Abstract
From January 1978 to December 2001, 133 patients with severe aplastic anemia (SAA) underwent non-T cell-depleted allogeneic bone marrow transplantation from an HLA-identical sibling donor, at the Hospital Saint Louis using either the combination of cyclophosphamide (Cy) and thoracoabdominal irradiation (TAI; n = 100) or Cy and antithymocyte globulin (ATG; n = 33), as a conditioning regimen. With 13.6 years of follow-up, the 10-year survival estimate was 64%. Four factors were associated with lower survival: older age, use of Cy-TAI, any form of treatment prior to transplantation (either androgens or immunosuppressive therapy, [IST]), and grade II to IV acute graft-versus-host disease (GvHD). TAI was the sole factor associated with the occurrence of acute GvHD. The risk of cancers (15-year cumulative incidence, 10.9%) was associated with older age and with the use of cyclosporine as IST before transplantation. Cumulative incidences and risk factors of nonmalignant late effect including avascular osteonecrosis and late bacterial, viral, and fungal infection were also analyzed. Improved results using Cy-ATG as conditioning can lead to more than 90% chance of cure in patients with SAA. Even if, in our experience, the role of Cy-ATG versus that of Cy-TAI remained inextricably related to the year of transplantation, the major detrimental role of the GvHD disease in the long-term outcome and its relation to TAI supports avoidance of irradiation in the conditioning regimen. Furthermore, avoidance of any IST before transplantation in patients with a sibling donor is a prerequisite for attaining such excellent results.
Introduction
Allogeneic bone marrow transplantation (BMT) from an HLA-identical sibling is a curative form of therapy for patients with acquired severe aplastic anemia (SAA). Survival has significantly improved over the past 3 decades, mainly with the introduction of cyclosporine A (CsA) in the early 1980s, which resulted in reduced graft rejection and deaths due to infections1-5 ; the actuarial risk of rejection has been reduced from 24% in the 1970s to the current 7%. Although irradiation before transplantation also reduces the incidence of graft rejection, it is associated with early and late sequelae such as chronic graft-versus-host disease (GvHD)3 and secondary tumors.6-8 Improved results with survival in excess of 90% has been reported in sensitized patients conditioned with the association of cyclophosphamide (Cy) and antithymocyte globulin (ATG) by the Seattle group and by other investigators.9-11 Even today, nearly a third of BMTs currently performed for SAA are done with irradiation-based conditioning both in Europe and in the United States (European Group for Blood and Marrow Transplantation and International Bone Marrow Transplant Registry [IBMTR], personal communication, July 2003).
Immunosuppressive therapy (IST) based on ATG and CsA has been given with increasing success to patients without an HLA-identical sibling, the results being correlated with the severity of neutropenia at the time of treatment.5,12-14 The combined use of ATG and CsA together with hematopoietic growth factors has produced promising results also in patients with very low neutrophil counts.15-17 Thus it turned out that some authors advocate the use of IST upfront, even in patients with severe disease and with a sibling donor, and use BMT only in case of IST failure or relapse.18
Factors previously related to lower survival after BMT include older age, interval between diagnosis and transplantation, number of transfusions, absence of CsA for GvHD prophylaxis.3-5 However, most studies were performed from retrospective analyses of registries or included few patients, and only one study reported detailed analysis on long-term outcome following BMT for SAA.19
In 1991, we reported our experience using the combination of Cy and thoracoabdominal irradiation (TAI) as conditioning regimen before BMT for SAA.20 In this previous analysis we showed that although leading to survival rate in the range of 70%, Cy-TAI was associated with an increased risk of development of secondary malignancies.6 We thus decided to definitively stop the use of irradiation-based conditioning for SAA. A few patients (n = 10) then received various busulfan-based conditioning regimens, but following the report by Storb et al in 1994 showing excellent survival and few rejections with the association of Cy and ATG,10 we uniformly used the Seattle conditioning regimen. The main aim of this study was to describe the long-term outcome in 133 patients who underwent BMT at the Hospital Saint Louis (Paris, France) after either Cy-TAI or Cy-ATG conditioning. With a 13.6-year follow-up, the main findings of the present study included decreased survival associated with the use of Cy-TAI and a detrimental effect in the long-term of any IST before transplantation for aplastic anemia.
Patients, materials, and methods
Aim
The study aim was to determine factors associated with long-term survival and those associated with late complications following BMT. For homogeneity purposes only, patients who received either Cy-TAI or Cy-ATG were included in this study. Reasons for this deliberate choice are discussed in the “Introduction.”
Patients
From January 1978 to December 2001, 143 patients (Table 1) underwent non-T cell-depleted allogeneic BMT from an HLA-identical sibling donor, at the Hospital Saint Louis. Ten patients receiving various busulfan-based conditionings during this period were excluded from this analysis. One hundred patients received the combination of Cy (150 mg/kg) and TAI (6 Gy). Thirty-three patients were given Cy-ATG as a conditioning regimen, as described by Storb and coworkers (4 daily doses of Cy, total dose 200 mg/kg, and 5 daily doses of ATG 2.5 mg/kg/d [Sangstat, Lyon, France]; with methylprednisolone being used during ATG [1 mg/kg/d]). Most patients had idiopathic aplastic anemia (n = 103, 77%). Known causes included drug-induced SAA (n = 2, 2%), SAA developing from hepatitis (n = 19, 14%), and paroxysmal nocturnal hemoglobinuria (PNH; as defined by a positive Ham test; n = 9, 7%). Nearly half the patients had received some form of treatment or IST before referral to our service. At diagnosis, 82 patients had severe disease and 26 had nonsevere aplastic anemia; disease severity at diagnosis could not be evaluated in 25 patients. At transplantation all patients reached the criteria for the severity of the disease. Graft-versus-host prophylaxis consisted of the combination of methotrexate (MTX) and CsA21,22 in 70 patients (53%). Transfusion policy, antibiotics, and antifungal prophylaxis and treatment, cytomegalovirus (CMV) preemptive therapy, and other management policies have been described previously.20,23,24
Statistical analysis
For the purpose of this analysis on long-term outcomes only data on patients with successful engraftment were considered. Four patients without engraftment were excluded for analysis (except for overall survival rate calculation). Two “nontakes” or rejections occurred after Cy-TAI and 2 after Cy-ATG. Survival rates were calculated using Kaplan-Meier estimates. Thus, data on 129 patients are the subject of this report. Main patient, disease, and transplant characteristics of these 129 patients are summarized in Table 2. To study the prognostic value of variables known at BMT on long-term survival, the proportional hazard model was used25 with backward elimination of nonprognostic factors based on the likelihood ratio test. In a first step, all variables were proposed to the model, except for disease severity at diagnosis and recipient CMV serologic status (due to missing value, 24 and 49, respectively). The following clinical parameters were tested: year of transplantation, recipient age, disease severity, infection within 3 months before transplantation, number of transfusions before transplantation, treatment before transplantation, and if yes, treatment type (ATG, CsA, or androgens), donor/recipient sex combination, ABO incompatibility between donor and recipient, and conditioning regimen. In a second step, it was tested if the initially excluded variables had an additive prognostic value to factors selected in the first step, provided these factors were still predictive of long-term survival in the subgroup of patients with known severity of the disease or recipient CMV serologic status. In a third step, the prognostic value of the selected variables was tested in parallel to acute or chronic GvHD and their severity by using the proportional hazard model with time-dependent covariates for GvHD occurrence. Finally, selected pretransplantation factors allowed us to define a prognostic index with decreasing probabilities of long-term survival. In multivariate analyses P < .05 was used for significance. The proportional hazard assumption was tested as proposed by Grambsch and Therneau.26
Cumulative incidences of late complications and acute and chronic GvHD were estimated in a competing risks setting, with death not related to late complications treated as the competing event.27,28 Risk factors for late complications and chronic GvHD were analyzed by proportional hazard model with time-dependent covariates. Covariates were the same as those analyzed for survival unless specified. In these multivariate analyses P values less than .05 were used for significance.
Results
Overall long-term survival
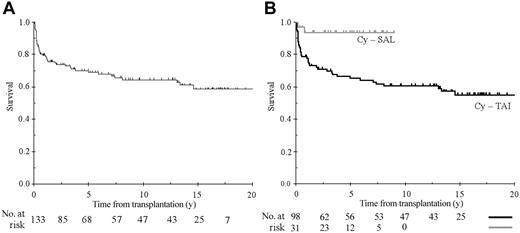
With a median follow-up of 13.6 ± 0.7 years the 5-, 10-, and 15-year survival estimates for the 133 patients who underwent BMT were 69.0% ± 4.0%, 64.5% ± 4.5%, and 58.7% ± 5.2%, respectively (Figure 1A). Analyses on long-term survival were then restricted to 129 patients with engraftment.
Overall survival. (A) Overall survival of 133 patients who underwent transplantation at the Hospital St Louis (median follow-up of 13.6 years). (B) Overall survival according to the conditioning regimen with cyclophosphamide plus either antithymocyte globulin (Cy-ATG) or thoracoabdominal irradiation (Cy-TAI).
Overall survival. (A) Overall survival of 133 patients who underwent transplantation at the Hospital St Louis (median follow-up of 13.6 years). (B) Overall survival according to the conditioning regimen with cyclophosphamide plus either antithymocyte globulin (Cy-ATG) or thoracoabdominal irradiation (Cy-TAI).
All long-term survivors had normal blood counts. After TAI we have already reported full donor engraftment.29 After ATG-Cy, all long-term survivors had more than 90% donor cell engraftment (as assessed by polymerase chain reaction analysis of mini- or micro-satellite sequences). Results of univariate analyses of factors affecting survival are included in Table 2. The role of conditioning regimen on survival merits particular attention (Figure 1B). Indeed, due to our change in treatment protocol, its role is inextricably related to the period of transplantation and, over the study period, patient, disease, and transplant characteristics may have changed. As expected, follow-up time was much longer after Cy-TAI (15.9 ± 0.7 years) than after Cy-ATG (4.4 ± 0.8 years). This variation in follow-up time has no influence on prognostic analysis because censoring data of patients who received Cy-TAI from 4 to 9 years did not change either the effect of the conditioning regimen or its magnitude (P ranging from .008 to .006; hazard ratio ranging from 5.5 to 5.8). As shown in Table 1, differences in patient, disease, and transplant characteristics between the 2 groups were limited to the use of CsA or androgens before transplantation, in the use of CsA plus MTX as GvHD prophylaxis, and in CMV monitoring/therapy. Among changes, introduction of CsA, search of CMV reactivation by pp65 antigenemia and preemptive therapy, and use of MTX plus CsA were probably the most important. However, none of these factors reached statistical significance in multivariate analysis. Indeed, when studying patients who received TAI, no effect of the regimen used for GvHD prophylaxis could be seen. Furthermore, when comparing Cy-TAI to Cy-ATG in those patients who received CsA plus MTX, the hazard ratio was 4.9 (95% CI, 1.1-22.2; P = .02) similar to what was observed globally, indicating that the role of Cy-ATG was not the consequence of changing practice in GvHD prophylaxis. Causes of death are summarized in Table 3. By multivariate analysis, including pretransplantation factors only, 3 factors remained independently associated with lower survival: patient age older than 15 years (relative risk [RR] = 2.4; 95% CI, 1.2-4.7; P = .011), use of Cy-TAI as conditioning regimen (RR = 6.2; 95% CI, 1.5-25.9; P < .001), and any form of treatment before transplantation (either androgens or IST; RR = 2.1; 95% CI, 1.1-3.9; P = .018). The proportional hazard assumption was not rejected (P = .720 for the global test; P = .280, .995, .664 for each of the 3 included factors, respectively). Previous treatment was not associated with calendar year of transplantation, use of CsA as GvHD prophylaxis, type of conditioning, age, or severity of the disease. However, it obviously leads to a longer interval from diagnosis to transplantation (95% of the patients with an interval more than a year have been pretreated as compared to 40% with the interval less than a year, P < .0001). Twenty-one patients had an interval before transplantation that exceeded a year, and 20 of them were treated before transplantation (16 of them with IST, including 14 with ATG). A significant association was found between treatment with ATG and time interval between diagnosis to transplantation more than a year (P = .004). Age was also associated with longer interval before transplantation (only 8% of patients younger than 15 years of age underwent transplantation after 1 year as compared to 5% in patients aged 15 to 20 years, 26% aged 20 to 30 years, and 29% in patients older than 30 years; P = .019). Finally, 33% of the patients with nonsevere disease underwent transplantation beyond a year as compared with 8% in patients with SAA (P < .001). When we restricted the analysis to patients receiving a transplant within a year after diagnosis (n = 107), the impact of prior treatment lost statistical significance (RR = 1.67, P = .16), but when testing a different hazard ratio of prior treatment in patients undergoing transplantation after 1 year and in those receiving a transplant before a year, statistical significance was not reached.
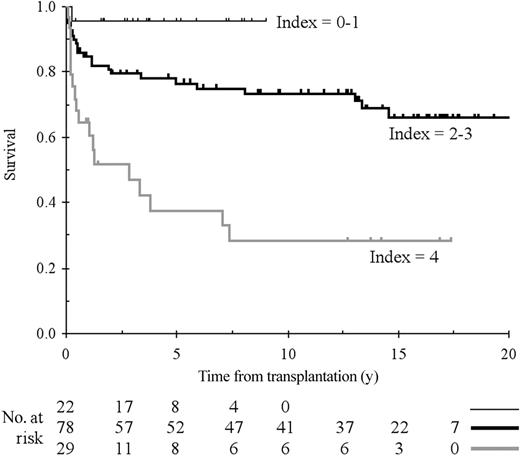
When adding GvHD as a time-dependent factor (acute and chronic and severe), the same 3 factors were found to affect survival and a fourth GvHD of grade II or more (RR = 3.6; 95% CI, 1.9-7.0; P < .0001). The RR associated with each factor was very stable, except for Cy-TAI for which the RR was 3.5 instead of 6.2 (P = .05). We then constructed a risk index using pretransplantation risk factors based on the results of the Cox model; this index affected a value of 1 if the patient was 15 years old or older, 1 if the patient was treated before transplantation, and 2 if the conditioning was Cy-TAI. Representative curves comparing patients with 0 to 1, 2 to 3, and 4 index values are presented in Figure 2. The proportional hazard assumption was still not rejected (P = .589 for the global test or P = .589 for the global test, P = .416 and .305 for each of the 2 categories relative to baseline category).
Chronic GvHD
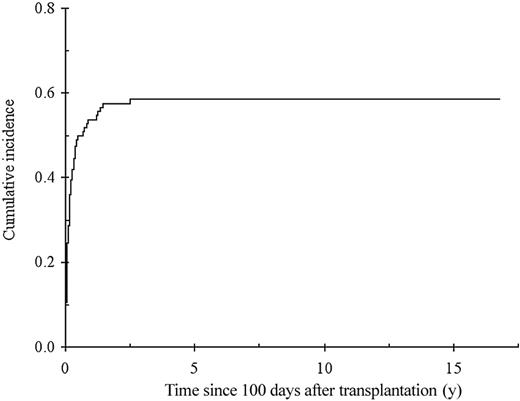
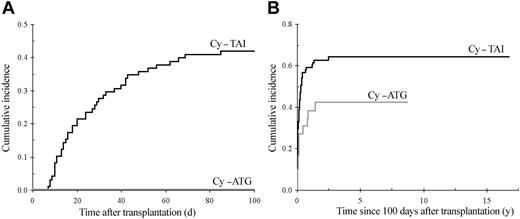
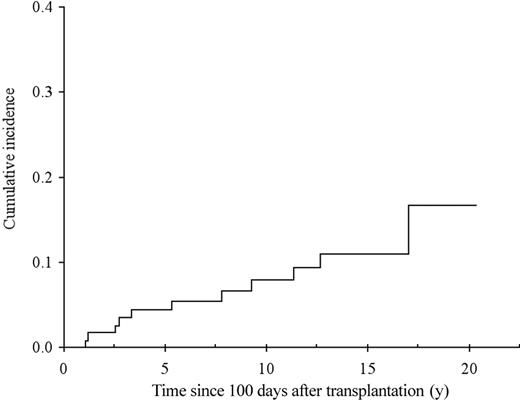
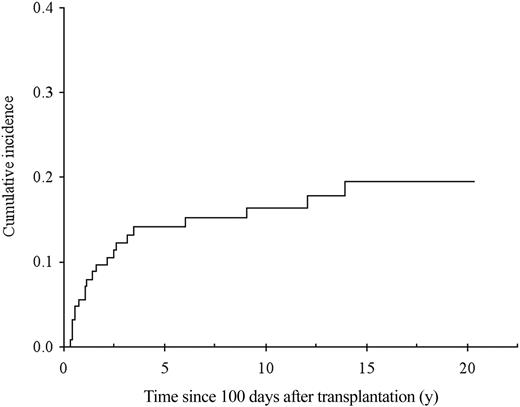
The cumulative incidence of chronic GvHD in 114 patients surviving beyond day 100, with engraftment is shown in Figure 3. Sixty-six patients developed chronic GvHD, which was extensive in 52 (79%). By multivariate analysis, previous acute GvHD of grade I (RR = 2.5; 95% CI, 1.4-4.7; P = .003), of grade II or higher (RR = 4.6; 95% CI, 2.5-8.7; P < .0001), and GvHD prophylaxis with MTX alone (RR = 2.9; 95% CI, 1.5-5.8; P = .007) were associated with an increased risk of developing chronic GvHD. Of note, all patients who received MTX alone as GvHD prophylaxis also received TAI. In addition, the use of TAI was associated with increased risk of chronic GvHD when pretransplantation factors only were included in the model (RR = 2.2; 95% CI, 1.2-4.0; P = 0.01), and TAI was the sole factor associated with the occurrence of acute GvHD (RR = 4.3; 95% CI, 2.1-8.9; P < .0001; Figure 4A). The impact of irradiation on the cumulative of chronic GvHD is illustrated in Figure 4B.
Cumulative incidence of chronic GvHD from day 100 after transplantation with death unrelated to chronic GvHD considered as a competing risk.
Cumulative incidence of chronic GvHD from day 100 after transplantation with death unrelated to chronic GvHD considered as a competing risk.
Cumulative incidence of GvHD. (A) Cumulative incidence of grade II or higher acute GvHD according to the conditioning regimen (death not related to acute GvHD considered as a competing risk), 100-day cumulative incidence Cy-TAI, 41.8% (SE, 5%); Cy-ATG, 0%; Gray test, P < .0001. (B) Cumulative incidence of chronic GvHD according to conditioning: Cy-TAI, 64.0% (SE, 5.3%); Cy-ATG, 42.2% (SE, 9.6%); Gray test, P = .025.
Cumulative incidence of GvHD. (A) Cumulative incidence of grade II or higher acute GvHD according to the conditioning regimen (death not related to acute GvHD considered as a competing risk), 100-day cumulative incidence Cy-TAI, 41.8% (SE, 5%); Cy-ATG, 0%; Gray test, P < .0001. (B) Cumulative incidence of chronic GvHD according to conditioning: Cy-TAI, 64.0% (SE, 5.3%); Cy-ATG, 42.2% (SE, 9.6%); Gray test, P = .025.
Late complications
Malignancies. Solid tumors were diagnosed in 11 patients (the 15-year cumulative incidence was 10.9%, SE = 3.4%, as shown in Figure 5). Nine of 11 cancers occurred in patients who received TAI within the conditioning regimen. Among the 2 patients who received Cy-ATG the type of cancer was basal cell carcinoma of the skin and squamous-cell carcinoma of the cervix developing 1 and 3.1 years after transplantation, respectively. Among the 9 cancers occurring after TAI, 5 were tumors of the head and neck developing within the border of the irradiation field. All but 2 tumors developed in patients with previous chronic GvHD. Cancers after TAI occurred 1.2 to 17 years after transplantation. Three of the 9 cancers developed more than 10 years after transplantation (at a period of time when none of the Cy-ATG patients are yet at risk because of shorter follow-up). The relative risk of cancer associated with the use of TAI conditioning was 1.49 (95% CI, 0.28-7.93). In multivariate analysis, the risk of cancers was associated with age older than 15 years at transplantation (RR = 6.1; 95% CI, 1.4-27.1; P = .032), and with the use of CsA as immunosuppressive therapy before transplantation (RR = 4.9; 95% CI, 1.0-23.4; P = .023).
Cumulative incidence of secondary cancers from transplantation with death not related to cancer considered as a competing risk.
Cumulative incidence of secondary cancers from transplantation with death not related to cancer considered as a competing risk.
Nonmalignant late effects. Numerous type of nonmalignant complications were recorded, but few were numerous enough to allow meaningful statistical analyses. Among nonfrequent complications, it was of note that overt hypothyroidism occurred in only 1 patient, and cataract needing surgical intervention in 2 (TAI spares the eyes). Depression needing medical treatment was noted in 12 long-term patients. Because gonadal failure and growth retardation have been previously reported,30-32 we focused analyses on late infections and avascular osteonecrosis.
Osteonecrosis was diagnosed in 21 patients, leading to a 15-year cumulative incidence of 19.6% (SE, 4.1%; Figure 6). The risk of osteonecrosis was associated with age older than 18.7 years at transplantation (RR = 9.5; 95% CI, 2.8-32.8; P < .0001). All cases of osteonecrosis were diagnosed in patients who received IST before transplantation with ATG or in patients with GvHD treated with steroids.
Cumulative incidence of avascular necrosis of bone from transplantation with death not related to necrosis considered as a competing risk.
Cumulative incidence of avascular necrosis of bone from transplantation with death not related to necrosis considered as a competing risk.
Late infections. Forty-two viral infections were diagnosed in 38 patients after day 100 (leading to a 15-year cumulative incidence of 43.9%; SE, 6.1%; Figure 7A). Types of infection included hepatitis C in 20 patients, hepatitis B (n = 4), HIV infection in 4, late CMV disease (n = 4), and varicella/zoster infection in 10 patients. By multivariate analysis, the risk of any viral infection beyond day 100 was associated with the use of ATG as pretransplantation IST (RR = 2.9; 95% CI, 1.4-6.0; P = .007) and with acute GvHD (RR = 2.1; 95% CI, 1.0-4.5; P = .04). All cases of HIV after transplantation and 18 of 20 of the hepatitis C cases were in the Cy-TAI cohort. Tests are now routinely done to exclude contamination of blood products, and only 2 new cases of hepatitis C infection occurred after Cy-ATG.
Cumulative incidence of infection from day 100 after transplantation with death not related to infection type considered as a competing risk. (A) Viral; (B) bacterial; (C) fungal.
Cumulative incidence of infection from day 100 after transplantation with death not related to infection type considered as a competing risk. (A) Viral; (B) bacterial; (C) fungal.
Microbiologic-proven bacterial infections were diagnosed, after day 100, in 31 patients (15-year cumulative incidence, 28.5%; SE, 4.4%; Figure 7B). The risk of late severe sepsis was associated with extensive chronic GvHD, as a time-dependent covariate (RR = 2.8; 95% CI, 1.3-5.7; P = .006), and with male sex (RR = 2.5; 95% CI, 1.1-5.7; P = .025).
Seven patients were diagnosed with fungal infections after day 100, leading to a 15-year cumulative incidence of 6.7% (SE, 2.5%; Figure 7C). In the overall cohort, 20 patients developed fungal infections after transplantation (aspergillosis n = 14, candidiasis, n = 7). The risk of fungal infection in the overall cohort was associated with age older than 15 years (RR = 6.7; 95% CI, 1.6-29.3; P = .002), previous treatment before transplantation (RR = 3.6; 95% CI, 1.4-9.5; P = .007), and infection before transplantation (RR = 2.7; 95% CI, 1.1-6.6; P = .03). When restricting the risk factor analysis to patients surviving beyond day 100, only age older than 18.7 years remained significantly associated with increased risk of fungal infection (RR = 7.4; 95% CI, 0.9-61.3; P = .03).
Discussion
To provide a long-term perspective of treatment results, we evaluated the outcome in patients with SAA undergoing transplantation at the Hospital Saint Louis and who had full engraftment. The major conclusions of this analysis of long term-outcome are 2-fold. First, the use of Cy-ATG instead of irradiation significantly improved survival in our patient cohort, and second, any trial of IST (or of androgens) before transplantation had a major detrimental effect on the long-term survival in patients receiving a graft from an HLA-identical sibling donor. During the long time period over which patients were accrued, many changes in transplantation and general supportive care occurred. Among changes, the introduction of CsA and the introduction of CMV reactivation screening and preemptive therapy were probably the most important factors and were thus introduced as risk factors in survival analyses, but did not reach significance. However, we cannot completely rule out that other modifications in daily care of patients during the time period of our study might have contributed to improved survival in the recent years in addition to the use of Cy-ATG as conditioning regimen whose effect cannot be separated from the time of transplantation.
By multivariate analysis, 4 factors influenced survival. Both older age and grade II to IV acute GvHD have been described in previous studies.2-5 The major impact of conditioning and of previous treatment was, however, less expected. Indeed, whereas survival rates in the range of 80% to 90% with Cy-ATG are in line with those reported by the Seattle group,9-11 a large retrospective analysis from the IBMTR did not find any impact of the type of conditioning regimen to explain improved outcome of BMT for aplastic anemia.4 The analysis of causes of death revealed that GvHD with or without infection was the leading cause of death after transplantation.33 When analyzing risk factors for acute GvHD, TAI as conditioning was the sole factor associated with increased risk of acute GvHD. In 1992, an IBMTR study already reported that the use of limited-field irradiation was associated with increased risk of acute, but not of chronic, GvHD.3 Thus decreased survival in patients who underwent transplantation after Cy-TAI conditioning was likely due to radiation-induced epithelial cell damage that triggered acute GvHD. This was reinforced by our findings that the cumulative incidence of grade II or more acute GvHD was 0% in patients who received Cy-ATG as compared to over 40% in patients who received Cy-TAI as conditioning regimen. Because all patients who received Cy-ATG for conditioning also received CsA and MTX for GvHD prophylaxis, the role of GvHD prophylaxis regimen could not be studied. Of note: (1) None of the GvHD prophylactic regimens (MTX alone, CsA alone, CsA plus MTX) had a significant impact on survival after Cy-ATG conditioning, and (2) when comparing Cy-ATG to Cy-TAI in patients who received CsA plus MTX, the impact of conditioning regimen was not changed. Thus improved survival with Cy-ATG cannot be explained by modifications of GvHD prophylactic regimens.
The other main finding of this study was the strong impact of previous treatment, by IST or androgens, on the long-term outcome of SAA patients after BMT. Previous studies have reported that the interval from diagnosis to transplantation over 1 year and number of transfusions (> 20) before transplantation increased the risk of death after BMT for SAA.4,5,34-37 These 2 factors are linked to the eventual use of IST before transplantation. In this study, previous treatment was not associated with the time of transplantation nor with the type of conditioning, age of the patients, nor severity of the disease. However, it obviously led to a longer interval from diagnosis to transplantation and more transfusions. Twenty-one patients had an interval before transplantation that exceeded a year and 20 of them had treatment with ATG with or without CsA, as compared to 43 of 107 previously treated patients who underwent transplantation within a year (P < .0001). A significant association was found between treatment with ATG and time interval between diagnosis to transplantation more than a year. Finally, a third of the patients with nonsevere disease underwent transplantation beyond a year as compared to fewer than 10% of the patients with severe or very severe disease. Thus, these results pointed out the choice of physicians who referred us some patients after IST failure. It is clear that this referral introduced an unavoidable bias because only those patients who failed to respond or had relapses were referred for transplantation. The survival of IST-treated patients with nonsevere disease is very good in the short-term (> 90% at 5 years),38 and results of BMT are better in children than in adults (here and in other studies4,5,34-37 ). This study and Seattle group results9 show that the association of Cy-ATG for conditioning and CsA plus MTX for GvHD prophylaxis for BMT offers patients with SAA a 90% chance of cure10,11 with reduced long-term toxicity indicating that there is no reason to postpone BMT, in patients with an HLA-identical sibling, even in adults and in patients with less severe disease.
The cumulative incidence of solid cancers at 15 years was in the range of 10%, with no evidence of a plateau. This reinforced our previous findings6,39 and fit with those of other studies.40 Radiation itself was not a risk factor in this analysis. However, it should be pointed out that the 2 cancer types that occurred in 2 patients who received Cy-ATG were not taken into account in other studies on late malignancies. Basal cell carcinoma of the skin is usually excluded from such analyses because the incidence of this tumor in the general population is imprecise and because its surgical excision in long-term patients may not always be reported. The patient with cervix carcinoma developed this cancer 3.1 years after transplantation, which is quite short to be considered as a “secondary” malignancy. Thus, if we had decided to exclude these 2 cancers, because all others occurred after TAI, irradiation would have been a strong risk factor for late secondary cancer. By multivariate analysis, older age and use of CsA before transplantation were independently associated with the risk of secondary malignancies. Older age has already been associated with cancer risk in aplastic recipients undergoing BMT.6,39 The finding concerning CsA fit with experimental studies,41 studies in recipients of solid organ transplants,42 and with one registry study in bone marrow transplant recipients.43 Thus, prolonged use of CsA (before and after transplantation for these patients) might be linked to increased risk of cancers. Because CsA dependence in over a third of patients with SAA who are treated with IST has been reported,14,16 it would be worthwhile to also closely follow in the long-term these patients who do not undergo transplantation because they might also be at higher risk of secondary solid tumors, as previously reported.
Finally, we studied some nonmalignant late effects. The cumulative incidence of avascular osteonecrosis reached nearly 20% and was higher in older patients. We previously reported that, for unknown reasons, patients with aplastic anemia have a higher risk than patients with other diagnoses who receive transplants.44,45 Furthermore, the Saint Georges Hospital group reported also that patients not receiving transplants who have been treated with IST also develop osteonecrosis.46 Finally, older age and use of steroids to treat GvHD are well-recognized risk factors for osteonecrosis.44,45,47 The fact that here GvHD and its treatment were not found as risk factors is probably due to lack of statistical power due to limited number of events.
The incidence of bacterial infection reached nearly 30% at 20 years. This was associated with extensive chronic GvHD and male sex. Late bacterial septicemia also accounted for nearly 20% of late deaths in patients surviving beyond 2 years in the Seattle series.19 As previously reported by us24 and others,48-50 extensive chronic GvHD is the major risk factor for late sepsis. The relation to male sex is, however, unexplained.
Fungal infections are of major concern in patients with aplastic anemia. Whereas their incidence in patients surviving beyond 3 months was rather low (6.7%), they were the leading cause of infectious death before 3 months. Overall, the risk was increased in patients who have been infected (whatever the cause) before transplantation and by the use of androgens or IST before transplantation. In patients surviving beyond 3 months, only older age was associated with increased risk of fungal infection due to steroid treatment for GvHD.
Finally, the incidence of viral infections reached over 43% at 15 years. This was mainly due to late detection of hepatitis C infection and to varicella zoster infections. We recently completed a study on the long-term outcome of patients receiving transplants and who are infected with hepatitis C. Patients with SAA represented 40% of these hepatitis C-infected patients, a finding probably related to the number of transfusions.51 ATG as IST before transplantation and acute GvHD were both strong risk factors for viral infection occurring beyond day 100. Both of these risk factors have been associated with long-lasting immune deficiency.
In conclusion, improved results using Cy-ATG as conditioning and CsA plus MTX as GvHD prophylaxis can today lead to a greater than 90% chance of long-term survival in patients with SAA. However, only avoidance of any IST before transplantation, avoidance of irradiation within the conditioning regimen, and urgent grafting before sensitization to blood cell transfusions are compatible with such excellent results. The main focus of clinical research in BMT for SAA should now be to reduce further the incidence of chronic GvHD, the leading factor of late complications in this disease.
Prepublished online as Blood First Edition Paper, December 4, 2003; DOI 10.1182/blood-2003-07-2546.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ”advertisement” in accordance with 18 U.S.C. section 1734.