Abstract
Lentiviral vectors are increasingly being used for transferring genes into hematopoietic stem cells (HSCs) due to their ability to transduce nondividing cells. Whereas results in in vitro studies and the nonobese diabetic/severe combined immunodeficiency (NOD/SCID) model have been highly encourgaging, studies in large animals have not confirmed the superior transduction of HSCs using lentiviral vectors versus oncoretroviral vectors. In contrast to the stable gene marking we have consistently achieved with oncoretroviral vectors in animals that received myeloablative conditioning, we observed the complete disappearance of genetically modified enhanced green or yellow fluorescent protein–expressing cells in 5 baboons that received transplants of HSCs transduced with lentiviral vectors alone or in combination with oncoretroviral vectors. Immune responses to transgene products have been found to be involved in the disappearance of gene-modified cells after nonmyeloablative conditioning. Thus, we examined whether the disappearance of genemodified cells after ablative conditioning may be due to an immune response. In 4 of 5 animals, cytotoxic T lymphocytes specific for the transgene protein were readily detected, demonstrating that immune reactions were responsible for the disappearance of the gene-marked cells in the animals. In summary, we report the induction of transgene-specific immune responses after transplantation of lentivirally transduced repopulating cells in a myeloablative setting.
Introduction
Whether for the treatment of genetic or acquired diseases such as cancer or AIDS, the efficacy of most gene therapy protocols will depend on persistent, high-level expression of transgene-encoded proteins. In many instances these proteins will constitute novel antigens, and thus the induction of immune responses against transgene products is of concern for the long-term success of these therapies. Clinical trials and large animal studies have shown that when cells expressing xenogenic reporter genes or selectable markers such as enhanced green fluorescent protein (EGFP), neomycin resistance, and HyTK are infused into human or large animal recipients without conditioning or after nonmyeloablative conditioning regimens, potent T-cell– and antibody-mediated immune responses capable of clearing the gene-marked cells from the host can develop.1-4 In contrast, no immune responses against gene-modified cells have been reported when cells are transplanted after myeloablative conditioning regimens, and recipients generally remain tolerant to the transgene products.5-11 The induction of tolerance after myeloablative and nonmyeloablative transplantation has not been carefully studied, but may in part reflect killing or inhibition of peripheral T cells specific for the foreign gene product by the conditioning and the deletion of T cells specific for the foreign gene product generated de novo in the thymus after transplantation.
Recently, a number of research groups have reported the use of lentiviral vectors for the transduction of hematopoietic stem cells. Because lentiviral vectors are able to transduce nondividing cells,12-14 they may be superior to oncoretroviral vectors for the transduction of relatively quiescent hematopoietic stem cells (HSCs), especially in relatively short transduction protocols or in the absence of cytokine stimulation. Very high lentiviral transduction of murine stem cells and human nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse repopulating cells (SRCs) have been reported, with gene transfer levels more than 50%.15-18 In contrast, gene-marking frequencies in nonhuman primates have been variable and generally quite low with lentiviral vectors.10,11,19,20 In a previous study of 6 baboons that received transplants of lentivirus-transduced HSCs after myeloablative conditioning, we achieved persistent long-term gene marking in only 2 animals, and only one showed long-term marking of more than 1% of peripheral blood leukocytes.10 The complete disappearance of genetically modified cells after myeloablative conditioning was unusual and prompted us to consider whether immune responses could have contributed to this disappearance. Therefore, in the current study we analyzed 5 additional animals that exhibited a complete loss of genetically modified cells after transplantation to determine if an immune response against the transgene played a role in the clearance of the gene-marked cells.
Materials and methods
Lentivirus vectors
The lentiviral transfer vectors RRLsin.cPPT.hPGK.GFP.Wpre and RRLsin. cPPT.hPGK.YFP.Wpre (kindly provided by Dr L. Naldini, San Raffaele Telethon Institute for Gene Therapy, Milan, Italy) are self-inactivating HIV-derived vectors expressing EGFP or its yellow variant (EYFP) from the internal human phosphoglycerate kinase promoter (hPGK), and include a woodchuck hepatitis pre-element and a central polypurine tract.17 VSV-G-pseudotype vector stocks were prepared by calcium phosphate–mediated 3-plasmid transfection of 293T cells as described previously.10 Briefly, 27 μg of the transfer vector construct, 17.5 μg second generation gag-pol packaging construct pCMVΔR8.74, and 9.5 μg VSV-G expression construct pMD.G were used for transfection of 12 × 106 293T cells overnight in 25 mL Dulbecco modified Eagle medium (DMEM) with 10% heat-inactivated fetal bovine serum (HIFBS). The cells were treated with 10 mM sodium butyrate during the first of three 12-hour vector collections. The supernatant was filtered through a 0.22-μm filter and concentrated 100-fold by centrifugation. A fourth vector stock of VSV-G/RRLsin.CMV.GFP.Wpre was obtained from Cell Genesys (San Francisco, CA) to compare with our vector preparations for the M00067 transplant. Vector titers were assessed by infection of human HT1080 cells at limiting dilution with analysis for EGFP/EYFP expression by flow cytometry. The titers of the concentrated VSV-G-pseudotype lentivirus vectors were between 2.0 × 108 and 1.4 × 109 IU/mL.
Oncoretrovirus vectors
The MNDEYFPSN vector encodes EYFP under the control of the 5′ modified viral long terminal repeat (LTR) and the bacterial neomycin phosphotransferase (neo) gene driven by the SV40 promoter (kindly provided by Dr Donald Kohn, USC Keck School of Medicine, Los Angeles). Virally conditioned medium (VCM) was harvested from confluent plates of Phoenix-GALV/MNDEYFPSN c28 packaging cells, filtered through a 0.45 μm filter and stored at –70°C. Vector harvests had titers of approximately 1 × 105 IU/mL as evaluated by transduction of HT1080 cells.
Gene transfer into baboon CD34-enriched bone marrow cells
Baboon marrow leukocytes were labeled with immunoglobulin M (IgM) monoclonal antibody (MoAb) 12.8 (anti-CD34) at 4°C for 20 minutes, washed, and incubated with rat monoclonal antimouse IgM microbeads (Miltenyi Biotec, Auburn, CA) for 20 minutes at 4°C, washed again and then separated using an immunomagnetic column technique (Miltenyi Biotec) according to the manufacturer's instructions. CD34 purities ranged from 76% to 91%. CD34-enriched cells were prestimulated for 18 to 48 hours, either in tissue-culture–treated 75 cm2 flasks (Corning, Corning, NY) or in non–tissue-culture–treated 75 cm2 flasks (Falcon, Franklin Lakes, NJ) that had been coated with CH-296 (RetroNectin; kindly provided by Takara Shuzo, Otsu, Japan), at 2 μg/cm in the presence of recombinant human growth factors, including interleukin 3 (IL-3), IL-6, stem cell factor (SCF), megakaryocyte growth and development factor (MGDF), FMS-like tyrosine kinase 3-ligand (Flt3-L), and granulocyte colony-stimulating factor (G-CSF) (kindly provided by Amgen, Thousand Oaks, CA). After prestimulation, cells were transduced with lentiviral vectors diluted in growth medium at a multiplicity of infection (MOI) of 10 or 100 or with undiluted oncoretroviral conditioned medium (MOI < 1) over the CH-296 fragment in the presence of growth factors. After transduction, the cells were harvested and infused into the animals; small aliquots were set aside for colony-forming units (CFUs) and fluorescence activated cell sorting (FACS).
Animals
Healthy juvenile baboons (Papio cynocephalus anubis) were housed at the University of Washington National Primate Research Center under conditions approved by the American Association for Accreditation of Laboratory Animal Care. Studies were conducted under protocols approved by the institutional review board and animal care and use committees. The autologous baboon cell transplants were performed as previously described.6 Before the bone marrow harvest, animals were treated with recombinant human SCF (rhSCF; 50 μg/kg) and rhG-CSF (100 μg/kg) (kindly provided by Graham Molineux, Amgen) as single, daily subcutaneous injections. After 5 days of growth factor administration, marrow (approximately 70 mL-80 mL) was aspirated from the humeri and/or femora and collected in preservative-free heparin. In preparation for transplantation, all animals received myeloablative total body irradiation (TBI), 1020 cGy, administered from a linear accelerator at 7 cGy/min as 2 equally divided doses 24 hours apart. After transplant, animals were given 100 μg/kg rhG-CSF intravenously once daily, starting at day 0 and continuing until their peripheral blood neutrophil counts were more than 1000/μL.
Transplantation of NOD/SCID mice with baboon CD34+ cells
Cells were transplanted into NOD/LtSz-scid/scid (NOD/SCID) mice similarly to a published standard protocol.21 All mice were bred from breeders purchased from The Jackson Laboratory (Bar Harbor, ME). All animals were handled under sterile conditions and maintained in microisolators. Before transplantation, 6- to 8-week-old mice received TBI with 375 cGy at 20 cGy/min from a linear accelerator source and received transplants by tail-vein injection within 24 hours with 2 × 106 CD34-enriched baboon cells after retroviral transduction, after mock transduction or uncultured, as noted.
Flow cytometric analysis of baboon hematopoietic cells
Leukocytes, isolated by ammonium chloride red cell lysis from heparinized peripheral blood and bone marrow samples drawn at multiple time points after transplantation, were analyzed for EGFP and EYFP expression on a FACS Vantage (Becton Dickinson, San Jose, CA). For each sample, at least 500 000 propidium iodide (2 μg/mL)–excluding, forward- and right-angle light scatter–gated events were evaluated. Flow cytometric data were analyzed using CELLQuest v3.3 software (BD Biosciences, San Jose, CA). Expression of EGFP and EYFP in granulocyte, monocyte, and lymphocyte populations was determined either by gating based on forward- and right-angle light scatter characteristics or on expression of lineage-specific CD markers. Murine antihuman phycoerythrin (PE)–conjugated monoclonal antibodies which have been shown to crossreact with baboon CD markers included anti-CD13 (clone L138), anti-CD20 (clone L27), and matched isotype control (clone X40) from BD Pharmingen, San Diego, CA, and anti-CD3 (clone FN18) from BioSource International, Carmarillo, CA. Red cells and platelets from whole blood diluted 1:100 in phosphate-buffered saline (PBS) were delineated by their forward- and right-angle light scatter properties and assessed for EGFP/EYFP expression.
Analysis of EGFP/EYFP expression in a CFU assay
CD34-enriched cells (1000 per 35-mm plate) were cultured at least in triplicate in a double layer agar culture system as previously described.6 Briefly, isolated cells were cultured in alpha minimal essential medium supplemented with 25% HIFBS, 0.1% bovine serum albumin (BSA; fraction V; Sigma, St Louis, MO), 0.3% (wt/vol) agar (BioWhittaker, Rockland, ME) overlaid on medium with 0.5% agar containing 100 ng/mL SCF, IL-3, IL-6, granulocyte-macrophage–CSF (GM-CSF), and G-CSF, and 4 U/mL Epo (provided by Amgen). Cultures were incubated at 37°C in 5% CO2 in a humidified incubator. Colonies were enumerated and evaluated for EGFP/EYFP expression at day 14 of culture using an inverted fluorescent microscope. DNA was isolated from colonies and subjected to polymerase chain reaction (PCR) analysis to determine the frequency of colonies positive for proviral DNA.
Fluorescent probe PCR assay (TaqMan)
PCR amplification and subsequent detection of the EYFP and EGFP transgenes was performed by using a quantitative real-time PCR assay (TaqMan). DNA (300 ng) was amplified at least in duplicate with EYFP-specific primers (5′-GGA TTG CAC GCA GGT TCT C-3′ and 5′-AGA GCA GCC GAT TGT CTGTT-3′) and a fluorescence-tagged probe (5′-FAM-TGC CCA GTC ATA GCC GAA TAG CCT CTC CAT-TAMRA-3′). For EGFP, the specific primers 5′-TAC ACA AAT CGC CCG CAG A-3′ and 5′-AGC CTG GTC GAA CGC AGAC-3′ were used with the probe 5′-FAM-CGA CTT CTA CAC AGC CAT CGG TCC AGA-TAMRA-3′. These primers and probes were designed using Primer Express software (Perkin-Elmer, Foster City, CA) and were obtained from Synthegen, Houston, TX. Standards consisted of dilutions of DNA extracted from cell lines transduced with a single copy of the EGFP or EYFP vector. Negative controls consisted of DNA extracted from peripheral blood leukocytes (PBLs) from control animals or water. Reactions were run using ABI master mix (Applied Biosystems, Branchburg, NJ) on the ABI Prism 7700 sequence detection system (Applied Biosystems) using the following thermal cycling conditions: 50°C for 2 minutes and 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute.
Cytotoxic T-lymphocyte chromium release assays
A T-cell–mediated immune response specific for EGFP/EYFP-expressing cells was assayed in 4 of the 5 animals by cytotoxic T-lymphocyte (CTL) assay. PBMCs were obtained from the peripheral blood of animals by Ficoll density gradient and were cultured with irradiated, autologous MNDEYFPSN-transduced CD34-enriched bone marrow cells at a ratio of 5:1 in RPMI-HEPES 10% HIFBS in the presence of low-dose IL-2 (10 U/mL) for 7 to 15 days. Alternatively, in a few experiments a panel of twenty 20-mer peptides constituting the entire EGFP sequence was used as an antigen source for the in vitro stimulation (kindly provided by Dr Paul Johnson, New England National Primate Center, Southborough, MA). Peptide was added to PBMCs at a concentration of 100 μg/mL total protein (5 μg/mL of each peptide) on day 0 and the cells were cultured as for the stimulation described above. Cytotoxicity of mock-transduced and EYFP-transduced autologous CD34-enriched cells by these in vitro–stimulated PBMCs was assessed in a chromium (Cr-51) release assay. Autologous EYFP-transduced or mock-transduced CD34-enriched bone marrow targets were loaded with Cr-51 overnight and then mixed with the in vitro–stimulated T cells at 1:1, 1:5, and 1:20 ratios in triplicate. Targets were cultured with either medium or NP40 detergent for the determination of minimum and maximum release, respectively. The cultures were incubated for 4 to 5 hours at 37°C, then an aliquot of supernatant was blotted onto a lumaplate for Cr-51 detection. Percent specific lysis was calculated according to the following formula: [(Experimental Release – Minimum Release)/(Maximum Release – Minimum Release)] × 100. Specific lysis of mock-transduced targets served as an internal negative control for each animal. Other negative controls included parallel CTL assays with PBMCs from a naive animal and an animal tolerant to EGFP/EYFP.
Intracellular cytokine staining assays
The presence of an EGFP/EYFP-specific immune response was also tested by intracellular cytokine staining assay in 2 of the 5 animals. The in vitro–stimulated PBMCs generated by coculture with irradiated, autologous EYFP-transduced CD34 cells as described above were incubated at 37°C with autologous EYFP-transduced or mock-transduced CD34 cells at a ratio of 1:1 in the presence of anti-CD28 (10 μg/mL; BD Pharmingen) and anti-CD49d (10 μg/mL; BD Pharmingen). After 3 hours, Brefeldin A (10 μg/mL; Sigma) was added to block release of interferon-γ, and the cell suspensions were incubated at 37°C for another 4 hours. After activation, the cells were divided, permeabilized, and stained with anti–interferon-γ–PE (clone 4S.B3; Pharmingen) and CD8-PERCP (clone SK1; BD Biosciences), and flow cytometric analysis was performed using the FACS Calibur (Becton Dickinson). The forward- and right-angle light scatter–gated events were analyzed to determine the percent of interferon-γ–producing CD8+ cells using CELLQuest v3.3 software. Phorbol 12-myristate 13-acetate (PMA; 10 ng/mL; Sigma)/ionomycin (1 μg/mL; Sigma) and/or staphylococcal enterotoxin B (SEB) stimulation were used as a positive control for each animal assayed. Internal negative controls for each animal consisted of in vitro–stimulated T cells not activated by antigen.
Phenotyping of lentivirally and oncoretrovirally transduced cells
Cryopreserved CD34-enriched baboon bone marrow cells were transduced with lentivirus vector, oncoretrovirus vector, or mock transduced with medium. On day 3 after transduction, the cells were harvested from liquid culture and phenotyped with PE-conjugated human lineage–specific antibodies previously shown to crossreact with baboon cells. Antibodies included IgG1 isotype control-PE (clone X40), CD34-PE (clone 563), CD3-PE (clone SP34), CD20-PE (clone L27), CD13-PE (clone L138), CD14-PE (clone TUK4), and CD83 (clone HB15e) all from BD Pharmingen except CD14-PE which was from Dako (Carpinteria, CA).
Results
Transplantation of transduced cells and follow-up
In an attempt to improve the efficiency of HSC transduction, we used a competitive repopulation assay in baboons to study the impact of different culture conditions on the level of in vivo marking achieved using lentiviral vectors. In some of the animals that received lentivirally transduced cells, we observed the complete disappearance of marked cells after an initial engraftment of transgene-expressing cells.10 This finding was in contrast to the results obtained with transplantations we have performed over the past 5 years using stem cells transduced by oncoretroviral vectors where we have always observed some degree of transgene persistence for the duration of our follow-up. To further investigate this finding, we analyzed in more detail 4 of 5 baboons that lost marking to determine whether an immune response to the transgene was responsible for the disappearance of gene-marked cells. Two of the 5 animals received both lentivirally and oncoretrovirally transduced cells, and the other 3 animals received lentivirally transduced cells only. Table 1 summarizes some of the experimental variables as well as the characteristics of the transplanted cell population. Transduction of the bulk CD34-enriched population as assessed by flow cytometry varied from less than 1% to more than 50% and the frequency of proviral-positive CFUs ranged from 1% to 80% as determined by PCR on single colonies. Although the percentage of CFUs positive for proviral DNA was generally higher than the percentage of bulk culture cells that expressed EGFP/EYFP, the relative marking under the 2 transduction conditions was similar when measured by either method. The animals were followed for approximately 6 months after transplantation (mean follow-up, 180 days).
Transduction of HSCs by VSV-G pseudotype vector does not interfere with engraftment
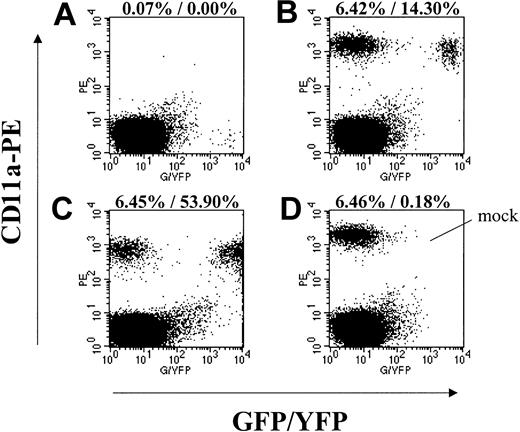
A possible explanation for the low frequency of transgene-expressing cells detected after transplantation in this study and in studies by An et al19,20 is that the VSV-G envelope protein or another component of the lentiviral vector preparation was toxic to the HSCs and reduced their engraftment ability. To address this possibility, we compared the engraftment kinetics of the 3 baboons that received only VSV-G pseudotype lentivirus-transduced cells (M00067, A00083, and A00074) to the 2 animals who received both oncoretrovirus- and lentivirus-transduced cells (K99307 and M99149) (Table 1), and also to a large group of historic controls that received transplants of EGFP/EYFP oncoretrovirus-transduced cells only. The average days to absolute neutrophil count (ANC) more than 500/μL for the lentivirus-only animals was 19.3 (range, 15 to 23), K99307 and M99149 both engrafted within 20 days, and the mean engraftment in the historic controls was 16.9 days (range, 12 to 28; n = 11). The similar engraftment kinetics of the cultured repopulating cell populations in these 3 groups of animals suggest that neither the VSV-G envelope nor any other component of the lentivirus preparations was toxic to short-term repopulating cells. In addition, aliquots of the transduced cells from animals A00074, A00083, and K99307 were transplanted into NOD/SCID mice. The engraftment of transduced baboon cells in these mice was similar to engraftment of mock-transduced cells (Figure 1).
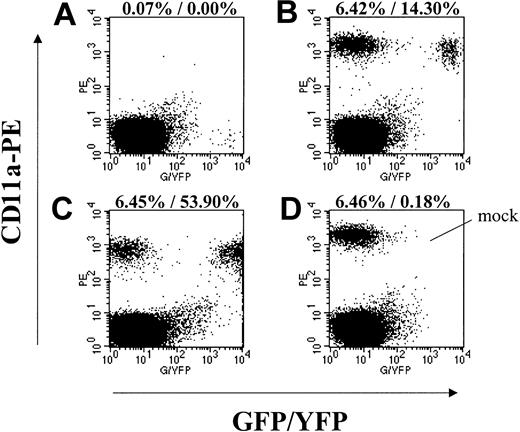
Engraftment of lentivirus-transduced EGFP/EYFP-expressing baboon CD34-enriched cells in NOD/SCID mice. Displayed are representative flow cytometry data from mice that received transplants in parallel with the autologous baboon transplants. Forward-scatter/side-scatter (FSC/SSC) gating to exclude debris and propidium iodide (PI) gating to exclude dead cells was applied to all plots. (A) Isotype control staining of a mouse that received a transplant of EGFP-expressing lentivirustransduced A00074 cells (MOI 10) showing GFP-positive events in the lower right quadrant. (B) CD11a-PE staining of the same mouse in panel A showing engraftment of transduced and nontransduced baboon cells. (C) CD11a-PE staining of a mouse that received a transplant of EYFP-expressing lentivirus-transduced A00083 cells (all growth factors). (D) CD11a-PE staining of a mouse that received a transplant of mock-transduced A00074 cells. Given are the percent engraftment (ie, % CD11a+) and the percent of CD11a+ cells expressing EGFP/EYFP.
Engraftment of lentivirus-transduced EGFP/EYFP-expressing baboon CD34-enriched cells in NOD/SCID mice. Displayed are representative flow cytometry data from mice that received transplants in parallel with the autologous baboon transplants. Forward-scatter/side-scatter (FSC/SSC) gating to exclude debris and propidium iodide (PI) gating to exclude dead cells was applied to all plots. (A) Isotype control staining of a mouse that received a transplant of EGFP-expressing lentivirustransduced A00074 cells (MOI 10) showing GFP-positive events in the lower right quadrant. (B) CD11a-PE staining of the same mouse in panel A showing engraftment of transduced and nontransduced baboon cells. (C) CD11a-PE staining of a mouse that received a transplant of EYFP-expressing lentivirus-transduced A00083 cells (all growth factors). (D) CD11a-PE staining of a mouse that received a transplant of mock-transduced A00074 cells. Given are the percent engraftment (ie, % CD11a+) and the percent of CD11a+ cells expressing EGFP/EYFP.
Transient expression/gene marking after transplantation
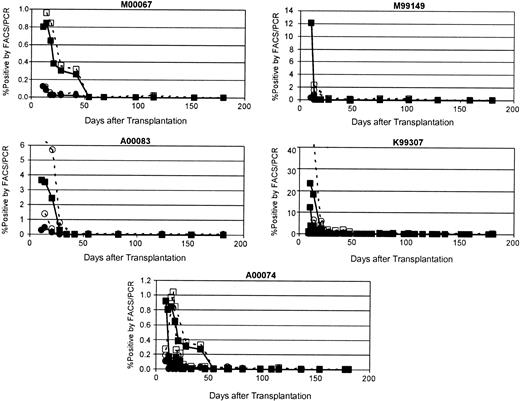
Although the transduced CD34-enriched cells appeared to engraft normally in these 5 animals based on neutrophil and platelet recovery, the frequency of EGFP/EYFP-expressing cells declined rapidly and disappeared entirely between 26 and 54 days after transplantation. Peak marking of 0.10% to 23.20% was detectable by flow cytometry in these animals (Figure 2).
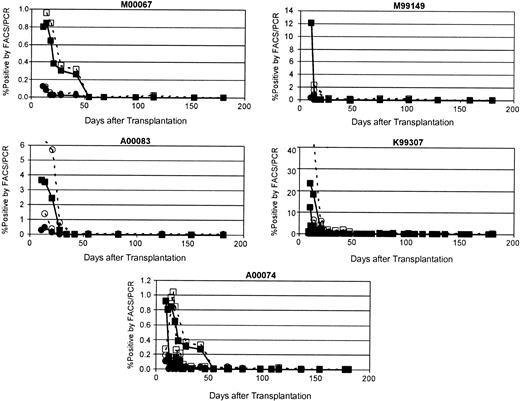
Disappearance of EGFP/EYFP-marked cells after transplantation. Shown for each of the 5 animals that underwent transplantation in this study are the percent of peripheral blood leukocytes positive for transgene as measured by flow cytometry (closed symbols) and by real-time PCR (open symbols). Squares indicate EYFP marking, whereas circles indicate EGFP marking. See Table 1 for vector details.
Disappearance of EGFP/EYFP-marked cells after transplantation. Shown for each of the 5 animals that underwent transplantation in this study are the percent of peripheral blood leukocytes positive for transgene as measured by flow cytometry (closed symbols) and by real-time PCR (open symbols). Squares indicate EYFP marking, whereas circles indicate EGFP marking. See Table 1 for vector details.
Generally, the highest marking was seen at the earliest time points after transplantation (9-14 days), and the marking levels decreased dramatically in the subsequent weeks. To determine whether the rapid decline and disappearance of EGFP/EYFP-expressing cells detected by flow cytometry resulted from clearance of these cells from the animal or simply silencing of the provirus, quantitative real-time PCR was performed on DNA extracted from peripheral blood and bone marrow leukocytes. The level of marking determined by PCR was slightly higher than that determined by flow cytometry at all time points; however, the dynamics of the marking and expression curves were very similar (Figure 1). All evidence of marking as determined by TaqMan PCR disappeared with the same kinetics as the disappearance of EGFP/EYFP expression detected by flow cytometry, indicating that the gene-modified cells were being cleared, not that the transgene was being silenced.
Detection of EGFP/EYFP-specific cytotoxic T lymphocytes
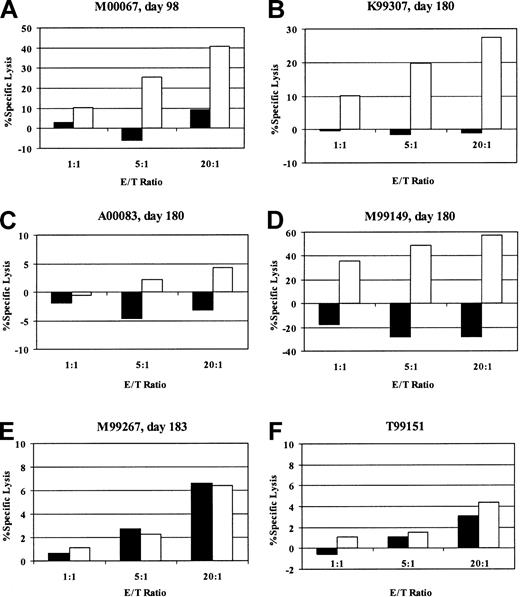
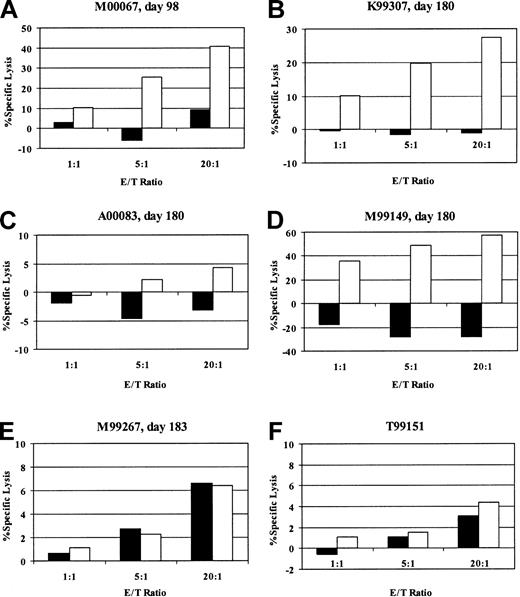
To determine whether the clearance of gene-marked cells was due to the induction of an immune response against the transgene products, we assayed for the presence of EGFP/EYFP-reactive cytotoxic T cells in the peripheral blood of 4 of 5 animals after transplantation. The fifth animal could not be tested because it was euthanized before the possibility of an immune response was considered, and appropriate samples were not cryopreserved. The CTL assays were performed with in vitro–stimulated fresh or cryopreserved PBMCs drawn 3 to 6 months after transplantation (2-5 months after the loss of marking). We were able to detect the presence of a strong EGFP/EYFP-specific cytotoxic T-cell response by chromium release assay in 3 of the 4 animals and a weak response in the remaining animal (Figure 3). Maximum specific lysis of autologous CD34-enriched EYFP-transduced cells was 4.3% (10:1), 27.4% (20:1), 57.3% (20:1), and 40.8% (20:1) in cultures from animals A00083, K99307, M99149, and M00067, respectively, at the E/T ratios indicated in parentheses. These assays were performed after in vitro stimulation and do not provide a quantitative assessment of EGFP- or EYFP-reactive T cells in vivo. However, since they were performed on cryopreserved samples obtained more than 2 months after the loss of marking, the findings suggest stronger responses were likely present during the active phase of rejection. To ensure that we were detecting a memory response and not priming a response against EGFP/EYFP by our in vitro stimulation conditions, we performed identical assays with PBMCs from a naive animal (T99151) and a transplant animal with stable EGFP/EYFP marking of more than 8% more than 1 year after transplantation (M99267). The cultures from these control animals did not show any specific lysis of EGFP/EYFP-expressing autologous targets. As additional evidence of the presence of an immune response against the transgene product, we were able to detect EGFP/EYFP-specific CD8+ T lymphocytes in in vitro–stimulated peripheral blood of 2 of the animals by staining for intracellular interferon-γ after brief stimulation with autologous EYFP-transduced cells (Figure 4). In the majority of experiments, the autologous CD34-enriched cells used as antigen presenting cells (APCs) for the in vitro stimulation and as targets for the CTL assays were transduced with the oncoretroviral vector MNDEYFPSN. The fact that we were able to detect cytotoxic activity in PBMCs from animals that received transplants of only lentivirally transduced cells confirms that the immune responses we were assaying were specific for the EGFP/EYFP transgene product and not for cryptic antigens expressed in the lentiviral vector or for any other protein components of the vector preparation which may have been transiently transferred to the repopulating cells during the transduction culture. In 2 of the animals, we were also able to detect specific killing when an EGFP peptide panel was the antigen source, corroborating that the immune responses are against epitopes derived from the EGFP or EYFP protein (data not shown). Given that EGFP and EYFP differ by only 4 of 240 amino acids,22 it is likely that any responses mounted against cells expressing one protein would be capable of killing cells expressing the other protein.
Detection of EGFP/EYFP-specific cytotoxic T-lymphocyte responses. Percent specific lysis of mock-transduced (▪) or EYFP-transduced (□) autologous targets by in vitro–stimulated PBMCs from M00067 (A), K99307 (B), A00083 (C), M99149 (D), EGFP/EYFP-tolerant transplanted control animal M99267 (E), and naive control animal T99151 (F). Assays were done in triplicate at 3 effector-to-target (E/T) ratios as indicated.
Detection of EGFP/EYFP-specific cytotoxic T-lymphocyte responses. Percent specific lysis of mock-transduced (▪) or EYFP-transduced (□) autologous targets by in vitro–stimulated PBMCs from M00067 (A), K99307 (B), A00083 (C), M99149 (D), EGFP/EYFP-tolerant transplanted control animal M99267 (E), and naive control animal T99151 (F). Assays were done in triplicate at 3 effector-to-target (E/T) ratios as indicated.
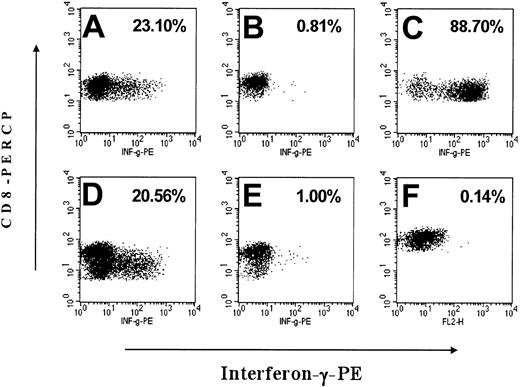
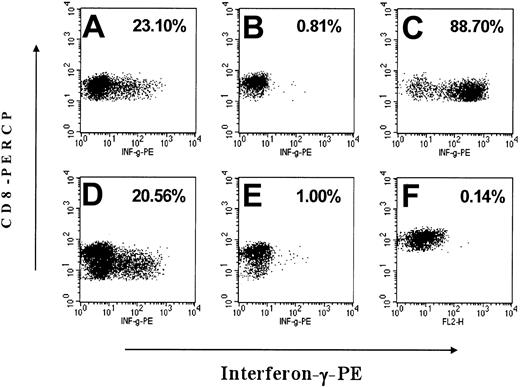
Interferon-γ release of CD8+ T lymphocytes in response to EYFP antigen. After a 7- to 15-day in vitro stimulation with irradiated, autologous EYFP-positive CD34-enriched bone marrow cells, FSC/SSC gated, CD8+ events were analyzed by flow cytometry for their expression of interferon-γ (IFN-γ) after exposure to mock-transduced or EYFP-transduced autologous targets with the percent of CD8+ cells positive for IFN-γ indicated in the upper right of the plots. Exposure to PMA/ionomycin was used as a positive control for IFN-γ production. (A) M99149 PBMCs exposed to EYFP+ antigen presenting cells (APCs), (B) M99149 PBMCs exposed to mock-transduced APCs, (C) M99149 PBMCs treated with PMA/ionomycin, (D) M00067 PBMCs exposed to EYFP-positive APCs, (E) M00067 PBMCs exposed to mock-transduced APCs, (F) tolerant control animal M99267 PBMCs exposed to EYFP-positive APCs.
Interferon-γ release of CD8+ T lymphocytes in response to EYFP antigen. After a 7- to 15-day in vitro stimulation with irradiated, autologous EYFP-positive CD34-enriched bone marrow cells, FSC/SSC gated, CD8+ events were analyzed by flow cytometry for their expression of interferon-γ (IFN-γ) after exposure to mock-transduced or EYFP-transduced autologous targets with the percent of CD8+ cells positive for IFN-γ indicated in the upper right of the plots. Exposure to PMA/ionomycin was used as a positive control for IFN-γ production. (A) M99149 PBMCs exposed to EYFP+ antigen presenting cells (APCs), (B) M99149 PBMCs exposed to mock-transduced APCs, (C) M99149 PBMCs treated with PMA/ionomycin, (D) M00067 PBMCs exposed to EYFP-positive APCs, (E) M00067 PBMCs exposed to mock-transduced APCs, (F) tolerant control animal M99267 PBMCs exposed to EYFP-positive APCs.
Phenotype of lentivirus- and oncoretrovirus-transduced cells
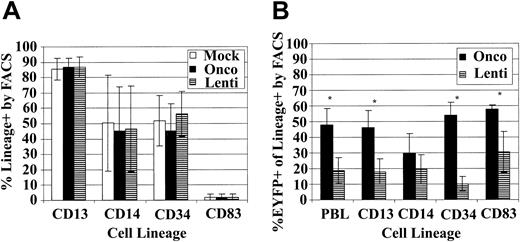
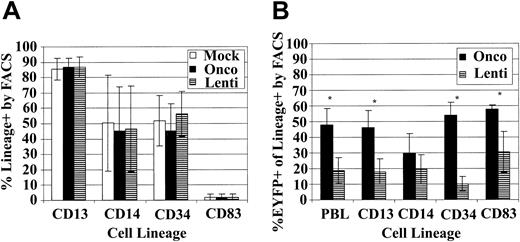
Because we have only observed the induction of transgene-specific immune responses after myeloablative TBI in animals that received transplants of lentivirally transduced cells, we compared our oncoretroviral and lentiviral transduction cultures to determine if the cell populations differed in the expression of differentiation markers or in the EGFP/EYFP transduction of specific subsets of the CD34-enriched population. We hypothesized that immune responses might develop in animals receiving lentivirally transduced cells because the lentiviral vectors transduce at higher efficiency a subset of cells capable of inducing an immune response or, alternatively, because they do not efficiently transduce cells important in promoting the development of tolerance to the transgene. In 4 separate experiments we transduced baboon CD34-enriched cells with mock vector, oncoretroviral, or lentiviral vector and then determined the percent of cells positive for CD3, CD13, CD14, CD20, CD34, and CD83 and also the expression of EGFP/EYFP in each of these subsets. We did not detect any CD3- or CD20-positive cells in our cultures, and the percent of cells positive for CD13, CD14, CD34, and CD83 was not significantly different for the 3 populations (Figure 5A), indicating that our vectors are not driving differentiation of the CD34-enriched population differently and that the overall subpopulation composition of the oncoretrovirally and lentivirally transduced transplant population is similar. However, we found statistically significant differences in transduction efficiency of the subsets between the oncoretroviral and lentiviral vectors. The oncoretroviral transduction rates into the total PBLs (P = .019), CD13 (P = .023), CD34 (P = .005), and CD83 (P = .025) populations were significantly higher than those mediated by lentiviral vectors (Figure 5B). Comparisons were carried out using paired t tests. All reported P values are 2-sided. Interestingly, it appears that the discrepancy between oncoretroviral- and lentiviral-mediated transduction efficiencies was most pronounced in the CD34+ fraction and perhaps least pronounced in the CD83+ population.
Phenotype of lentivirus- and oncoretrovirus-transduced baboon CD34-enriched cells. (A) Percent of cells positive for the indicated CD markers in the mock (□), oncoretrovirus (▪), and lentivirus (▤) transduction cultures. (B) Percent of oncoretrovirally transduced (▪) and lentivirally transduced (▤) cells in the total leukocyte (PBL), granulocyte/monocyte (CD13 and CD14), progenitor (CD34), and dendritic (CD83) subsets. Asterisks denote a significant difference (P < .05) in transduction efficiency between oncoretrovirus and lentivirus vectors. For both analyses, over 50 000 FSC/SSC gated, PI-negative events were analyzed for each sample.
Phenotype of lentivirus- and oncoretrovirus-transduced baboon CD34-enriched cells. (A) Percent of cells positive for the indicated CD markers in the mock (□), oncoretrovirus (▪), and lentivirus (▤) transduction cultures. (B) Percent of oncoretrovirally transduced (▪) and lentivirally transduced (▤) cells in the total leukocyte (PBL), granulocyte/monocyte (CD13 and CD14), progenitor (CD34), and dendritic (CD83) subsets. Asterisks denote a significant difference (P < .05) in transduction efficiency between oncoretrovirus and lentivirus vectors. For both analyses, over 50 000 FSC/SSC gated, PI-negative events were analyzed for each sample.
Discussion
In the current study we demonstrate for the first time the induction of transgene-specific CTL responses to gene-modified cells after a myeloablative conditioning regimen in baboons. These immune responses were detected after transplantation of CD34-enriched cells transduced with lentiviral vectors alone or in combination with oncoretroviral vectors and resulted in the complete disappearance of gene-modified cells. Our data suggest that the use of lentiviral vectors may contribute to the development of antitransgene immune responses in these animals.
A potential explanation for the low marking in our animals could be a toxic effect of the VSV-G envelope protein or some other component of the virus preparations on the engrafting cells. VSV-G protein has been shown to be toxic to numerous cell types.23,24 While this was a concern in our study, the timely recovery of neutrophil counts after transplantation in the 5 animals reported here and especially in the 3 animals receiving only VSV-G pseudotype lentivirally transduced cells suggests that the lentiviral vectors did not have a toxic or damaging effect on repopulating cells. The lack of toxicity on repopulating cells was also supported by the finding that there was no negative effect of VSV-G–transduced cells on engraftment in the NOD/SCID model. Thus, the use of the VSV-G pseudotype was not responsible for the disappearance of marking. As additional evidence of lack of toxicity, mock-transduced VSV-G pseudotype lentivirus-transduced and GALV pseudotype oncoretrovirus-transduced populations had similar expansion and CFU clonogenicity (Table 1), indicating comparable maintenance of progenitors during transduction with the different vectors.
Whereas our long-term marking levels after oncoretroviral transductions vary from very low (< 1% of PBLs) to high (> 20%), we have never observed complete disappearance of gene-modified cells in these studies.6-8,11,25-27 We have performed transplantations on more than 14 baboons with oncoretrovirally transduced cells encoding EGFP/EYFP, and all 14 animals showed long-term persistence of EGFP/EYFP-expressing cells after transplantation, as measured by both flow cytometry and quantitative PCR (marking range: 0.4% to 18.8% by FACS; follow-up range: 1.5 to 42 months). In contrast, in the current study, 5 of 5 animals that received lentivirally transduced cells completely lost marking. Based on our experience with immune responses after nonmyeloablative transplants, we hypothesized that immune responses against EGFP/EYFP could be responsible for this disappearance of genemodified cells. In 4 of these 5 animals we were able to document T-cell–mediated immune responses against EGFP/EYFP by both cytotoxicity assay of in vitro–cultured T cells and intracellular cytokine staining of peripheral blood T cells. Identical assays from a tolerant animal (M99267) and a naive animal (T99151) proved that our assay conditions were not capable of priming a response in vitro and that we were detecting a memory response.
The disappearance of marked cells due to an immune response was previously shown to occur after nonmyeloablative conditioning (240 cGy TBI).2 Our current results show that immune responses to transduced cells can also occur after myeloablative conditioning (1020 cGy TBI). The T cells causing the disappearance of gene-marked cells after myeloablative conditioning may originate from (1) T cells infused together with the CD34 cell graft; (2) grafted hematopoietic cells differentiating into T cells in the thymus; or (3) T cells that survived the conditioning. The origin from T cells infused with the graft is unlikely since we detected no T cells in the grafts by flow cytometry (suggesting that < 5 × 103 T cells/kg were infused, assuming 0.1% sensitivity of flow cytometry). The origin from grafted hematopoietic cells is also unlikely since in baboons T cells start to be generated de novo after 2 months after transplantation,28 whereas the disappearance of the marked cells occurred between day 26 and day 54. Thus, the T cells causing the disappearance likely originate from T cells that survived the 1020 cGy TBI. Consistent with that, there appears to be a marked proliferation of the surviving T cells in the first month after transplantation so that by day 28, CD4 T-cell counts reach approximately 30% of pretransplant levels, and CD8 T-cell counts near 100% pretransplant levels (Storek et al28 ; and J. S. and H.-P. K., unpublished data, August 2003). Given that T cells that survive 1020 cGy TBI likely take part in the immune response against the transgene-expressing cells, the immune response might be avoided by a more T-cell–ablative or suppressive conditioning regimen (eg, by adding a cytotoxic drug or an anti–T-cell antibody to the TBI).
Why did immune responses occur after infusion of lentivirally and not oncoretrovirally transduced cells? Some investigators have suggested that there is a threshold level of marking necessary to induce tolerance to transgenes, and if this level is not achieved, rejection rather than the induction of tolerance will occur. Higher levels of overall marking may induce peripheral tolerance in surviving cells perhaps by a veto mechanism29 and are likely to lead to higher antigenic expression in thymic tissues and more efficient deletion of reactive T cells.
Another possibility for the induction of immune responses after infusion of lentivirally versus oncoretrovirally transduced cells could be that the lentiviral vectors induce differentiation of the CD34-enriched population such that there are more immune response promoting cells or fewer tolerizing cells at the end of the culture period. Our analysis of differentiation marker expression on transduction cultures in 4 independent in vitro experiments suggests that the oncoretrovirally and lentivirally transduced cell populations are not significantly different in their expression of CD3 (T cell), CD13 and CD14 (granulocyte/monocyte), CD20 (B cell), CD34 (progenitor), or CD83 (mature dendritic cell)—and not different from mock-transduced cultures—and therefore our vectors are not driving differentiation differently. However, when we analyzed EYFP expression mediated by lentiviral or oncoretroviral vectors in these subsets, we found significant differences in transduction rates. The oncoretroviral vectors transduced the whole leukocyte (PBL), CD13, CD34, and CD83 populations at a significantly higher rate than the lentiviral vectors. The difference was particularly remarkable for CD34+ cells, which were transduced about 5 times more efficiently by oncoretroviral vectors than by lentiviral vectors. Although not statistically significant (P = .15), the phenotyping data suggests that the lentiviral transduction of CD34+ cells is lower than that of the whole leukocyte population. Other investigators have suggested that sustained expression of foreign gene products in HSCs may be critical for the induction of tolerance.30-32 Perhaps the transduction rate into CD34+ cells by our lentiviral vectors was not sufficient to induce tolerance. In that case, the use of recently described modified HIV-based lentiviral vectors may circumvent this problem.33
In conclusion, we have shown that baboons that received transplants of gene-marked bone marrow cells have developed potent immune responses to the EGFP/EYFP transgenes despite the use of a myeloablative conditioning regimen. The use of lentiviral vectors to transduce cells may play a role in the development of immune responses in this setting. These results have important implications for the development of gene therapy protocols.
Supported by National Institutes of Health grants HL54881, HL53750, DK47754, DK56465, CA18029, and RR00166. H.-P.K. is a Markey Molecular Medicine Investigator.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, September 25, 2003; DOI 10.1182/blood-2003-07-2324.
The authors wish to thank Mike Gough and the staff of the University of Washington National Primate Research Center for assistance with the animals, and Dr R. Paul Johnson for providing EGFP peptides, as well as Bonnie Larson and Helen Crawford for their help in preparing the manuscript.