmune diseases such as Sjögren syndrome and systemic lupus erythematosus.15
An interesting question is the nature of the receptors involved in the BAFF and APRIL activity on myeloma cells. BAFF and APRIL bind, respectively, 3 (BAFF-R, TACI, and BCMA) and 2 (TACI and BCMA) distinct receptors. In addition, another receptor for APRIL probably exists, as reported for adenocarcinoma cells.49 We show here that, although BCMA was expressed by 5 of 5 normal plasmablasts, 13 of 13 HMCLs, and 11 of 11 primary myeloma cells, TACI and BAFF-R expression was heterogeneous. In some HMCLs expressing only BCMA (XG-5 and XG-6 HMCLs, for example), BCMA RNA was not associated with a functional membrane receptor since these HMCLs were unable to bind BAFF–murine CD8 fusion protein. This is in agreement with the described retention of BCMA in the Golgi complex in HMCLs50 and with the lack of B-cell deficiency in BCMA-/- mice.51 On the contrary, HMCLs that highly expressed TACI or BAFF-R bound BAFF–murine CD8 complex, confirming the presence of functional membrane receptors for BAFF. BAFF- and BAFF-R–deficient mice present a similar loss of follicular and marginal zone B cells in secondary lymphoid organs, suggesting that BAFF-R is the predominant stimulatory receptor for BAFF.52-55 The expression of TACI and BAFF-R is tightly regulated during the B-cell maturation process, and one can hypothesize that these 2 molecules could have different biologic activities depending on the cell type. Interestingly, XG-14 cells expressing a low level of TACI and no BAFF-R were sensitive to APRIL only. One hypothesis is that XG-14 expresses a receptor specific for APRIL that has not yet been identified, as reported for adenocarcinoma cells.49 Novak et al reported that all myeloma cells they tested bound soluble BAFF.48 In that study, BAFF-R was not detectable on the cell surface of HMCLs using an anti–BAFF-R antibody, whereas purified primary myeloma cells expressed BAFF-R. Thus, these data and our results indicate that we cannot yet draw firm conclusions on the respective role of TACI and BAFF-R in myeloma cells that express the 2 receptor genes, that is, the majority of purified primary myeloma cells.
In order to better understand the effect of BAFF and APRIL on myeloma cells, we examined intracellular signaling pathways. BAFF was reported to induce NF-κB activation in B cells23 and an overexpression of BCMA in human 293 cells activates the Rel/NF-κB, JNK, Elk-1, and p38 kinase transcription factors.56,57 Activation of TACI in Jurkat T cells also results in activation of AP-1, NF-κB, and nuclear factor of activated T cells (NF-AT).58 In myeloma cells, we and others have shown that IL-6 activates 3 essential pathways: the JAK/STAT, MAPK, and PI3K/Akt cascades, whereas IGF-1 activates MAPK and PI3K/Akt.36,41,59-62 IGF-1 also activates NF-κB.41 We show here that neither BAFF nor APRIL was able to induce STAT3 phosphorylation but did activate ERK1/2 and PI3K/Akt pathways. An inhibitor of PI3K/AKT abrogated the growth of myeloma cells induced by BAFF and APRIL. Of interest, BAFF and APRIL were also able to efficiently activate NF-κB, as was TNF-α. Finally, we found that NF-κB activation was critical for the myeloma cell proliferation activity of BAFF or APRIL since it was dramatically blocked by an NF-κB peptide inhibitor.
BAFF and APRIL induced an up-regulation of Bcl-2 and Mcl-1 antiapoptotic proteins in myeloma cells, whereas the level of Bcl-xL remained unchanged. In murine B cells, the survival effect of BAFF was associated with induction of A1, Bcl-2, and Bcl-xL.23,26,27 This is in agreement with the activation of NF-κB, which enhances the transcription of a number of antiapoptotic genes, including A1, Bcl-2, Bcl-xL, cIAP2, and cFLIP. Previously, we demonstrated that among 10 antiapoptotic and proapoptotic proteins, including A1, Bcl-xl, and Bcl-2, only Mcl-1 was regulated by IL-6 or IFN-α in myeloma cells.45 Furthermore, oligonucleotide antisenses to Mcl-1, but not to Bcl-2 or Bcl-xL, were able to induce apoptosis of myeloma cell lines,47 and constitutive Mcl-1 expression strongly reduced apoptosis induced by IL-6 withdrawal.46 Collectively, these data suggest that Mcl-1 is the major antiapoptotic protein involved in IL-6–mediated survival in myeloma. An overexpression of Bcl-2 in myeloma cell lines could also confer protection from apoptosis induced by dexamethasone,63 etoposide, or doxorubicin64 and may therefore contribute to tumor cell survival and a multidrug-resistant phenotype. Thus, BAFF and APRIL up-regulate in myeloma cells the expression of the 2 major antiapoptotic proteins, Mcl-1 and Bcl-2, known to be involved in the rescue from apoptosis induced by growth-factor removal or DEX treatment.
The data mentioned previously in this paper that were obtained with myeloma cell lines could be extended to primary myeloma cells that expressed BAFF-R and/or TACI. As primary myeloma cells rapidly apoptosed as soon as they were purified,65 they were cultured together with their bone marrow environment. The TACI-Fc fusion protein, able to block both BAFF and APRIL, reduced the survival of primary myeloma cells, and increased inhibition was obtained when TACI-Fc was used together with DEX or anti–IL-6 MoAb. Interestingly, when the 3 inhibitors were combined, virtually all primary myeloma cells died, whereas the cocultured nonmyeloma cells were unaffected. These in vitro data suggest that inhibitors of BAFF and TACI could be very useful to induce apoptosis of myeloma cells when used alone or in combination with DEX and/or anti–IL-6 MoAb. The advantage of using BAFF/APRIL inhibitors in MM is emphasized by the current finding that serum levels of BAFF and APRIL were increased roughly 5-fold in patients with MM as compared with age-related healthy individuals. These serum concentrations were in the range of those able to promote myeloma cell growth in vitro. The presence of circulating APRIL has not been reported in humans. The circulating serum levels of BAFF reported here were close to those found in autoimmune diseases, where it was correlated with the autoantibody level.66,67 Further studies are necessary to determine whether BAFF or APRIL serum levels are prognostic factors in patients with multiple myeloma.
New therapeutic agents have now been developed to inhibit BAFF/APRIL in B-cell neoplasia and autoimmune diseases, such as anti-BAFF MoAb and the TACI-Fc, or the BAFF/APRIL signaling pathway, such as the PS-1145 IκB kinase inhibitor.55,68-70 Thus, the present report suggests that these novel inhibitors may be promising elements in the treatment of patients with MM, possibly in association with DEX and/or anti–IL-6 MoAb.
Prepublished online as Blood First Edition Paper, December 4, 2003; DOI 10.1182/blood-2003-06-1984.
Supported by grants from the Ligue Nationale Contre le Cancer (équipe labellisée), Paris, France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.