Abstract
Although FLT3 mutations are essentially found in myeloid-lineage leukemia cells, a high level of FLT3 expression was recently observed in MLL gene-rearranged acute lymphoblastic leukemia without FLT3 mutations. Here, we analyzed the biologic and clinical significance of the FLT3 transcript level in comparison with several gene alterations in 181 de novo acute myeloid leukemia (AML) cases. The mean expression level in AML was higher than that in normal mononuclear cells, whereas the range varied widely. A high level of FLT3 is related to internal tandem duplication of the FLT3 gene (FLT3/ITD), the mutations within the activation loop of FLT3 (FLT3/D835Mt), and tandem duplication of the MLL gene (MLL-TD) but not to p53 or N-RAS gene mutations. Furthermore, a high expression level in AML cases with FLT3 mutations was not related to MLL-TD. Overexpressed FLT3 revealed autophosphorylation and had the same sensitivity to the FLT3 inhibitor as FLT3/ITD. Overexpression of FLT3 (more than 200 000 copies/μgRNA) was an unfavorable prognostic factor for overall survival in 91 AML cases without FLT3/ITD. These results indicated that FLT3 overexpression may distinguish a novel disease entity in AML without FLT3 mutations and serve as a therapeutic target for FLT3 inhibitors.
Introduction
The prevalence and significance of several genetic abnormalities in patients with acute myeloid leukemia (AML) have been reported. The most powerful prognostic factor in AML has been the karyotype of the leukemia cells.1 Three cytogenetic risk groups (favorable, intermediate, and poor) are widely accepted, but there is a practical limitation to the definition of cytogenetic risk, especially in patients falling in the intermediate group. Additional prognostic factors are therefore required. It has been reported that abnormalities in the RAS and p53 genes as well as the FLT3 gene are implicated in the pathogenesis of AML.2-7 Mutations in FLT3, RAS, and p53 have been found in approximately 30%, 20%, and 5% to 10% of adult AML cases, respectively, indicating that mutations in these 3 genes are the most frequent genetic alterations in AML.
We and several groups have demonstrated that FLT3 mutations are a strong prognostic factor in AML.8-18 To date, several large-scale analyses have revealed that FLT3 mutations are essentially found in myeloid-lineage leukemia cells.16,19,20 However, FLT3 mutations within an activation-loop were found in 5 of 30 acute lymphoblastic leukemia (ALL) cases with mixed-lineage leukemia (MLL) gene-rearranged ALL.21 It is notable that FLT3 was highly expressed in MLL gene-rearranged ALL, leading to the constitutive activation of wild-type FLT3 kinase, and that primary ALL cells and an ALL cell line SEMK2-M1, which strongly expressed FLT3 but did not carry FLT3 mutations, had the same sensitivity to a potent FLT3 inhibitor as leukemia cells and a cell line with FLT3 mutations.21,22
FLT3 is preferentially expressed on hematopoietic stem cells as well as in the brain, placenta, and liver.23,24 The ligand to FLT3 (FL) is expressed as a membrane-bound or soluble form by bone marrow stroma cells and stimulates the stem cells alone or in cooperation with other cytokines.25-32 FL-FLT3 interaction, therefore, plays an important role in the survival, proliferation, and differentiation of stem cells. In FLT3-expressing leukemia cells, FL stimulation enhances proliferation and reduces apoptosis.
Although FLT3 is expressed on the surface of a high proportion of AML cells as well as B-lineage ALL cells,33-37 little is known about the clinical significance of the FLT3 expression level in acute leukemia. In this study, we analyzed the expression level of the FLT3 transcript quantitatively in comparison with several gene alterations in 181 de novo AML cases. Because the prevalence of the MLL gene rearrangement is lower in adult de novo AML than in therapy-related AML and in infant or childhood acute leukemia, the present cohort did not contain cases with MLL gene translocation. Chromosomal translocations involving the MLL gene result in the production of a chimeric protein in which MLL is fused to more than 30 different fusion partners.38,39 These MLL-fusion products have been thought to contribute to leukemogenesis by conferring gain of function or interfering with normal MLL function dominant negatively.40-42 However, tandem duplication of the MLL gene (MLL-TD) was reported in AML with normal karyotypes as well as with trisomy 11.43-47 The duplicated region of the MLL gene essentially spans exons 3 to 9 or exons 3 to 11, and MLL-TD also results in a fusion product. Although the biologic mechanism remains unclear, the prognostic implication of MLL-TD has been demonstrated by the analysis of 387 AML cases,45 suggesting the involvement of MLL-TD in the pathogenesis of leukemia. Furthermore, coduplication of the MLL and FLT3 genes was recurrently found in pediatric AML cases.47 Therefore, we also analyzed the prevalence of MLL-TD and demonstrated the effects of MLL-TD on the prevalence of the FLT3 gene mutations and the FLT3 expression, as well as the prognostic significance of the FLT3 expression level in AML.
Patients, materials, and methods
Patients and samples
The diagnosis of AML was based on the morphology, histopathology, expression of leukocyte differentiation antigens, and/or the French-American-British (FAB) classification. The study population included 181 patients with newly diagnosed de novo AML consisting of 7 M0, 31 M1, 52 M2, 39 M3, 29 M4, 9 M5, 6 M6, and 8 M7 FAB types. Bone marrow (BM) samples from patients with AML were subjected to Ficoll-Hypaque (Pharmacia LKB, Uppsala, Sweden) density gradient centrifugation. All samples were confirmed to contain more than 90% leukemia cells and then cryopreserved in liquid nitrogen before use. For the normal control, each of 5 BM and cord blood (CB) mononuclear cells (MNCs) was used. We obtained informed consent from all patients and volunteers to use their samples in this study. CB was collected after full-term deliveries with informed consent approved by the Review Board of Tokai Cord Blood Bank. CD34+ cells from CB were separated from MNCs by using Dynabeads M-450 conjugated with an anti-CD34 monoclonal antibody and DETACHaBEAD (Dynal, Oslo, Norway) according to the manufacturer's instructions.48 Each separated aliquot was confirmed to contain more than 95% CD34+ cells by flow cytometry (data not shown).
Cytogenetic G-banding analysis was performed with standard methods. In this study, cytogenetic risk groups were determined as follows: a favorable risk group was defined by t(8; 21) or inv16; a poor risk group by t(9; 22), del5, or del7; and an intermediate risk group by normal or other karyotypes and karyotype unknown.
Screening for mutations of the FLT3, N-RAS, and p53 genes and MLL-TD
High–molecular weight DNA was extracted from the samples by the standard method. FLT3 gene mutations of the internal tandem duplication (FLT3/ITD) and activation loop (FLT3/D835Mt); N-RAS gene mutations of codons 12, 13, and 61; and p53 gene mutations of exons 5 to 8 were examined as previously reported and were confirmed by the sequencing procedure.8,16,49-51 MLL-TD was examined by reverse transcriptase–polymerase chain reaction (RT-PCR) with the primer pairs 6.1 (5′-GTCCAGAGCAGAGCAAACAG-3′) and E3AS (5′-ACACAGATGGATCTGAGAGG-3′), and 6.1 and 4.2R (5′-GGAGCAAGAGGTTCAGCATC-3′) according to published conditions.45,52 Amplified products were cut from the gel, purified with a QIAquick gel extraction kit (Qiagen, Chatsworth, CA), and directly sequenced on a DNA sequencer (310; Applied Biosystems, Foster City, CA) using a BigDye terminator cycle sequencing kit (Applied Biosystems).
Quantitation of FLT3 transcript expression
Total RNA was extracted from the samples by using a QIAamp RNA Blood Mini Kit (Qiagen). cDNA was synthesized from each RNA by using a random primer and Moloney murine leukemia virus reverse transcriptase (Super-Script II; Gibco BRL, Gaithersburg, MD) according to the manufacturer's recommendations. The expression level of the FLT3 transcript was quantitated by using a real-time fluorescence detection method on an ABI Prism 7000 sequence detection system (Applied Biosystems). The primer and probe sequences for real-time PCR of FLT3 were sense primer, 5′-TTTCACAGGACTTGGACAGAGATTT-3′; antisense primer, 5′-GAGTCCGGGTGTATCTGAACTTCT-3′; and TaqMan probe, 5′-FAMTCCAAATTCCAGCATGCCTGGTTCAAG-TAMRA-3′. The housekeeping gene, GAPDH, served as a control for cDNA quality. Relative gene expression levels were calculated by using standard curves and adjusted on the basis of the expression level of the GAPDH gene. Each gene expression level was analyzed in triplicate, and the mean was subjected to analysis. Full-length human wild-type (Wt)–FLT3 cDNA cloned into the pCDHF3 vector, kindly provided by Dr Oliver Rosnet (Institut National de la Santé et de la Recherche Médicale (INSERM), France), was used as the standard. The copy number of the plasmid was calculated from the DNA concentration and the molecular weight of the plasmid. The copy number of FLT3 in each sample was calculated by comparing the Ct values of samples with that of the standard.
Because our quantitative condition detected both Wt- and mutant-FLT3 transcripts, the results from the samples with FLT3 gene mutations revealed the total amount of both transcripts, and we then compared the relative abundance of Wt- and mutant-FLT3 transcripts by RT-PCR. In the samples with FLT3/ITD, we amplified the juxtamembrane domain by using the primer pair R5 (5′-TGTCGAGCAGTACTCTAAACATG-3′) and 12R (5′-CTTTCAGCATTTTGACGGCAACC-3′) as previously described.49 Amplified products were separated on agarose gels and stained with ethidium bromide. The relative proportion of Wt and ITD fragments in each sample was determined by the intensities of both fragments.
Flow cytometry
Cryopreserved AML cells were thawed, washed twice with phosphatebuffered saline, and incubated with an antihuman FLT3 monoclonal antibody (SF1.340; Immunotech, Marseille, France), followed by a fluorescein isothiocyanate (FITC)–conjugated antimouse immunoglobulin antibody (Immunotech). The surface expression of FLT3 products was analyzed by flow cytometer (FACSCalibur; Becton Dickinson, San Jose, CA).53 The relative expression level of FLT3 on the cell surface was adjusted to the level of the isotype control.
Western blot
Cell lysates from primary AML cells were extracted as previously described. Lysates were immunoprecipitated with a rabbit antihuman FLT3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and protein G Sepharose (Amersham Pharmacia Biotech, Piscataway, NJ). The precipitated samples were separated by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis and electroblotted onto Immobilon polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA). Immunoblotting was performed with an antiphosphotyrosine antibody (4G10; Upstate Biotechnology, Lake Placid, NY). The membranes were incubated with stripping buffer and then reprobed with an anti-FLT3 antibody. Signals were developed by using an enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech). To examine the total FLT3 product level, whole cell lysates from each AML cell were subjected to immunoblotting with an anti-FLT3 antibody.53,54 The relative expression level of FLT3 was adjusted to the expression of Actin protein. The expression level was determined by densitometry.
Treatment with an FLT3 inhibitor
A potent FLT3 inhibitor, AG1296, was purchased from Calbiochem (San Diego, CA) and dissolved in dimethyl sulfoxide (DMSO) at an appropriate concentration. Primary AML cells were suspended in RPMI-1640 medium (Gibco BRL) containing 10% fetal calf serum (FCS), and 2 × 104 cells per well were seeded in 96-well culture plates with or without potent FLT3 inhibitors. Cell viability was measured by using the CellTiter96 Proliferation Assay (Promega, Madison, WI) according to the manufacturer's instructions. These procedures were performed 3 times independently.
To examine the effect of the FLT3 inhibitor on the phosphorylation status, leukemia cells were incubated with RPMI-1640 medium containing 10% FCS and 10 μM AG1296 for 3 hours, then subjected to immunoblot analysis as described in “Western blot.”
Analysis of clinical characteristics
It is necessary to analyze the clinical characteristics in a well-documented cohort. Among the 181 patients analyzed, there were 39 with acute promyelocytic leukemia (APL). APL has been considered as a separate disease entity among AML, and the introduction of all-trans retinoic acid (ATRA) has dramatically improved its clinical outcome.55 Because 26 patients with APL were treated with ATRA-based therapy, but the remaining patients were not included in this cohort, we excluded patients with APL from the analysis for clinical characteristics. In addition, 23 patients with AML, except for APL, were treated with independent regimens. We, therefore, analyzed the clinical characteristics of 119 patients with AML, excluding those with APL, who were treated with the AML87, AML89, and AML92 protocol of the Japan Adult Leukemia Study Group (JALSG).56-58
Statistical analysis
Differences in median variables in age, peripheral white blood cell (WBC) counts, platelet counts, and copy numbers of FLT3 were analyzed with the Mann-Whitney U test for distribution among 2 groups or the Kruskal-Wallis test and the Bonferroni test for distribution among more than 3 groups. Analysis of the distribution between 2 continuous variables was performed by using the Spearman rank correlation test. Analysis of frequencies was performed by using Fisher exact test for 2 × 2 tables or Pearson chi-square test for larger tables. Survival probabilities were estimated by the Kaplan-Meier method, and differences in the survival distributions were evaluated by using the log-rank test. The prognostic significance of the clinical variables was assessed by using the Cox proportional hazards model. These statistical analyses were performed with StatView-J 5.0 (Abacus Concepts, Berkeley, CA). For all analyses, the P values were 2-tailed, and a P value of less than .05 was considered statistically significant.
Results
High-level expression of the FLT3 transcript in AML
We quantitated the expression level of the FLT3 transcript in AML cells by using a real-time fluorescence detection method. As a control of normal hematopoietic cells, we also quantitated the expression level in each of 5 normal BM and CB MNCs. Each mean expression level was 3709 (range, 2352-9240) and 4736 (range, 2285-7916) copies/μgRNA in BM and CB MNCs, respectively, and there was no significant difference among them. Because FLT3 is known to be highly expressed on hematopoietic stem cells, we examined the expression level in CD34+ and CD34– cells separated from 5 CB samples. Each mean expression level was 6643 (range, 3814-8668) and 3712 (range, 1169-3955) copies/μgRNA, respectively. The expression level in the CD34+ cells was about 1.7 times higher than that in the CD34– cells, whereas it did not deviate far from the mean level of BM MNCs.
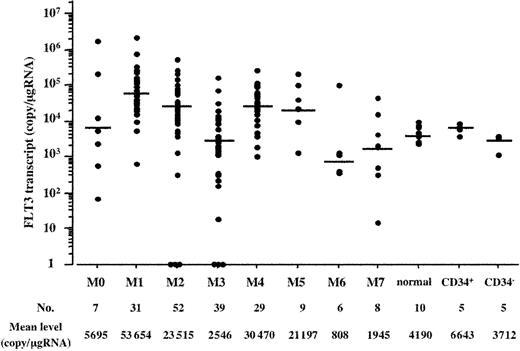
In AML cells, the mean expression level of the FLT3 transcript was 20 203 copies/μgRNA. However, because each expression level varied from 0 to 2 322 706 copies/μgRNA, we compared the expression level of the FLT3 transcript with the percentage of leukemia cells in BM and found no significant association between them. In addition, because we first enriched leukemia cells from BM samples by density gradient centrifugation, we did not adjust the expression level by the percentage of leukemia cells in BM. According to the FAB classification, each mean expression level was 5695 copies/μgRNA in M0, 53 654 in M1, 23 515 in M2, 2546 in M3, 30 470 in M4, 21 197 in M5, 808 in M6, and 1945 in M7, and the distribution among the FAB types was significant (P < .0001 by the Kruskal-Wallis test) (Figure 1). The Bonferroni test revealed that distributions were significantly higher in the M1, M2, and M4 FAB types than in normal hematopoietic cells but not in other FAB types.
Expression level of the FLT3 transcript according to the FAB type. Distribution of the expression level of the FLT3 transcript is indicated according to the FAB type. There was a significant difference among the FAB types and normal hematopoietic cells (P < .0001 by the Kruskal-Wallis test). The Bonferroni test revealed that distributions were significantly higher in the M1, M2, and M4 FAB types than in normal hematopoietic cells but not other FAB types. Normal hematopoietic cells included each of 5 BM and CB MNCs. CD34+ and CD34– cells were isolated from CB MNCs. Horizontal bars indicate each mean value.
Expression level of the FLT3 transcript according to the FAB type. Distribution of the expression level of the FLT3 transcript is indicated according to the FAB type. There was a significant difference among the FAB types and normal hematopoietic cells (P < .0001 by the Kruskal-Wallis test). The Bonferroni test revealed that distributions were significantly higher in the M1, M2, and M4 FAB types than in normal hematopoietic cells but not other FAB types. Normal hematopoietic cells included each of 5 BM and CB MNCs. CD34+ and CD34– cells were isolated from CB MNCs. Horizontal bars indicate each mean value.
To examine the association of the FLT3 expression level with gene alterations, we analyzed the mutations of the FLT3, N-RAS, and p53 genes in 181 patients with AML. We found FLT3/ITD in 38 cases (21.0%), FLT3/D835Mt in 8 (4.4%), N-RAS mutations in 22 (12.2%), and p53 mutations in 10 (5.5%). In addition, we examined MLL-TD by RT-PCR. It has been reported that MLL-TD was detected in normal BM and peripheral blood lymphocytes (PBLs) by nested RT-PCR but not by single-step RT-PCR.47,52 To confirm this and our own RT-PCR conditions, we examined each of 5 normal BM and CB MNCs for MLL-TD by single-step RT-PCR. However, we obtained no band in these normal samples even under our conditions. We, therefore, used single-step RT-PCR for screening MLL-TD and found it in 19 cases (10.5%). Direct sequencing of the amplified products revealed an exon 9/exon 3 fusion in 14 cases, exon 10/exon 3 fusion in 1 case, exon 11/exon 3 fusion in 3 cases, and exon 9/exon 5 fusion in 1 case. It was noteworthy that 7 and 2 of the 19 MLL-TD–positive cases had FLT3/ITD and N-RAS gene mutations, respectively. In addition, 2 MLL-TD–positive cases had both FLT3/ITD and p53 gene mutations. MLL-TD tended to be associated with FLT3/ITD (P = .07 by Fisher exact test) but not at all with FLT3/D835Mt, N-RAS, or p53 gene mutations.
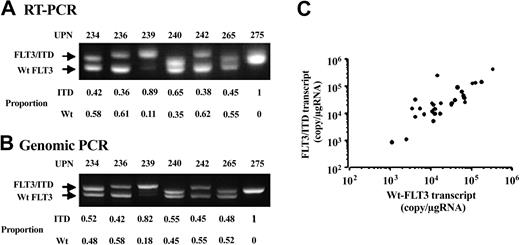
Because our quantitative condition detected both Wt- and mutant-FLT3 transcripts, we compared the relative abundance of Wt- and mutant-FLT3 transcripts in cases with FLT3 gene mutations. In this cohort, there were 38 FLT3/ITD cases. We semiquantitatively determined the relative proportions of Wt- and mutant-FLT3 transcripts in these cases and calculated each expression level according to the proportions (Figure 2A). Furthermore, we compared the results obtained by RT-PCR with those by genomic PCR and found that the relative proportions of Wt- and mutant-FLT3 transcripts were consistent with those of Wt- and ITD-alleles (Figure 2B). Although the relative proportions of Wt- and mutant-FLT3 transcripts varied, they were not related to the total expression level of the FLT3 transcript (data not shown). In addition, the expression levels of Wt- and mutant-FLT3 transcripts were closely correlated (P < .0001) (Figure 2C). These results indicated that the expression levels of the FLT3 transcript in cases with FLT3 gene mutations depended not only on the mutant-transcripts levels, but also the Wt-transcript levels. We, therefore, subjected the total expression levels to further analyses.
Proportion of the Wt- and mutant-FLT3 fragments. Representative results of RT-PCR (A) and genomic PCR (B) in 7 AML samples with FLT3/ITD are shown. The proportion given for each sample denotes the relative intensity of Wt-FLT3 and FLT3/ITD fragments. Each relative proportion obtained by RT-PCR was consistent with that by genomic PCR. (C) The correlation between the expression levels of the Wt-FLT3 and FLT3/ITD transcripts in 34 AML samples with FLT3/ITD is shown. Four AML samples, which lost the Wt-FLT3 allele, were excluded from this analysis. Both expression levels were closely correlated (P < .0001 by the Spearman rank correlation test).
Proportion of the Wt- and mutant-FLT3 fragments. Representative results of RT-PCR (A) and genomic PCR (B) in 7 AML samples with FLT3/ITD are shown. The proportion given for each sample denotes the relative intensity of Wt-FLT3 and FLT3/ITD fragments. Each relative proportion obtained by RT-PCR was consistent with that by genomic PCR. (C) The correlation between the expression levels of the Wt-FLT3 and FLT3/ITD transcripts in 34 AML samples with FLT3/ITD is shown. Four AML samples, which lost the Wt-FLT3 allele, were excluded from this analysis. Both expression levels were closely correlated (P < .0001 by the Spearman rank correlation test).
A high expression level of FLT3 was related to FLT3/ITD (P = .0020), MLL-TD (P = .0121), and FLT3/D835Mt (P = .0463) but not to N-RAS or to p53 gene mutations (Table 1). To clarify the effects of MLL-TD and the FLT3 gene mutations on the expression level of the FLT3 transcript, we divided the 181 AML cases into 5 genotypes according to the presence of MLL-TD, FLT3/ITD, and FLT3/D835Mt. Of these patients, 7 (3.9%) had both MLL-TD and FLT3/ITD, 12 (6.6%) had only MLL-TD, 31 (17.1%) had only FLT3/ITD, 8 (4.4%) had only FLT3/D835Mt, and 123 (68.0%) had neither. The FLT3 expression level in each genotype group ranged from 16 229 to 759 110 (mean, 121 219), 1325 to 1 731 514 (mean, 69 742), 416 to 258 336 (mean, 27 490), 1885 to 219 489 (mean, 63 756), and 0 to 2 322 706 (mean, 13 705) copies/μgRNA, respectively. There was a significant difference among these mutations (P = .0001 by the Kruskal-Wallis test). The Bonferroni test revealed that distributions were significantly higher in AML cases with FLT3/ITD, FLT3/D835, MLL-TD, or both MLL-TD and FLT3/ITD than in those without mutations. However, there were no significant differences among cases with FLT3/ITD, FLT3/D835, MLL-TD, or both MLL-TD and FLT3/ITD.
Overexpressed Wt-FLT3 of AML cells was tyrosine phosphorylated
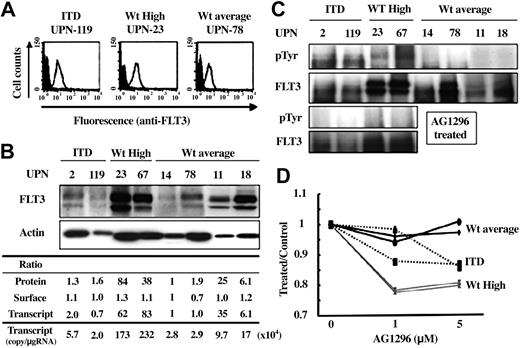
To analyze the biologic effects of the expression level of the FLT3 transcript, we analyzed the expression level and tyrosine-phosphorylation status of FLT3 protein in primary AML cells. Although a limited number of AML cells was available for this analysis, we selected 8 primary AML samples harboring a normal karyotype: 2 (unique patient numbers [UPNs] 2 [FAB, M4] and 119 [M4] whose FLT3 expression levels were 56 635 and 19 541 copies/μgRNA, respectively) had FLT3/ITD and 6 (UPNs 14 [M2], 78 [M2], 11 [M1], 18 [M5], 23 [M0], and 67 [M1] whose expression levels were 28 152, 29 146, 97 373, 170 237, 1 731 514, and 2 322 706 copies/μgRNA, respectively) had Wt-FLT3. Of these AML samples, UPN 23 had both MLL-TD and N-RAS mutations, but the others did not have FLT3/D835, MLL-TD, p53, or N-RAS mutations.
Although the surface expression level of FLT3 protein was not related to the FLT3 transcript level, immunoblot analysis using whole cell lysates from leukemia cells revealed that the total amount of FLT3 protein essentially reflected the transcript level (Figure 3A-B). Of note is that FLT3 products from UPNs 23 and 67, who expressed extremely high levels of FLT3 transcripts, were tyrosine phosphorylated, although those from UPNs 14, 78, 11, and 18 were not (Figure 3C). To exclude the possibility that leukemia cells from UPNs 23 and 67 had novel FLT3 gene mutations, we sequenced the entire coding region of FLT3 and found no mutations.
Biologic effects of the expression level of the FLT3 transcript. Biologic effects of the FLT3 transcript level were analyzed by using 8 AML samples harboring a normal karyotype. Two—UPNs 2 (FAB, M4) and 119 (M4)—had FLT3/ITD, and 6—UPNs 14 (M2), 78 (M2), 11 (M1), 18 (M5), 23 (M0), and 67 (M1)—had Wt-FLT3. Of these AML samples, UPN-23 had both MLL-TD and N-RAS mutations, but the others did not have FLT3/D835, MLL-TD, p53, or N-RAS mutations. (A) Surface expression level of FLT3 was examined by flow cytometry. There was no marked difference according to the expression level of the FLT3 transcript. Open and filled histograms indicate staining with anti-FLT3 and isotype control, respectively. (B) Immunoblot analysis revealed that the total cellular level of FLT3 protein reflected the expression level of the FLT3 transcript. Each expression level of FLT3 protein was adjusted to that of Actin protein. Ratio indicates the relative expression level of the whole cellular protein (Protein), surface protein (Surface), and the transcript when compared with that of UPN 14. (C) Tyrosine phosphorylation of each FLT3 protein was examined. Overexpressed Wt-FLT3 was phosphorylated as well as FLT3/ITD (upper 2 panels). These phosphorylations were inhibited by treatment with AG1296 for 3 hours (lower 2 panels). (D) Cell viability was measured by using the CellTiter96 Proliferation Assay 72 hours after the addition of AG1296 at the indicated concentration to primary AML cells. The y-axis indicates the ratio of absorbance for AG1296-treated cells to untreated cells. Wt-average indicates the results from UPNs 14 and 78 cases, ITD from UPNs 2 and 119, and Wt-High from UPN-23 and -67.
Biologic effects of the expression level of the FLT3 transcript. Biologic effects of the FLT3 transcript level were analyzed by using 8 AML samples harboring a normal karyotype. Two—UPNs 2 (FAB, M4) and 119 (M4)—had FLT3/ITD, and 6—UPNs 14 (M2), 78 (M2), 11 (M1), 18 (M5), 23 (M0), and 67 (M1)—had Wt-FLT3. Of these AML samples, UPN-23 had both MLL-TD and N-RAS mutations, but the others did not have FLT3/D835, MLL-TD, p53, or N-RAS mutations. (A) Surface expression level of FLT3 was examined by flow cytometry. There was no marked difference according to the expression level of the FLT3 transcript. Open and filled histograms indicate staining with anti-FLT3 and isotype control, respectively. (B) Immunoblot analysis revealed that the total cellular level of FLT3 protein reflected the expression level of the FLT3 transcript. Each expression level of FLT3 protein was adjusted to that of Actin protein. Ratio indicates the relative expression level of the whole cellular protein (Protein), surface protein (Surface), and the transcript when compared with that of UPN 14. (C) Tyrosine phosphorylation of each FLT3 protein was examined. Overexpressed Wt-FLT3 was phosphorylated as well as FLT3/ITD (upper 2 panels). These phosphorylations were inhibited by treatment with AG1296 for 3 hours (lower 2 panels). (D) Cell viability was measured by using the CellTiter96 Proliferation Assay 72 hours after the addition of AG1296 at the indicated concentration to primary AML cells. The y-axis indicates the ratio of absorbance for AG1296-treated cells to untreated cells. Wt-average indicates the results from UPNs 14 and 78 cases, ITD from UPNs 2 and 119, and Wt-High from UPN-23 and -67.
Overexpressed FLT3 is sensitive to a potent FLT3 inhibitor
To examine whether overexpressed FLT3 is sensitive to a FLT3 inhibitor, we analyzed the change in its phosphorylation status after treatment with a potent FLT3 inhibitor, AG1296. Leukemia cells with FLT3/ITD or overexpressing Wt-FLT3 were treated with 10 μM AG1296 for 3 hours, then subjected to Western blot analysis. All FLT3 products showed dephosphorylation at tyrosine residues by AG1296 (Figure 3C). Furthermore, we examined whether inhibition of FLT3 by AG1296 led to cytotoxicity in these leukemia cells. We assessed metabolically active cells 72 hours after the addition of AG1296 and found that leukemia cells that overexpressed Wt-FLT3 were more sensitive to treatment with AG1296 than those with FLT3/ITD (Figure 3D).
Clinical characteristics of AML cases expressing a high level of the FLT3 transcript
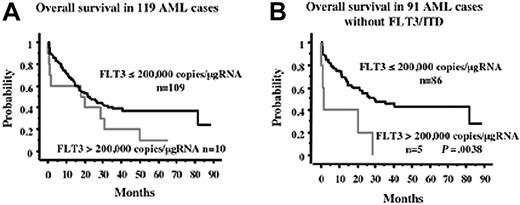
To analyze the effect of the expression level of the FLT3 transcript on the clinical characteristics, it is necessary to determine the cutoff level of overexpression. Because this and previous studies demonstrated that FLT3 products from leukemia cells with an extremely high expression level of the transcript were constitutively tyrosine phosphorylated, it seemed logical that the transcript level, where the product was phosphorylated, was defined as overexpression. Although we could not examine the phosphorylation status of the FLT3 products from clinical samples, Western blot analysis revealed that the Wt-FLT3 product from the AML sample, whose transcript level was 170 237 copies/μgRNA, was not tyrosine phosphorylated (Figure 3C). Therefore, we tentatively determined the cutoff level of overexpression as 200 000 copies/μgRNA and analyzed the effect of FLT3 overexpression on the prognosis of 119 patients with AML, excluding those with APL, who were treated with the JALSG protocol. In this cohort, there were 10 AML cases whose FLT3 levels were more than 200 000 copies/μgRNA. Kaplan-Meier analysis for overall survival showed that overexpression of the FLT3 transcript tended to be a worse prognostic factor, although it was not statistically significant (P = .1067) (Figure 4A). However, because a high expression level of FLT3 transcripts was associated with FLT3/ITD, which was one of the poor prognostic factors, we analyzed the clinical effect of overexpression of the FLT3 transcript within the 91 cases without FLT3/ITD. In this group, there were 5 cases whose FLT3 levels were more than 200 000 copies/μgRNA (Table 2). Among them, 2 cases—UPNs 23 and 67—revealed an extremely high level of the FLT3 transcript. The FLT3 products of these 2 cases were confirmed to be tyrosine phosphorylated (Figure 3C), although those of the other cases could not be analyzed. Overexpression of the FLT3 transcript was not related to other gene mutations, age, WBC count, cytogenetical findings, or FAB type (Table 3). Overexpression was related to a lower complete remission (CR) rate, although not significantly (P = .0686), probably because of the small number in this cohort. Kaplan-Meier analysis for overall survival showed that the worse prognosis was the overexpression of FLT3 (Figure 4B). Univariate analysis showed that the unfavorable prognostic factors for overall survival were age 60 years or older (P = .0006), overexpression of the FLT3 transcript (P = .0068), and a high WBC count (more than 100 × 109/L) (P = .0074) (Table 4). However, cytogenetical findings, mutations of p53 and N-RAS genes, and MLL-TD were not unfavorable prognostic factors. Multivariate analysis showed that overexpression of the FLT3 transcript was the strongest unfavorable factor (relative risk [RR], 4.216; P = .003), followed by age 60 years or older (RR, 2.607; P = .001), and a high WBC count (RR, 2.101; P = .0386) (Table 4).
Overall survival according to overexpression of FLT3. Overall survival according to the overexpression of FLT3 in all AML cases (A) and in 91 AML cases without FLT3/ITD (B) are indicated. In the total cases, overexpression of the FLT3 transcript tended to indicate a worse prognosis, although not significantly. However, it was a poor prognostic factor within the cases without FLT3/ITD. Statistical difference was evaluated using the log-rank test.
Overall survival according to overexpression of FLT3. Overall survival according to the overexpression of FLT3 in all AML cases (A) and in 91 AML cases without FLT3/ITD (B) are indicated. In the total cases, overexpression of the FLT3 transcript tended to indicate a worse prognosis, although not significantly. However, it was a poor prognostic factor within the cases without FLT3/ITD. Statistical difference was evaluated using the log-rank test.
Discussion
An association of high FLT3 expression was first reported for MLL-rearranged ALL.22 Recently, Libura et al59 reported that AML with FLT3/D835Mt and the monocytic lineage AML with FLT3/ITD expressed a high level of the FLT3 transcript. In addition, they also reported that both FLT3/ITD and FLT3/D835Mt were frequently found in AML with MLL gene abnormalities, which involved DNA double-strand breakage at a topoisomerase II site, and such AML cases expressed a high level of the FLT3 transcript. Because our cohort did not contain cases with MLL rearrangements, we focused on MLL-TD as the MLL gene alteration. Seven of the 19 MLL-TD–positive cases had FLT3/ITD, but none had FLT3/D835Mt. When the 181 AML cases were divided into 5 genotypes according to the presence of MLL-TD, FLT3/ITD, and FLT3/D835Mt, there were no significant differences in FLT3 transcript level among the cases with FLT3/ITD, FLT3/D835Mt, MLL-TD, or both MLL-TD and FLT3/ITD. These results indicated that the high level of FLT3 transcript in AML cases with FLT3 gene mutations was not related to MLL-TD, although we could not confirm the effects of MLL rearrangements on the prevalence of FLT3 gene mutations and the FLT3 transcript level.
Previous analyses revealed that most of the primary AML cells expressed FLT3 mRNA as detected by Northern blotting or the RT-PCR method, and that 60% to 70% cells expressed FLT3 protein on their surface.35 However, these expression levels were not quantitatively determined, and the relationship between the mRNA and protein expression levels was not indicated. The MLL-rearranged ALL cell line, SEMK2-M1, was demonstrated to undergo intrachromosomal amplification of FLT3 by fluorescence in situ hybridization (FISH) using BAC probes spanning the FLT3 locus, suggesting that gene amplification is a possible mechanism leading to overexpression of the transcripts.21 Unfortunately, we could not examine whether FLT3 gene amplification occurred in the AML cells harboring high levels of the FLT3 transcript, although cytogenetic analysis by G-banding revealed no alterations involving chromosome 13, where the FLT3 gene is located,13q12 in any cases analyzed.
Although the surface expression level of FLT3 was not related to the expression level of the FLT3 transcript, the total cellular protein level essentially reflected the transcript level. It was reported that FLT3 proteins on the cell surface were internalized when exogenous FL stimulation was administered.60 Furthermore, FL was preferentially expressed by AML cells, and the FL/FLT3 autocrine mechanism may contribute to the antiapoptotic effect in leukemia cells.36,60,61 At present, little is known about the relationship between FL and FLT3 expression levels. However, it is possible that the overexpressed FLT3 proteins are internalized by inducing the FL expression. Alternatively, part of the overexpressed FLT3 proteins may be processed at the cell surface.
It is particularly important that the overexpressed Wt-FLT3 proteins were tyrosine phosphorylated. Previously, tyrosine phosphorylation of overexpressed ALL cells harboring the MLL rearrangement has been demonstrated.21 In addition, 3 of the 27 primary AML cells without FLT3/ITD were reportedly shown to be tyrosine phosphorylated even in the absence of FL stimulation.60 Although these 3 cases were not examined for other FLT3 gene mutations, including FLT3/D835Mt and FLT3 expression levels, overexpression may be involved in autophosphorylation in these cases. Because it was suggested that the FL-FLT3 autocrine mechanism contributes to the antiapoptotic effect in leukemia cells as described earlier, and overexpression may be related to the lower CR rate in induction chemotherapy, it seems possible that the FL expression induced by FLT3 overexpression leads to the receptor being phosphorylated by an autocrine mechanism. It was reported that the autophosphorylation of Wt-FLT3 induced by its overexpression was inhibited by a potent FLT3 kinase inhibitor at the same sensitivity as that of mutated FLT3.21 A previous study demonstrated this only in MLL-rearranged ALL cells, although our study demonstrated that it is also applicable to AML cells without MLL gene alterations. FLT3 kinase serves as an important molecular target in the treatment of leukemia, and several potent inhibitors have been subjected to clinical phase 1 and 2 trials.62 These inhibitors mainly target mutated FLT3 kinases, although our study suggested that the target could be extended to AML cases overexpressing FLT3 even without mutations.
Because FLT3/ITD was closely associated with high expression levels of the FLT3 transcript, high WBC counts, and a poor prognosis, we examined the clinical effect of FLT3 overexpression among the 91 cases without FLT3/ITD. As shown in Table 4, overexpression of FLT3 was not related to any clinical variables with statistical significance, although it tended to be related to a high WBC count and low CR rate. Because the cutoff level of overexpression used here was tentative and there were only 5 patients overexpressing FLT3 in this cohort, larger scale analysis is required to clarify the clinical significance of overexpression. However, our study suggested that overexpression may be an unfavorable prognostic factor for overall survival in AML without FLT3/ITD.
Prepublished online as Blood First Edition Paper, November 6, 2003; DOI 10.1182/blood-2003-06-1845.
Supported by Grants-in-Aid from the Japanese Ministry of Health, Labour and Welfare and the Ministry of Education, Culture, Sports, Science and Technology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
This study was performed in cooperation with JALSG. We thank Ms Manami Kira and Ms Kyoko Aoyama for secretarial and technical assistance.