Abstract
We recently identified the chimeric kinase FIP1L1-platelet-derived growth factor receptor α (PDGFRα) as a cause of the hypereosinophilic syndrome and of chronic eosinophilic leukemia. To investigate the role of FIP1L1-PDGFRA in the pathogenesis of acute leukemia, we screened 87 leukemia cell lines for the presence of FIP1L1-PDGFRA. One cell line, EOL-1, expressed the FIP1L1-PDGFRA fusion. Three structurally divergent kinase inhibitors—imatinib (STI-571), PKC412, and SU5614—inhibited the growth of EOL-1 cells. These results indicate that the fusion of FIP1L1 to PDGFRA occurs rarely in leukemia cell lines, but they identify EOL-1 as an in vitro model for the study of FIP1L1-PDGFRA-positive chronic eosinophilic leukemia and for the analysis of small molecule inhibitors of FIP1L1-PDGFRα. (Blood. 2004;103:2802-2805)
Introduction
The hypereosinophilic syndrome (HES) is a hematologic disease characterized by prolonged eosinophilia, exclusion of reactive eosinophilia, and organ damage.1,2 HES is reclassified as chronic eosinophilic leukemia (CEL) when clonality is demonstrated.3 We recently identified the kinase FIP1L1-platelet-derived growth factor receptor α (PDGFRα) as the cause and the therapeutic target of imatinib in 56% of HES cases.4 These results demonstrate that most HES cases are clonal in origin and could be reclassified as FIP1L1-PDGFRA-positive CEL. The FIP1L1-PDGFRA fusion gene is created by an interstitial chromosomal deletion on chromosome 4q12 that is not apparent using standard karyotypic analysis.4 Expression of the FIP1L1-PDGFRA fusion is under control of the ubiquitous FIP1L1 promoter, suggesting the possibility that FIP1L1-PDGFRα may be involved in the pathogenesis of other hematologic malignancies. To get insight into this, we screened 87 leukemia cell lines for the presence of the FIP1L1-PDGFRA fusion gene. Leukemia cell lines have been proven to be a valuable resource for the study of hematologic malignancies,5 and our results now identify the EOL-1 cell line as an in vitro model for the study of FIP1L1-PDGFRA-positive CEL.
Study design
PCR and RT-PCR
FIP1L1-PDGFRA fusion was amplified from DNA with primers FIP1L1-F9 (5′-tggggcaattgatgttatcg) and PDGFRA-RI12 (5′-gtgcaagggaaaagggagtct). RNA was isolated from cell lines from the DSMZ collection (http://www.dsmz.de), as described.6 Reverse transcription-polymerase chain reaction (RT-PCR) was performed with primers FIP1L1-F7 (5′-acctggtgctgatctttctgat) and PDGFRA-R14 (5′-tgagagcttgtttttcactgga) for the detection of FIP1L1-PDGFRA and primers PDGFRA-F11 (5′-ggtgctgttggtgattgtga) and FIP1L1-R10 (5′-cagctcctggagggaaaaac) for the detection of PDGFRA-FIP1L1. Primers PDGFRA-F11 and PDGFRA-R14 were used to detect PDGFRA expression, and primers FIP1L1-F7 and FIP1L1-R10 were used to detect FIP1L1 expression.
Cell culture and dose-response curves
The EOL-1 cell line (DSMZ ACC386) was grown in RPMI 1640 medium with 10% fetal bovine serum. Imatinib and PKC412 were kindly provided by Novartis; SU5614 was purchased from Calbiochem (San Diego, CA). Kinase inhibitors were stored in water (imatinib) or dimethyl sulfoxide (DMSO) (PKC412, SU5614) and diluted in RPMI 1640 medium. For dose-response curves, EOL-1 cultures were initiated at 3 × 105 cells/mL, and viable cell numbers were determined at the beginning and after 48 hours using the Celltiter AQueousOne solution (Promega, Madison, WI). Dose-response curves were fitted using Origin (OriginLab, Northampton, MA).
Detection of apoptosis
Apoptotic cells were detected by flow cytometric analysis using a FACSCalibur Cytometer (Becton Dickinson, Mountain View, CA) after staining with annexin V-fluorescein and propidium iodide (Roche, Indianapolis, IN).
Western blotting and immunoprecipitation
EOL-1 cells were treated with kinase inhibitors for 3 hours and then lysed in lysis buffer (Cell Signaling Technology, Beverly, MA) for immunoprecipitation or in sodium dodecyl sulfate (SDS) sample buffer (Cell Signaling Technology) for whole-cell lysates. Immunoprecipitation was performed using anti-PDGFRα (C-20) antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and Protein A agarose (Roche). Antibodies used were anti-phospho-signal transducer and activator of transduction 5 (STAT5) (Cell Signaling), anti-PDGFRα (951), anti-STAT5a (Santa Cruz Biotechnology), antiphosphotyrosine (4G10) (Upstate Biotechnology, Lake Placid, NY), antimouse-peroxidase (PO), and antirabbit-PO (AP Biotech, Piscataway, NJ).
Results and discussion
We performed RT-PCR on RNA obtained from 67 acute myeloid leukemia (AML) and 20 acute lymphoid leukemia (ALL) cell lines, with a primer combination that would detect all known FIP1L1-PDGFRA fusion variants.4,7 In 1 AML cell line, EOL-1 (and derivative EOL-3), a fusion between FIP1L1 and PDGFRA was detected (Figure 1). No other cell lines harbored this fusion gene (data not shown).
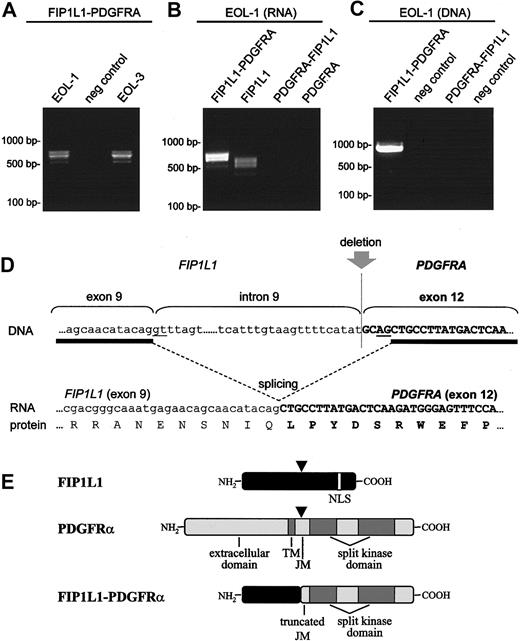
Fusion of FIP1L1 to PDGFRA in the EOL-1 cell line. Detection of the FIP1L1-PDGFRA fusion transcript in the EOL-1 and EOL-3 cell lines (A), detection of FIP1L1 expression in the EOL-1 cell line (B), and amplification of the FIP1L1-PDGFRA fusion gene on DNA from the EOL-1 cell line (C). Different transcripts are observed for FIP1L1 and FIP1L1-PDGFRA (A-B) because of alternative splicing. Expressions of PDGFRA-FIP1L1 and native PDGFRA were not detected (B). The sequence of the fusion gene surrounding the deletion is shown at the DNA level, at the RNA level (after splicing), and at the protein level (D). Splice donor and acceptor sites are underlined. A cryptic splice site is used in exon 12 of PDGFRA. Schematic representation of FIP1L1, PDGFRα, and FIP1L1-PDGFRα proteins (E). Points at which the proteins are interrupted by the deletion are indicated by arrowheads.
Fusion of FIP1L1 to PDGFRA in the EOL-1 cell line. Detection of the FIP1L1-PDGFRA fusion transcript in the EOL-1 and EOL-3 cell lines (A), detection of FIP1L1 expression in the EOL-1 cell line (B), and amplification of the FIP1L1-PDGFRA fusion gene on DNA from the EOL-1 cell line (C). Different transcripts are observed for FIP1L1 and FIP1L1-PDGFRA (A-B) because of alternative splicing. Expressions of PDGFRA-FIP1L1 and native PDGFRA were not detected (B). The sequence of the fusion gene surrounding the deletion is shown at the DNA level, at the RNA level (after splicing), and at the protein level (D). Splice donor and acceptor sites are underlined. A cryptic splice site is used in exon 12 of PDGFRA. Schematic representation of FIP1L1, PDGFRα, and FIP1L1-PDGFRα proteins (E). Points at which the proteins are interrupted by the deletion are indicated by arrowheads.
In consonance with our previous observations in patients with FIP1L1-PDGFRA-positive CEL,4,7 the EOL-1 cell line expressed in-frame FIP1L1-PDGFRA fusion transcripts, with the fusion of exon 9 of FIP1L1 to a truncated exon 12 of PDGFRA. Because of splice variation within FIP1L1, different fusion transcripts were observed in the EOL-1 cells (exon 8a can be present or absent between exon 8 and exon 9, but in both variants an open-reading frame is present) (Figure 1). Cloning of the fusion gene at the DNA level confirmed that the breakpoint in FIP1L1 was located in intron 9 and that the breakpoint in PDGFRA was located in exon 12. No reciprocal PDGFRA-FIP1L1 fusion gene could be detected (Figure 1). Taken together, these data indicate that FIP1L1-PDGFRA fusion in the EOL-1 cell line is the consequence of the del(4)(q12q12) interstitial chromosomal deletion, as observed in FIP1L1-PDGFRA-positive CEL patients.4 The EOL-1 cell line did not express wild-type PDGFRA but did express wild-type FIP1L1 (Figure 1).
EOL-1 was originally derived from the blasts of a 33-year-old man with CEL, when disease progressed to AML (54% blasts; karyotype, 48,XY,+6,+8,9q-).8 We recently discovered that this cell line harbors a partial tandem duplication within the MLL gene.9 Translocations involving MLL and partial tandem duplication within the MLL gene were described as leukemogenic mutations involved in the pathogenesis of AML.10,11 Mouse models have demonstrated that MLL fusion proteins are necessary but insufficient for leukemogenesis.10 Based on these and other observations, it has been proposed that AML cells harbor at least 2 mutations, 1 that confers a proliferative or survival advantage, or both, and 1 that results in impaired differentiation of hematopoietic progenitors.12 Taken together, these findings suggest that FIP1L1-PDGFRα and mutated MLL may cooperate to cause the progression of CEL to AML.
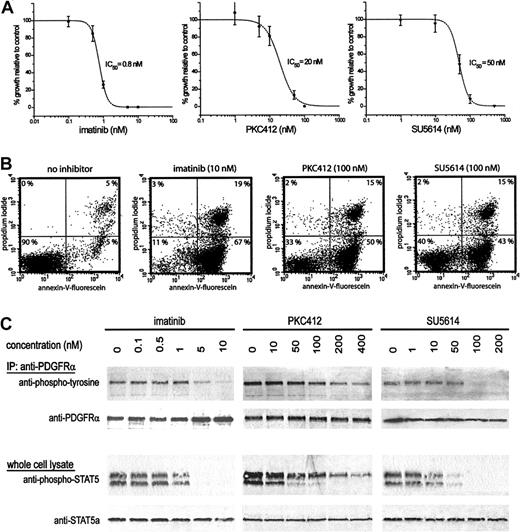
We next tested for dose-dependent inhibition of the growth of EOL-1 cells by the kinase inhibitors imatinib13 and PKC412,14 both known to inhibit FIP1L1-PDGFRα,4,15 and SU5614,16 known to inhibit FMS-like tyrosine kinase 3 (FLT3).17,18 The growth of EOL-1 cells was inhibited by these drugs, with cellular IC50 of approximately 0.8 nM for imatinib, 20 nM for PKC412, and 50 nM for SU5614 (Figure 2A). Twenty-four hours after drug treatment, most EOL-1 cells were apoptotic (Figure 2B), indicating that growth inhibition by these 3 drugs was caused by apoptosis. FIP1L1-PDGFRα has recently been identified as the major phosphorylated protein in EOL-1 cells.19 Our results suggest that all 3 inhibitors directly affect FIP1L1-PDGFRα activity, as indicted by the dose-dependent decrease of phosphorylation of FIP1L1-PDGFRα and STAT5, a downstream effector of FIP1L1-PDGFRα-mediated signal transduction. A 50% reduction of phosphorylation of FIP1L1-PDGFRα was reached at approximately 5 nM imatinib, 100 nM PKC412, and 50 nM SU5614 (Figure 2C). Because these 3 inhibitors also inhibit KIT and because PKC412 and SU5614 also inhibit FLT3, we investigated whether the inhibition of FLT3 or KIT could be involved in the growth inhibition of EOL-1 cells. However, we did not find evidence for the phosphorylation of FLT3 or KIT. Although we cannot exclude that the inhibition of other native tyrosine kinases may contribute to the inhibition of cell growth, our results suggest that growth inhibition and induction of apoptosis are primarily the result of direct FIP1L1-PDGFRα inhibition.
Inhibition of cell growth, induction of apoptosis, and inhibition of FIP1L1-PDGFRα kinase activity in EOL-1 cells treated with various kinase inhibitors. (A) Dose-response curves of EOL-1 cells treated with imatinib, PKC412, or SU5614. The percentage of growth relative to untreated cells is plotted for increasing drug concentrations (48-hour incubation). (B) Detection of apoptotic EOL-1 cells after 24-hour incubation with imatinib (10 nM), PKC412 (100 nM), or SU5614 (100 nM). Apoptotic cells (lower right quadrant), necrotic cells (upper right quadrant), and viable cells (lower left quadrant) were detected. (C) Phosphorylation of FIP1L1-PDGFRα was analyzed using antiphosphotyrosine antibody on immunoprecipitated FIP1L1-PDGFRα. Phosphorylation of STAT5 was analyzed using anti-phospho-STAT5 antibody, which recognizes phosphorylated STAT5a (upper band) and STAT5b (lower band). Membranes were blotted with anti-PDGFRα or anti-STAT5a antibodies as loading control.
Inhibition of cell growth, induction of apoptosis, and inhibition of FIP1L1-PDGFRα kinase activity in EOL-1 cells treated with various kinase inhibitors. (A) Dose-response curves of EOL-1 cells treated with imatinib, PKC412, or SU5614. The percentage of growth relative to untreated cells is plotted for increasing drug concentrations (48-hour incubation). (B) Detection of apoptotic EOL-1 cells after 24-hour incubation with imatinib (10 nM), PKC412 (100 nM), or SU5614 (100 nM). Apoptotic cells (lower right quadrant), necrotic cells (upper right quadrant), and viable cells (lower left quadrant) were detected. (C) Phosphorylation of FIP1L1-PDGFRα was analyzed using antiphosphotyrosine antibody on immunoprecipitated FIP1L1-PDGFRα. Phosphorylation of STAT5 was analyzed using anti-phospho-STAT5 antibody, which recognizes phosphorylated STAT5a (upper band) and STAT5b (lower band). Membranes were blotted with anti-PDGFRα or anti-STAT5a antibodies as loading control.
In conclusion, although CEL has been observed to progress to AML,4,8,20 our results suggest that FIP1L1-PDGFRα is not frequently involved in the pathogenesis of AML. We identified EOL-1 as the first cell line expressing the FIP1L1-PDGFRA fusion gene and as a valuable in vitro model for the screening for new FIP1L1-PDGFRα inhibitors in the context of a human cell line expressing the fusion protein from its endogenous promoter. EOL-1 cells may provide a unique reagent for understanding lineage involvement in FIP1L1-PDGFRA-positive CEL, FIP1L1-PDGFRα-mediated signaling, and the transcriptional targets of FIP1L1-PDGFRα that contribute to the CEL phenotype.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-07-2479.
Supported by National Institutes of Health grant DK50654 and CA66996 and the Leukemia and Lymphoma Society (D.G.G), and by research funding from Novartis Pharma AG (J.D.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
J.C. is a “postdoctoraal onderzoeker” of the “Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.” B.J.P.H. is a senior clinical fellow of the Leukaemia Research Fund (United Kingdom). D.G.G. is an associate investigator of the Howard Hughes Medical Institute.