Abstract
The absence of expanded numbers of hepatitis C virus (HCV)-reactive CD8+ T lymphocytes (CTLs) in patients chronically infected with HCV has led to the investigation of dendritic cell (DC) function in this population as a potential cause for this defect. Several studies have shown evidence for impaired monocyte-derived DCs in chronically infected patients. As it is difficult to reconcile these data with the fact that patients with chronic HCV are immune competent, we re-evaluated this finding, carefully assessing phenotypic markers and functional activity of patient DCs as compared with noninfected controls. In contrast to these prior studies, DCs from 13 of 13 chronic HCV patients expressed typical maturation markers. These mature DCs were capable of priming allogeneic T lymphocytes, as well as stimulating influenza-specific memory T cells. This finding is consistent with clinical and immunologic data that the deficit in the patient's immune repertoire is HCV-specific and suggests that refined models are required for understanding the role of DCs in HCV pathogenesis. (Blood. 2004;103:1026-1029)
Introduction
Hepatitis C virus (HCV) infects more than 170 million people worldwide posing a significant public health risk. Approximately 30% of people infected with HCV clear the virus while 70% progress to chronic infection.1 Many studies have explored the mechanisms by which the virus circumvents both humoral and cell-mediated immune responses. Although HCV-specific humoral responses are commonly seen in HCV-infected chimpanzees and humans, HCV-specific antibodies are not capable of conferring protection.2 Instead, protection in chimpanzees correlates with expanded populations of HCV-reactive CD8+ T cells. Similarly in humans, tetramer analysis of CD8+ T cells indicates the presence of HCV-specific clones in patients who successfully cleared the virus, but not in individuals chronically infected with HCV.3
To define these distinct immunologic outcomes, several groups have explored the role of dendritic cells (DCs) in HCV pathogenesis. DCs are the most potent antigen-presenting cells (APCs) and are responsible for the priming of naive T lymphocytes.4 While it is difficult to isolate native DCs owing to low numbers of circulating cells, it is possible to differentiate peripheral blood monocytes into DCs, which show similarity to those present in peripheral tissue.5,6 Termed immature DCs (iDCs), these cells are characterized by the lack of macrophage markers such as CD14, absence of the mature DC marker CD83, and low surface expression of costimulatory molecules and major histocompatibility complex II (MHC II). Upon exposure to inflammatory cytokines or microbial stimuli, the iDCs undergo a programmed phenotypic change and differentiate into stellate, nonadherent cells termed mature DCs (mDCs), which have down-regulated their ability to capture antigens and are prepared to prime naive T cells. These cells are characterized by the surface expression of CD83 as well as high levels of costimulatory molecules and MHC II.4 As a measure of this activity, it is possible to assay the ability of mDCs in vitro to prime allogeneic T lymphocytes.7
Notably, several groups have reported a maturation defect in monocyte-derived DCs generated from chronic HCV-infected donors.8-10 These observations led to a model where DC dysfunction results in the lack of HCV-specific CD8+ T cells in chronically infected patients.8 This model, however, fails to account for the HCV specificity of the defect, as chronically infected HCV patients are not globally immunocompromised (as would be expected if there were generalized DC dysfunction11 ). Additionally, in vivo data indicating the absence of HCV RNA in peripheral blood mononuclear cells (PBMCs) are inconsistent with a defective DC precursor.12 Here, we have re-examined the phenotype and function of monocyte-derived DCs generated from chronically infected HCV patients using stringent criteria for the definition of DC maturation. In contrast to these prior findings, we observe no defect in DCs from chronic HCV patients and offer an alternative model for the role of DCs in HCV pathogenesis.
Study design
Chronically infected HCV patients
Patient material was obtained as per a protocol approved by the institutional review boards (IRBs) of The New York Presbyterian Hospital (New York, NY) and The Rockefeller University Hospital (New York, NY), and all patients gave written informed consent. All donors tested negative for HIV and hepatitis B virus. Clinical characteristics of the patients are summarized in Table 1. In all cases, antiviral therapy had been discontinued at least 6 months prior to sample collection.
Isolation and preparation of cells
PBMCs, DCs, and T cells were prepared as previously described.13 PBMCs were isolated from whole blood by sedimentation over Ficoll-Hypaque (Amersham Pharmacia Biotech, Piscataway, NJ). T-cell-enriched and T-cell-depleted fractions were prepared by adherence to plastic in 1% single-donor plasma. The iDCs were prepared from the T-cell-depleted fraction by culturing cells in the presence of 1000 U/mL granulocyte-marcrophage colony-stimulating factor (GM-CSF) (Berlex, Seattle, WA) and 500 to 1000 U/mL interleukin 4 (IL-4) (R&D Systems, Minneapolis, MN) for 6 days.6,14 To generate mature DCs, cultures were stimulated on day 6 with 50 ng/mL tumor necrosis factor-α (TNF-α) (Alexis Biochemicals, San Diego, CA) and 0.1 μM prostaglandin E2 (PGE-2) (Sigma, St Louis, MO) for 36 to 48 hours.15
Allogeneic mixed leukocyte reaction (allo-MLR)
First, 2 × 105 purified T cells per well were plated with mDCs at indicated dilutions in 5% pooled human serum (Labquip, Woodbridge, ON, Canada). Cultures were incubated for 4 to 5 days at 37°C and pulsed with 1 μCi (0.037 MBq) 3H-thymidine during the last 14 to 16 hours of culture. 3H-thymidine incorporation was measured by means of a liquid scintillation counter (Perkin Elmer Life Sciences, Shelton, CT).
Detection of influenza-specific T cells by ELISPOT
The mDCs were infected with influenza A/PR/8 (Spafas, Norwich, CT) for 1 hour at 37°C, as described.13 DCs and T cells were plated in 96-well plates (Millipore, Billerica, MA) coated with 5 μg/mL α-IFN-γ monoclonal antibody (mAb) (Mab-1-D1K) (Mabtech, Nacka Strand, Sweden). Cultures were incubated for 24 to 36 hours at 37°C, washed with mild detergent, and incubated with 1 μg/mL biotin-conjugated α-IFN-γ mAb (Mabtech, Mab 7BG-1). Spots were visualized by means of Vectastain Elite Kit (Vector Laboratories, Burlingame, CA). Evaluation was performed in a blinded fashion by an independent service (Zellnet Consulting, New York, NY) by means of an automated enzyme-linked immunospot (ELISPOT) reader (Carl Zeiss, New York, NY). Spots represent IFN-γ production by single cells and are reported as spot-forming cells (SFCs) per 106 cells.
Results and discussion
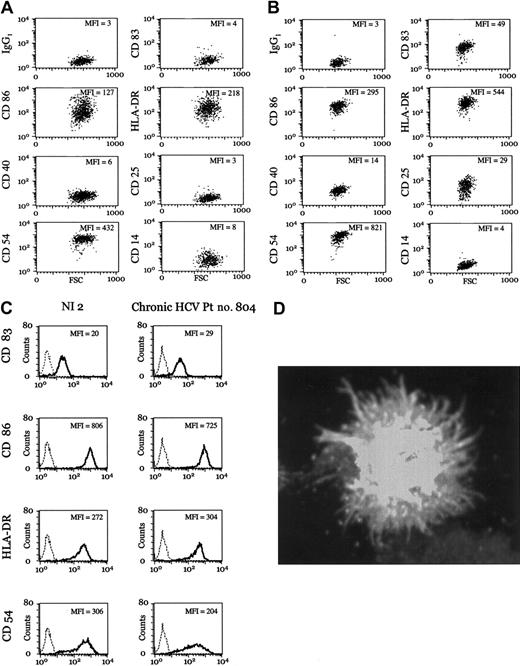
PBMCs were isolated from chronic HCV patients and used for the generation of iDCs. Surface expression of CD14, CD25, CD40, CD54, CD83, CD86, and human leukocyte antigen-DR (HLA-DR), measured by FACSCalibur (BD Biosciences, San Jose, CA), showed similarity to normal iDCs (Figure 1A; see the Supplemental Data Set link at the top of the online article on the Blood website; Table S1). Following exposure to TNF-α and PGE-2, patient DCs expressed surface markers characteristic of mDCs (Figure 1B; Supplementary Table S1). Direct comparison of mDCs from patients with chronic HCV and noninfected donors showed similar expression of CD83, CD86, HLA-DR, and CD54 (Figure 1C; Supplementary Table S1). Furthermore, patient DCs displayed a stellate morphology characteristic of mDCs (Figure 1D). Maturation was TNF-α-dependent, as PGE-2 alone did not induce phenotypic maturation.
Monocyte-derived DCs from chronic HCV patients undergo normal TNF-α-dependent maturation. (A-B) Representative examples of iDCs (A) and mDCs (B) from chronic HCV patient no. 3. Geometric mean fluorescence intensity (MFI) is indicated. See Supplementary Table S1 for numeric values and additional data on all patients. (C) Representative examples of mDCs from a noninfected (NI) donor and a patient with chronic HCV. Expression of the indicated surface marker (dark lines) is overlaid with the isotype control (dotted lines). All antibodies were obtained from BD Biosciences. (D) The mDCs from chronic HCV patient no. 936 were adhered to Alcian blue-treated coverslips, fixed with 3.4% paraformaldehyde (PFA), and stained with anti-MHC II (clone 93C9), followed by secondary antimouse immunoglobulin G (IgG) Alexa 488. Cells were visualized with a Zeiss Axioplan 200. Original magnification, × 630.
Monocyte-derived DCs from chronic HCV patients undergo normal TNF-α-dependent maturation. (A-B) Representative examples of iDCs (A) and mDCs (B) from chronic HCV patient no. 3. Geometric mean fluorescence intensity (MFI) is indicated. See Supplementary Table S1 for numeric values and additional data on all patients. (C) Representative examples of mDCs from a noninfected (NI) donor and a patient with chronic HCV. Expression of the indicated surface marker (dark lines) is overlaid with the isotype control (dotted lines). All antibodies were obtained from BD Biosciences. (D) The mDCs from chronic HCV patient no. 936 were adhered to Alcian blue-treated coverslips, fixed with 3.4% paraformaldehyde (PFA), and stained with anti-MHC II (clone 93C9), followed by secondary antimouse immunoglobulin G (IgG) Alexa 488. Cells were visualized with a Zeiss Axioplan 200. Original magnification, × 630.
We next evaluated patient DCs for their ability to prime allogeneic T lymphocytes as a measure of their functional capacity. Notably, proliferation was robust (more than 105 cpm at 90:1 T/DC ratio) (Figure 2A; Supplementary Table S2) and specific, as demonstrated by the lack of proliferation in syngeneic T cells (data not shown). Comparison of priming capacity of phenotypically normal iDCs and mDCs from chronic HCV and noninfected donors provides functional data to support the normal TNF-α-dependent maturation in both patient populations (Figure 2A; Supplementary Table S2). Normalized patient data are shown with heavy lines representing the respective mean allostimulatory capacity in noninfected individuals versus chronic HCV patients (Figure 2B; Supplementary Table S2). We conclude that monocyte-derived DCs generated from patients chronically infected with HCV are phenotypically and functionally normal.
Mature DCs derived from chronic HCV patients prime allogeneic T cells and stimulate antigen-specific syngeneic memory T cells. (A) Allo-MLR T-cell priming by iDCs and mDCs from noninfected (NI) donor no. 3 and chronic HCV patient no. 794 are shown. Error bars indicate ± SD. T cells cultured alone are represented by a filled triangle. (B) Allostimulatory responses of mDCs from all tested noninfected donors and chronic HCV patients were normalized to values of the noninfected donor at 30:1 to generate an allostimulatory index and were plotted as thin lines with patient identification numbers noted. Thick black line represents mean allostimulatory capacity. See Supplementary Table S2 for numeric values and additional data on iDC stimulation. (C) IFN-γ production by influenza-specific T cells stimulated with either uninfected (white portion of bar) or infected mDCs (black portion of bar) from all patients tested. Data from noninfected, responder, and chronic HCV patients are shown as spot-forming cells (SFCs) per 106 T cells, plotted on a logarithmic scale. NR indicates fewer than 50 SFCs per 106 T cells. Influenza responsiveness is patient specific and dependent on the individual's prior exposure to the virus. Therefore, patient responses may not be averaged across the sample population. Data on noninfected donors (buffy coats) present a range of influenza T-cell precursor frequencies (fewer than 50 to 2300 cells per 106 T cells) and the percentage of responders as based on a signal greater than 50 spot-forming cells per 106 T cells (88% responders; n = 25).
Mature DCs derived from chronic HCV patients prime allogeneic T cells and stimulate antigen-specific syngeneic memory T cells. (A) Allo-MLR T-cell priming by iDCs and mDCs from noninfected (NI) donor no. 3 and chronic HCV patient no. 794 are shown. Error bars indicate ± SD. T cells cultured alone are represented by a filled triangle. (B) Allostimulatory responses of mDCs from all tested noninfected donors and chronic HCV patients were normalized to values of the noninfected donor at 30:1 to generate an allostimulatory index and were plotted as thin lines with patient identification numbers noted. Thick black line represents mean allostimulatory capacity. See Supplementary Table S2 for numeric values and additional data on iDC stimulation. (C) IFN-γ production by influenza-specific T cells stimulated with either uninfected (white portion of bar) or infected mDCs (black portion of bar) from all patients tested. Data from noninfected, responder, and chronic HCV patients are shown as spot-forming cells (SFCs) per 106 T cells, plotted on a logarithmic scale. NR indicates fewer than 50 SFCs per 106 T cells. Influenza responsiveness is patient specific and dependent on the individual's prior exposure to the virus. Therefore, patient responses may not be averaged across the sample population. Data on noninfected donors (buffy coats) present a range of influenza T-cell precursor frequencies (fewer than 50 to 2300 cells per 106 T cells) and the percentage of responders as based on a signal greater than 50 spot-forming cells per 106 T cells (88% responders; n = 25).
While it is difficult to demonstrate DC function in vivo, one measure used is the evaluation of circulating memory T cells. We show that influenza-reactive T cells derived from patients can be effectively restimulated with syngeneic mDCs, as measured in an IFN-γ ELISPOT (Figure 2C). Precursor frequency of influenza-reactive T cells in HCV patients (n = 5), measured by spot-forming cells (SFCs) per 106 T cells, fell within the range of noninfected individuals (precursor CTL [pCTL] range in humans is 0.02% to 0.15% on the basis of 7 control patients studied here and more than 350 ELISPOT assays performed in our laboratory; R.S.L. and M.L.A., unpublished data16 ). This finding suggests that in vivo, chronic HCV patients maintain the ability to support central memory T cells. It also offers additional evidence for functionally competent mDCs in chronic HCV patients and illustrates that the observed defect in the HCV-specific T-cell repertoire does not extend to other viral antigens.
In this study, we have characterized HCV patient DCs, demonstrating phenotypically normal and functionally active mDCs. This finding differs from previous reports; we suggest that this difference is due to differences in culturing conditions. It is possible that incomplete maturation resulted in a perceived functional defect in the study by Auffermann-Gretzinger et al,8 as suggested by the presence of contaminating CD83- cells in the fluorescence-activated cell sorter (FACS) plots of mDC cultures of noninfected individuals. Specifically, we do not find evidence for a defect in TNF-α-mediated DC maturation as demonstrated by a clear shift in allostimulatory capacity when iDCs are effectively matured (Figure 2A). T-cell priming with mDCs (Figure 2A-B) contrasts with other studies where reported thymidine incorporation was 10-fold lower than typically observed,9 possibly reflecting poor growth conditions for T-cell/DC allo-MLR cultures. Here, using conditions adequate for DC maturation and T-cell priming, mDCs from chronic HCV patients are found to be both phenotypically normal and functionally active. These data are consistent with the current understanding of immune function and pathogenesis of HCV, which suggest that the immune defect is HCV specific (Figure 2C). These results are also consistent with the growing belief that in vivo, PBMCs are not typically infected by HCV (Boisvert et al12 and our own unpublished work, November 2002). Although this work does not rule out a possible in vivo DC (or DC subset) defect, our data support the investigation of alternative roles for the DCs in HCV pathogenesis, as we must account for the HCV-selective defect in the T-cell repertoire of chronic HCV patients. These may include DC induction of HCV-specific CD8 T-cell tolerance secondary to the absence of specific cell populations (eg, HCV-specific CD4 T cells), the presence of regulatory T cells, or the modulation of responses as a result of HCV binding to DC-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN).17,18
Prepublished online as Blood First Edition Paper, October 2, 2003; DOI 10.1182/blood-2003-04-1339.
Supported in part by Public Health Service grants 5F32CA88568 (M.L.A), CA57973, and AI40034 (C.M.R.); The Greenberg Medical Research Institute (I.M.J. and C.M.R.); The Burroughs Wellcome Foundation (M.L.A.); The Doris Duke Charitable Foundation (M.L.A.); National Institutes of Health (NIH) Medical Scientist Training Program (MSTP) grant GM07739; The W. M. Keck Foundation Medical Scientist Fellowship (R.S.L.); the NIH General Clinical Research Center (GCRC) grant M01-RR00102; and The Rockefeller University Hospital (RUH) Program Office.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Dr Robert Darnell for insightful discussions and critical review of the manuscript. We are also grateful to Nicole Taylor, NP, and Karen Weisz, NP, at The New York Presbyterian Hospital (NYPH) and General Clinical Research Center (GCRC) staff at The Rockefeller University Hospital (RUH) for assistance with sample collection. Most important, we acknowledge the generosity of the individuals who participated in the study.