Abstract
The graft-versus-leukemia (GVL) effect, mediated by donor T cells, has revolutionized the treatment of leukemia. However, effective GVL remains difficult to separate from graft-versus-host disease (GVHD), and many neoplasms are GVL resistant. Murine studies aimed at solving these problems have been limited by the use of leukemia cell lines with limited homology to human leukemias and by the absence of loss-of-function leukemia variants. To address these concerns, we developed a GVL model against murine chronic-phase chronic myelogenous leukemia (mCP-CML) induced with retrovirus expressing the bcr-abl fusion cDNA, the defining genetic abnormality of chronic-phase CML (CP-CML). By generating mCP-CML in gene-deficient mice, we have studied GVL T-cell effector mechanisms. mCP-CML expression of Fas or tumor necrosis factor (TNF) receptors is not required for CD8-mediated GVL. Strikingly, maximal CD4-mediated GVL requires cognate interactions between CD4 cells and mCP-CML cells as major histocompatibility complex-negative (MHC II-/-) mCP-CML is relatively GVL resistant. Nevertheless, a minority of CD4 recipients cleared MHC II-/- mCP-CML; thus, CD4 cells can also kill indirectly. CD4 GVL did not require target Fas expression. These results suggest that CPCML's GVL sensitivity may in part be explained by the minimal requirements for T-cell killing, and GVL-resistance may be related to MHC II expression. (Blood. 2004;103:4353-4361)
Introduction
The graft-versus-leukemia (GVL) effect has revolutionized the treatment of leukemia and lymphoma.1-9 In GVL, donor T cells recognize host antigens as non-self and thereby attack neoplastic cells. Chronic-phase chronic myelogenous leukemia (CP-CML) is the prototypical GVL-sensitive neoplasm in which complete molecular remissions are achieved in nearly 80% of patients with CP-CML who receive donor leukocyte infusions (DLIs).2-4 Despite this success, alloimmune therapy for cancer has 2 principle drawbacks. First, many neoplasms, including CML in blast crisis (BC-CML), are relatively GVL resistant.2,3,10-22 The basis for this differential susceptibility, even between such closely related leukemias as CP-CML and BC-CML, is unknown. Second, GVL has been difficult to separate from graft-versus-host disease (GVHD), the broad attack by donor T cells on recipient tissues. These 2 problems remain unsolved even though they have been recognized for nearly 50 years.23
A major obstacle to overcoming these limitations has been the absence of murine models of clinically relevant GVL-sensitive leukemias. Most murine GVL studies have used cell lines with limited resemblance to human leukemias and even less relevance to CP-CML, which is the most GVL sensitive of human leukemias.24-45 In addition, most studies have used major histocompatibility complex (MHC)-incompatible models, whereas most human allogeneic hematopoietic stem cell transplantations (alloSCTs) are MHC matched and multiple minor histocompatibility antigen (miHA) mismatched.
A detailed mechanistic understanding of GVL against a clinically relevant murine leukemia would be an important step in understanding differential GVL sensitivity and in developing better strategies for separating GVL from GVHD. To do so, we have adopted a murine model of CP-CML (murine chronic-phase CML; mCP-CML) generated by way of retroviral insertion into murine hematopoietic progenitors of the bcr-abl (p210) fusion cDNA, the defining genetic abnormality in human CP-CML.46-50 When irradiated mice receive p210-transduced hematopoietic progenitors, a myeloproliferative disease ensues marked by a high peripheral white blood cell (WBC) count and extensive infiltration of bone marrow (BM) and spleen. Most peripheral WBCs are maturing granulocytes with few blasts, whereas the spleen and bone marrow are replaced by myeloid cells in varying states of differentiation. mCP-CML is oligoclonal and is dependent on bcr-abl tyrosine kinase activity.48,51 A difference between mCP-CML and human CP-CML is that mCP-CML mice succumb to leukemic infiltration of the lung.
A major advantage of this retroviral model is that mCP-CML can be induced in hematopoietic progenitors from any mouse strain, including mice genetically deficient in pathways that might be important for GVL sensitivity. Thus, we have been able to study GVL against gene-deficient mCP-CML and in multiple strain pairings. Here, we use mCP-CML to demonstrate T-cell effector mechanisms in GVL against mCP-CML in clinically relevant MHC-matched, miHA-mismatched models.
Materials and methods
Mice
All mice were between 7 and 10 weeks of age. Male or female C3H.SW, B10.BR, and AKR/J mice were obtained from the Jackson Labs (Bar Harbor, ME). B6 and BALB/c mice were obtained from the National Cancer Institute (Frederick, MD). B6 TNFR1/R2-/- mice (TNFR-/-) were created by us by crossing TNFR1-/- and TNFR2-/- mice (C57BL/6-Tnfrsf1atm1Imx and B6.129S2-Tnfrsf1btm1Mwm; Jackson Labs). These mice were screened by way of polymerase chain reaction for both the wild-type and knock-out alleles. RAG-/-/Faslpr mice were generated by crossing B6 RAG-/- and B6-lpr mice (Jackson Labs). Mice were screened by flow cytometry of peripheral blood looking for the absence of B cells, T cells, and Fas expression. In the text, these mice are referred to as Faslpr. B6 IA beta chain-deficient mice (IAb-/-) were obtained from Taconic (German-town, NY).
Retrovirus production
MSCV2.2 expressing the human p210 cDNA and a nonsignaling truncated form of the human low-affinity nerve growth factor receptor driven by an internal ribosome entry site (Mp210/NGFR) was a gift from Warren Pear. Retroviral supernatants were generated by way of transient transfection of the BOSC ecotropic retrovirus-producing line as described except for use of lipofection instead of calcium phosphate transfection.52 Briefly, on day -1, 4 × 106 BOSC cells were seeded on 6-cm plates in Dulbecco modified Eagle (DME) with 10% fetal calf serum (FCS). On day 0, the cells were transfected with 7.5 μg Mp210/NGFR using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Thirty-six hours after transfection the media was changed (4 mL). Retroviral supernatants were harvested 12 hours later, filtered through 0.45 μ screens, and stored in aliquots at -80°C. Virus was titered on 3T3 cells as described.52 Infected cells were enumerated with use of flow cytometry to identify NGFR-expressing cells.
Hematopoietic progenitor infections
To create p210-infected progenitors, recipient strain or gene-deficient mice backcrossed to the recipient strain were injected on day -6 with 5 mg 5-fluorouracil (5FU; Pharmacia & Upjohn, Kalamazoo, MI). On day -2, bone marrow (BM) cells harvested from femurs and tibias were cultured overnight at 2 × 106 nucleated cells/mL in prestimulation media (DME, 15% fetal bovine serum [FBS], 5% WEHI culture supernatant, interleukin-3 [IL-3; 6 ng/mL], IL-6 [10 ng/mL], and stem cell factor [SCF; 10 ng/mL]; all cytokines were from Peprotech [Rocky Hill, NJ]). On day -1, cells underwent “spin infection” with p210-expressing retrovirus. Cells were resuspended at 2 × 106/mL in prestimulation media with the addition of retroviral supernatant, polybrene (4 μg/mL; Sigma, St Louis, MO), and HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; 100 mm). The final dilution of retrovirus was calculated to be that which would infect 30% of 3T3 cells. BM cells were divided into aliquots in 6-well plates (4 mL/well) and spun in a swinging bucket rotor at 1000g for 90 minutes at 37°C. The plates were returned to the incubator for 2 hours. Cells were then harvested and cultured overnight in prestimulation media without polybrene or retrovirus. On day 0, the spin infection was repeated. Cells were harvested, washed, counted, and resuspended in injection buffer (phosphate-buffered saline [PBS], 100 mm HEPES).
Cell purifications
CD8 cells were purified by way of depletion from lymph node (LN) cells. LNs were crushed through metal screens, and red blood cells (RBCs) were lysed with ACK (0.15 M NH4CL, 1 mM KHCO3, and 0.1 mM Na2 EDTA). Cells were washed and stained with biotin-conjugated antibodies against CD4, B220, and CD11b. Cells were washed and incubated with streptavidin-conjugated magnetic beads (Miltenyi Biotec, Auburn, CA) and separated on an AutoMACS (Miltenyi Biotec) magnetic cell separator. CD4 T cells were purified from LN by using the same protocol as for CD8+ T cells, except anti-CD4 was omitted and biotin-conjugated anti-CD8 was used in its place. BM was flushed from tibias and femurs, followed by RBC lysis with ACK. BM T-cell depletion was performed with anti-Thy1.2-conjugated magnetic beads (Miltenyi Biotec) and the AutoMACS.
Transplantation protocol
In these experiments, all recipients were wild type. On day 0, B6 and AKR hosts received 900 cGy in 2 450-cGy fractions. BALB/c mice received 800 cGy in two 400 cGy fractions. Recipients were reconstituted with 5 to 7 × 106 T-cell-depleted donor type BM with 7 × 105 or 1 × 106 cells that underwent spin infection, with or without a source of donor T cells. In some experiments, BM cells from gene-deficient mice on a B6 background were infected to generate gene-deficient mCP-CML. Mice were followed for the development of mCP-CML, manifest by increased respiratory rate, hunched posture, and death. In most experiments mice were bled weekly for analysis of WBC counts and the presence of NGFR+ cells by flow cytometry.
Antibodies and flow cytometry
Antibodies used to characterize mCP-CML were Gr-1 fluorescein isothiocyanate (FITC), CD11b FITC, TER119, phycoerythrin (PE), Thy1.2 FITC (all from Pharmingen, San Diego, CA); B220 (clone 6B2, multiple colors; lab conjugated); and biotin-conjugated anti-NGFR (clone 20.4; lab conjugated). Antibodies used for cell separations were anti-CD4 (clone GK1.5; lab conjugated) and anti-CD8 (clone TIB105; lab conjugated). Whole blood was stained with appropriate antibodies, followed by RBC lysis with ACK. Propidium iodide was added to exclude dead cells. Cells were analyzed on a FACSCalibur (Becton Dickinson Immunocytometry Systems, San Jose, CA).
Statistics
P values for differences in survival were calculated by log-rank Mantel Cox if events occurred in the compared groups or by chi-square test if there were no events.
Results
GVL in 3 different MHC identical, multiple miHA incompatible donor/recipient pairs
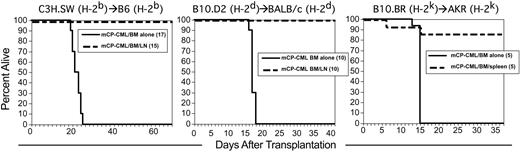
We initially tested GVL in the C3H.SW (H-2b)→B6 (H-2b) MHC-identical, multiple miHA disparate strain pairing. We chose an experimental design intended to model GVL against residual leukemia that survives lethal conditioning (model is described in Figure 1). B6 recipients were irradiated and reconstituted with Mp210/NGFR-infected B6 BM cells, T-cell-depleted C3H.SW BM, with or without C3H.SW unfractionated lymph node (LN) cells. Addition of 1.5 × 107 LN cells resulted in complete protection from death by mCP-CML (Figure 2). Mice were killed between 5 and 6 weeks after transplantation, and spleens from all LN recipients were free of NGFR+ cells (not shown). Significant GVHD did not develop, as we find in most experiments in this strain pairing when a mix of CD4 and CD8 cells is used.53
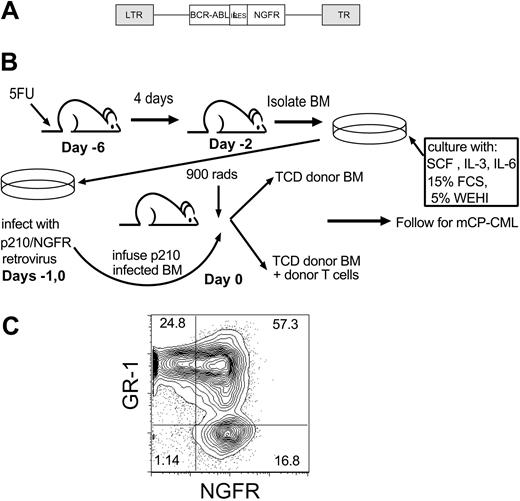
GVL anti-mCP-CML experimental design. (A) Retroviral construct (Mp210/NGFR). LTR indicates long terminal repeat; IRES, internal ribosome entry site. (B) GVL against mCP-CML model. On day -6, mice were injected intravenously with 5 mg 5-FU, and after 4 days BM was isolated and prestimulated (day -2) with SCF, IL-3, and IL-6. Cells underwent spin infection with Mp210/NGFR retrovirus on days -1 and 0. Cells were infused into irradiated syngeneic wild-type recipients along with T-cell-depleted (TCD) BM from allogeneic donors, with or without donor T cells. (C) Representative flow cytometry of peripheral blood from a mouse with mCP-CML.
GVL anti-mCP-CML experimental design. (A) Retroviral construct (Mp210/NGFR). LTR indicates long terminal repeat; IRES, internal ribosome entry site. (B) GVL against mCP-CML model. On day -6, mice were injected intravenously with 5 mg 5-FU, and after 4 days BM was isolated and prestimulated (day -2) with SCF, IL-3, and IL-6. Cells underwent spin infection with Mp210/NGFR retrovirus on days -1 and 0. Cells were infused into irradiated syngeneic wild-type recipients along with T-cell-depleted (TCD) BM from allogeneic donors, with or without donor T cells. (C) Representative flow cytometry of peripheral blood from a mouse with mCP-CML.
GVL in 3 different MHC-identical, miHA-disparate strain pairings. Transplantations were performed as described in “Materials and methods”; numbers of mice per group are in parentheses. Survival in C3H.SW (H-2b)→B6 (H-2b) (left panel; GVL mediated by LN cells), B10.D2 (H-2d)→BALB/c (H-2d) (middle panel; GVL mediated by LN cells), and B10.BR (H-2k)→AKR (H-2k) (right panel; GVL mediated by spleen cells).
GVL in 3 different MHC-identical, miHA-disparate strain pairings. Transplantations were performed as described in “Materials and methods”; numbers of mice per group are in parentheses. Survival in C3H.SW (H-2b)→B6 (H-2b) (left panel; GVL mediated by LN cells), B10.D2 (H-2d)→BALB/c (H-2d) (middle panel; GVL mediated by LN cells), and B10.BR (H-2k)→AKR (H-2k) (right panel; GVL mediated by spleen cells).
A key feature of GVL against human CP-CML is that it is effective regardless of HLA type. That is, no individual HLA molecule has been associated with a better or worse outcome after alloSCT. For our GVL model to be representative of human GVL against CP-CML, we should also observe GVL in multiple MHC-matched, miHA-mismatched donor/recipient pairs. We therefore tested GVL in 2 additional strain pairings in which we have studied GVHD: B10.D2 (H-2d)→BALB/c (H-2d)54 and B10.BR (H-2k)→AKR (H-2k).55 GVL was highly effective in both strain pairings (Figure 2). Thus, as in human GVL against CP-CML, GVL against mCP-CML is active on different MHC backgrounds.
p210 and NGFR are insufficient as target antigens
Because both p210 and NGFR are human proteins, it was possible that epitopes from these proteins, and not miHAs, were targeted by donor T cells. In principle, these epitopes would be similar to the epitopes generated around the junction between bcr and abl which would be non-self in humans with CP-CML. Nevertheless, we did experiments to ask whether p210 and NGFR alone would be sufficient for GVL. To do so we performed a syngeneic transplantation in which the only non-self antigens were derived from p210 and NGFR. B6 recipients were irradiated and reconstituted with Mp210/NGFR-infected B6 progenitors, B6 T-cell-depleted BM, with or without unfractionated B6 LN cells. As a positive GVL control, we simultaneously performed a parallel C3H.SW→B6 experiment. Syngeneic LN cells provided no protection from mCP-CML, and all syngeneic recipients died between days 16 and 18 from mCP-CML (not shown). In contrast, all evaluable C3H.SW→B6 mCP-CML/LN survived without the development of mCP-CML. Therefore, even if p210 and NGFR are included among target antigens, at a minimum, miHA differences are absolutely required for GVL. This finding is consistent with the high relapse rate seen in identical sibling transplantations for CP-CML.56
GVL can be mediated by CD4+ or CD8+ T cells
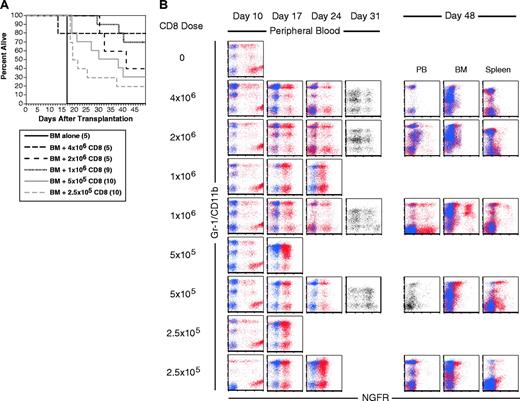
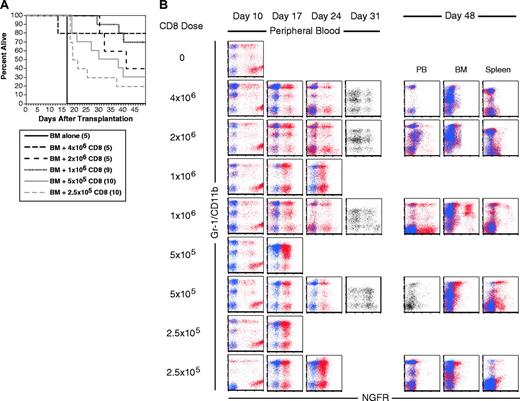
To ask whether CD8 cells alone can mediate GVL against mCP-CML, we perormed GVL experiments in the C3H.SW→B6 pairing with 3 to 4 × 106 purified CD8 cells, a number similar to that contained in the unfractionated LN cell experiments. In 2 independent experiments, no CD8 recipients died of mCP-CML, although some mice died of GVHD without evidence of leukemia (data not shown). To better evaluate the CD8 GVL response, we tested the efficacy of graded doses of donor C3H.SW CD8 cells by using 4 × 106, 2 × 106, 1 × 106, 5 × 105, 2.5 × 105, or 0 purified C3H.SW CD8 cells (Figure 3). As few as 2.5 × 105 donor CD8 cells were able to prolong survival, but most of these mice eventually succumbed to mCP-CML (Figure 3A). All recipients of 2 × 106 and 4 × 106 CD8 cells completely cleared their leukemia, although 3 of 5 recipients of 2 × 106 CD8 cells died of severe GVHD. Absence of leukemia was confirmed in killed mice by flow cytometry of peripheral blood, BM, and splenocytes. Mice that died spontaneously were scored as dying of leukemia if they had a positive assay of peripheral blood at more than 2 weeks after transplantation and an abnormal-sized spleen at necropsy.
CD8 cells alone mediate GVL. B6 mice were irradiated and reconstituted with T-cell-depleted C3H.SW BM, B6 mCP-CML with 0, 2.5 × 105, 5 × 105, 106, 2 × 106, or 4 × 106 C3H.SW CD8+ T cells. (A) Survival. P < .003 for each CD8 recipient group versus BM alone. (B) Serial flow cytometry of peripheral blood. Mice were bled on days 10, 17, 24, and 31 after transplantation, and cells were stained with antibodies against the myeloid marker Gr-1 (except for day 31 when anti-CD11b was used) and anti-NGFR or an isotype control for NGFR. Shown are superimposed dot plots from anti-NGFR (red dots) and isotype for NGFR (blue dots) stained samples. Single-color dot plots indicate that an isotype control was not available. Each row is a representative single mouse. Results from 2 representative recipients of 1 × 106, 5 × 105, and 2.5 × 105 CD8 cells are shown to capture the types of responses we observed. Flow cytometry of blood, BM, and spleen cells at time of killing is presented for the same mice. Note that mCP-CML develops prior to its elimination by donor CD8 cells.
CD8 cells alone mediate GVL. B6 mice were irradiated and reconstituted with T-cell-depleted C3H.SW BM, B6 mCP-CML with 0, 2.5 × 105, 5 × 105, 106, 2 × 106, or 4 × 106 C3H.SW CD8+ T cells. (A) Survival. P < .003 for each CD8 recipient group versus BM alone. (B) Serial flow cytometry of peripheral blood. Mice were bled on days 10, 17, 24, and 31 after transplantation, and cells were stained with antibodies against the myeloid marker Gr-1 (except for day 31 when anti-CD11b was used) and anti-NGFR or an isotype control for NGFR. Shown are superimposed dot plots from anti-NGFR (red dots) and isotype for NGFR (blue dots) stained samples. Single-color dot plots indicate that an isotype control was not available. Each row is a representative single mouse. Results from 2 representative recipients of 1 × 106, 5 × 105, and 2.5 × 105 CD8 cells are shown to capture the types of responses we observed. Flow cytometry of blood, BM, and spleen cells at time of killing is presented for the same mice. Note that mCP-CML develops prior to its elimination by donor CD8 cells.
Serial flow cytometric analysis of peripheral blood demonstrated that mCP-CML developed in all mice in all groups prior to eradication by donor CD8 cells (Figure 3B). Most of the CD8 recipients had NGFR+ cells in peripheral blood (PB) on day +31. None of 10 recipients of 2.5 and 5 × 105 CD8 cells cleared their leukemia, whereas 7 of 10 recipients of 1 × 106 and all recipients of 2 × 106 or 4 × 106 CD8 cells were free of leukemia when killed.
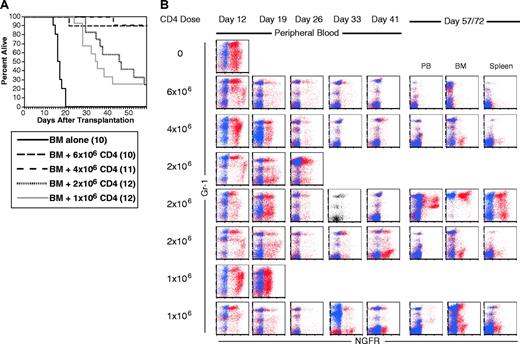
Like recipients of CD8 cells, in 3 independent experiments in the C3H.SW→B6 strain pairing, no recipients of 7 × 106 CD4 cells died of mCP-CML, and most mice had no evidence of residual NGFR+ cells (not shown). When we tested graded doses of C3H.SW CD4 cells (6 × 106, 4 × 106, 2 × 106, and 1 × 106), as few as 1 × 106 cells gave prolonged survival (Figure 4A). However, the only mice that cleared all NGFR+ cells were in the groups that received 6 × 106 or 4 × 106 donor CD4 cells. We also observed CD4-mediated GVL in the B10.D2→BALB/c donor/recipient pair (data not shown). As was the case with GVL mediated by only CD8 cells, all donor CD4 recipients developed mCP-CML prior to eradication by donor CD4 cells (Figure 4B).
CD4 cells alone mediate GVL. B6 mice were irradiated and reconstituted with T-cell-depleted C3H.SW BM, B6 mCP-CML with 0, 106, 2 × 106, or 4 × 106 C3H.SW CD4+ T cells. (A) Survival. P < .0001 comparing BM alone versus 1 × 106 CD4 cells analyzed to day 43 after transplantation. (B) Serial flow cytometry of peripheral blood. Mice were bled and cells were stained as described in “Materials and methods.” As in Figure 3, staining with anti-NGFR is shown in red, and isotype for NGFR is shown in blue. Isotype staining was not done on the sample with a single-color dot plot. Each row is a single mouse. Three and 2 representative mice are shown for the 2 × 106 and 1 × 106 CD8 doses to capture the types of responses we saw. The middle 2 × 106 mouse nearly clears mCP-CML at day 41, but it returns by day 72 after transplantation. Note that mCP-CML develops prior to its elimination by donor CD4 cells.
CD4 cells alone mediate GVL. B6 mice were irradiated and reconstituted with T-cell-depleted C3H.SW BM, B6 mCP-CML with 0, 106, 2 × 106, or 4 × 106 C3H.SW CD4+ T cells. (A) Survival. P < .0001 comparing BM alone versus 1 × 106 CD4 cells analyzed to day 43 after transplantation. (B) Serial flow cytometry of peripheral blood. Mice were bled and cells were stained as described in “Materials and methods.” As in Figure 3, staining with anti-NGFR is shown in red, and isotype for NGFR is shown in blue. Isotype staining was not done on the sample with a single-color dot plot. Each row is a single mouse. Three and 2 representative mice are shown for the 2 × 106 and 1 × 106 CD8 doses to capture the types of responses we saw. The middle 2 × 106 mouse nearly clears mCP-CML at day 41, but it returns by day 72 after transplantation. Note that mCP-CML develops prior to its elimination by donor CD4 cells.
CD8-mediated GVL is intact against Faslpr and TNFR1/R2-/- mCP-CML
Cytotoxic CD8+ T cells primarily kill by way of FasL and perforin/granzyme, although tumor necrosis factor α (TNF-α) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) can also mediate target cell death.57-60 Prior studies into GVL effector mechanisms have relied primarily on reagents that block a pathway (such as infusion of blocking antibodies to TNF-α) or on donor T cells deficient in a killing mechanism (TNF-α, perforin, or FasL-deficient T cells).28,30,42,43 Both TNF-α and FasL have important roles in regulating T-cell responses.61-64 Thus, results with these approaches might reflect effects on the development of the T-cell response and not only on the roles of these molecules on T-cell killing. We, therefore, chose to impair death receptors on mCP-CML cells. Irradiated B6 recipients were reconstituted with T-cell-depleted C3H.SW BM and Mp210/NGFR-infected progenitors from wild-type, Faslpr, or TNFR-/- B6 mice. One group of mice for each of the different mCP-CML types received 1 × 106 purified C3H.SW CD8 cells. We deliberately chose a dose of CD8 cells that does not result in 100% leukemia-free survival to minimize the possibility that we would miss an important contribution from an individual cytolytic pathway by infusing an overwhelming number of CD8 cells.
GVL was similar regardless of whether the mCP-CML cells lacked Fas or TNFR1/2 or expressed these molecules (Figure 5). Four of 14 recipients of wt CML and donor CD8 and 1 of 14 recipients of TNFR-/- or Faslpr died of leukemia; the remaining deaths were due to GVHD. Again, mCP-CML clearly developed in CD8 recipients prior to eradication (Figure 5B). All recipients of TNFR-/- and Faslpr mCP-CML and donor CD8 cells that survived until killing on day 42 after transplantation cleared all NGFR+ cells from blood, BM, and spleen. Similarly, 5 of 8 recipients of wt mCP-CML and donor CD8 cells completely cleared NGFR+ cells (not shown). Recipients of wild-type, TNFR-/-, and Faslpr mCPCML without donor CD8 cells died with similar kinetics and spleen weights, suggesting both that comparable numbers of infected progenitors were infused and that the basic biology of mCP-CML was not effected by the gene deletions. This latter point is supported by the similar immunophenotype of gene-deficient and wild-type mCP-CML (Figure 5B).
mCP-CML expression of TNFR1/TNFR2 or Fas is not required for CD8-mediated GVL. B6 recipients were irradiated and reconstituted with Mp210/NGFR-infected wild-type, TNFR1/TNFR2-/-, or Faslpr progenitors, T-cell-depleted C3H.SW BM, with or without 106 C3H.SW CD8+ T cells. (A) Survival. P < .0001 for each CD4 recipient group versus BM alone. (B) Serial analysis of peripheral blood. Each row is an individual mouse. Staining with anti-NGFR is shown in red, and isotype for NGFR is shown in blue. Each row is a single mouse. Note the similarity among wild-type, Faslpr, and TNFR-/- mCP-CML.
mCP-CML expression of TNFR1/TNFR2 or Fas is not required for CD8-mediated GVL. B6 recipients were irradiated and reconstituted with Mp210/NGFR-infected wild-type, TNFR1/TNFR2-/-, or Faslpr progenitors, T-cell-depleted C3H.SW BM, with or without 106 C3H.SW CD8+ T cells. (A) Survival. P < .0001 for each CD4 recipient group versus BM alone. (B) Serial analysis of peripheral blood. Each row is an individual mouse. Staining with anti-NGFR is shown in red, and isotype for NGFR is shown in blue. Each row is a single mouse. Note the similarity among wild-type, Faslpr, and TNFR-/- mCP-CML.
Optimal CD4-mediated GVL requires cognate interactions with mCP-CML targets and is independent of target Fas expression
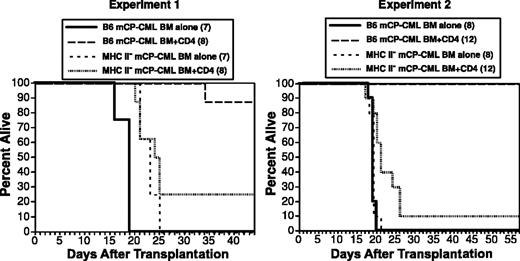
CD4 cells could mediate GVL against mCP-CML cells by direct, indirect, or both mechanisms. Cytotoxic CD4 cells (cytotoxic T lymphocytes; CTLs) that kill by way of FasL and perforin/granzyme are well described.57,58,65,66 Such cells would require T-cell receptor (TCR)-mediated cognate interaction with MHC II-peptide complexes on mCP-CML targets. Alternatively, CD4 cells might act indirectly by way of activation of macrophages that present miHAs or by way of elaboration of cytokines after contacting antigen-presenting cells displaying host miHAs. To distinguish these possibilities, we asked if CD4 cells could mediate GVL against MHC II deficient (MHC II-) mCP-CML. Because TCRs on CD4 cells recognize peptide antigen presented by MHC II, alloreactive CD4 cells would be unable to interact directly with MHC II- mCP-CML cells. To create MHC II- mCP-CML, we infected progenitors from B6 IA beta chain deficient mice (IAb-/-), which do not express MHC II.67 GVL was significantly reduced against MHC II- mCP-CML in 2 independent experiments (Figure 6), demonstrating that CD4 cells require cognate interactions for maximal GVL and that CD4 CTLs are important effectors. However, a small number of IAb-/- mCP-CML recipients were protected by donor CD4 cells, which suggests that CD4 cells are also capable of indirectly mediating GVL. Our CD4 preparations contained no more than 0.3% CD3+CD4- cells; thus, we at most transferred 18 000 CD8 cells, a number unlikely to have mediated GVL against MHC II- mCP-CML (Figure 3A).
Cognate interactions are required for maximal CD4-mediated GVL. B6 mice were irradiated and reconstituted with T-cell-depleted C3H.SW BM, wild-type, or IAb-/- mCP-CML, with or without 6 × 106 C3H.SW CD4+ T cells. Survival was significantly reduced in recipients of IAb-/- mCP-CML and CD4 cells as compared with wt mCP-CML and CD4 cells. Each panel is an independent experiment. Experiment 1: IAb mCP-CML BM alone versus IAb mCP-CML BM/CD4, P < 0.11; wt mCP-CML BM/CD4 versus IAb-/- mCP-CML BM/CD4, P < .0001. Experiment 2: IAb mCP-CML BM alone versus IAb mCP-CML BM/CD4, P < .02; wt mCP-CML BM/CD4 versus IAb-/- mCP-CML BM/CD4, P < .0001.
Cognate interactions are required for maximal CD4-mediated GVL. B6 mice were irradiated and reconstituted with T-cell-depleted C3H.SW BM, wild-type, or IAb-/- mCP-CML, with or without 6 × 106 C3H.SW CD4+ T cells. Survival was significantly reduced in recipients of IAb-/- mCP-CML and CD4 cells as compared with wt mCP-CML and CD4 cells. Each panel is an independent experiment. Experiment 1: IAb mCP-CML BM alone versus IAb mCP-CML BM/CD4, P < 0.11; wt mCP-CML BM/CD4 versus IAb-/- mCP-CML BM/CD4, P < .0001. Experiment 2: IAb mCP-CML BM alone versus IAb mCP-CML BM/CD4, P < .02; wt mCP-CML BM/CD4 versus IAb-/- mCP-CML BM/CD4, P < .0001.
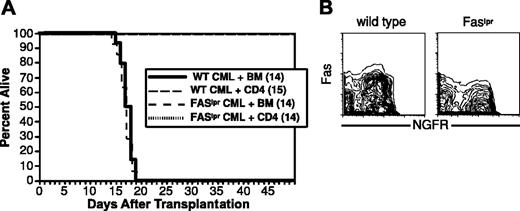
To determine whether FasL-mediated killing is important in CD4-mediated GVL, we asked whether donor CD4 cells could mediate GVL against Faslpr mCP-CML. Recipient B6 mice were irradiated and reconstituted with T-cell-depleted C3H.SW BM, wild-type, or Faslpr mCP-CML progenitors, with or without 4 × 106 purified C3H.SW CD4 cells. Donor CD4 cells mediated equivalent GVL against Fas+/+ and Faslpr mCP-CML (Figure 7). Despite the lack of evidence for FasL-mediated killing, mCP-CML cells from spleen clearly expressed Fas (Figure 7). As in prior experiments, serial flow cytometry confirmed that Faslpr and wild-type mCP-CML developed in all mice prior to eradication by donor CD4 cells (not shown).
FasL-mediated killing is not required for CD4 GVL. B6 mice were irradiated and reconstituted with T-cell-depleted C3H.SW BM, wild-type, or Faslpr Mp210/NGFR infected progenitors, with or without 4 × 106 C3H.SW CD4+ T cells. (A) Survival. (B) Fas expression. Note Fas expression in wild-type but not Faslpr NGFR+ cells.
FasL-mediated killing is not required for CD4 GVL. B6 mice were irradiated and reconstituted with T-cell-depleted C3H.SW BM, wild-type, or Faslpr Mp210/NGFR infected progenitors, with or without 4 × 106 C3H.SW CD4+ T cells. (A) Survival. (B) Fas expression. Note Fas expression in wild-type but not Faslpr NGFR+ cells.
Discussion
The 2 principle challenges in improving the efficacy of alloSCT in treatment of malignancy are decreasing GVHD and overcoming the relative GVL resistance of many neoplasms. Adetailed understanding of the killing mechanisms in GVL is key for developing strategies to overcome these obstacles, and this was the goal of the work presented. Herein, we describe for the first time GVL against a clinically relevant murine model of CP-CML. There have been numerous murine GVL models in which important observations have been made.24-45 However, nearly all of those studied GVL against cell lines that shared neither phenotype nor genetic etiology with common human leukemias. In particular, these cell lines do not recapitulate chronic-phase CML, which is the most GVL sensitive of human leukemias. Differences between these cell lines and authentic CP-CML could affect mechanisms and outcome of GVL. Critically, the mCP-CML we used is phenotypically and genotypically an excellent model for human CP-CML.46-50
The GVL model described here replicates key features of human GVL against CP-CML, in addition to the use of an appropriate leukemic target. To simulate a clinically relevant situation, our experiments modeled GVL against residual leukemia after lethal conditioning in MHC-matched, multiple miHA mismatched strain pairings. As in human alloSCT, in addition to mCP-CML, hematopoietic reconstitution can be derived from conditioning regimen resistant nonmalignant recipient hematopoiesis and engrafting donor BM. It was important that mCP-CML develop prior to its elimination by alloreactive T cells. Serial analysis of peripheral blood (Figures 3, 4, 5) and analysis of cohorts of mice killed at different time points (not shown) clearly confirm that this indeed occurred. Syngeneic transplantations demonstrated that p210 and NGFR were insufficient as target antigens. Thus, as is the case in human identical twin transplantations, p210 expression is insufficient for GVL. There is good precedent for immune competent mice not rejecting syngeneic malignant cells expressing mutant proteins or even model antigens.68-74 Specific to this work, 32D myeloid leukemic cells that express human p210 are also not spontaneously rejected.75
We found effective GVL against mCP-CML in 3 different MHC-compatible, multiple miHA-disparate strain pairings. This is consistent with human GVL data in which no HLA preference has been reported and GVL is clearly observed in patients with numerous HLA genotypes. Because different MHC present different peptides, the human data and our murine experiments suggest that no single immunodominant epitope is likely to be required for GVL.
We investigated the T-cell types necessary and sufficient for GVL. Results of human alloSCT with CD8-depleted BM suggested that CD4 cells alone were sufficient for GVL against CP-CML.76,77 Similarly, CD8-depleted DLI was effective in treating relapsed CP-CML.76,77 However, in each case, CD8 depletion was incomplete, and significant numbers of CD8 cells were infused. CD4 cells in CD8-depleted DLI might also have provided further help to alloreactive CD8 cells derived from the initial T-cell-replete transplantation, which in turn could have been the direct mediators of GVL. Our results clarify this point by showing that GVL can be mediated by highly purified CD4 cells.
That CD4 cells alone are capable of mediating GVL has implications for recognition as well as killing mechanisms. Surprisingly, maximal CD4-mediated GVL required cognate interactions with mCP-CML cells, as GVL was greatly reduced against MHC II- mCP-CML. It is possible that engrafting mCP-CML cells are critical because they directly prime naive alloreactive CD4 cells. However, for several reasons we think that the need for cognate interactions is more likely in the effector phase of GVL when the T-cell receptor of CD4+ CTLs bind to target MHC II-peptide complexes. First, it is key to note that in these experiments, both radiation-resistant wild-type host antigen-presenting cells and engrafting donor-derived antigen-presenting cells (APCs) are MHC II+ and are, therefore, available for donor CD4 cell priming.78,79 Second, alloimmune T-cell activation begins early after transplantation,80-85 and this activation would be prior to significant engraftment by leukemic cells. Finally, there is no reason to expect that leukemic cells would be more efficient at priming rare alloreactive CD4+ T cells than donor or host dendritic cells in T-cell areas of secondary lymphoid tissues. Rendering cells MHC II- is the only definitive way to prevent CD4+ T-cell priming. Therefore, to formally exclude a role for mCP-CML-mediated T-cell priming will require a series of experiments that ask whether mCP-CML priming is sufficient (ie, donor and host are MHC II- and priming can only occur on leukemic cells). This will entail extensive backcrossing of gene-deficient mice; thus, such experiments are beyond the scope of this report.
Despite a dominant role for direct recognition of MHC II on leukemic cells, a small number of mice that received MHC II- mCP-CML and donor CD4 cells survived, suggesting that CD4 cells can also mediate GVL without cognate recognition of leukemic cells. In these experiments no more than 18 000 CD8 cells could have contaminated our CD4 cell preparations. Because leukemia-free survival was only 20% (Figure 3A) with 250 000 CD8 cells, we think it is unlikely that 18 000 contaminating CD8 cells were responsible for survival. Thus, CD4 cells can promote GVL through multiple mechanisms, possibly including cytokines or macrophage activation, in addition to direct cytotoxicity.
Purified CD8+ T cells alone were also effective, which demonstrates that CD8-mediated GVL is helper T-cell independent as is GVHD in the C3H.SW→B6 strain pairing.79 Because Faslpr and TNFR-/- mCP-CMLs were equally susceptible to CD8-mediated GVL, neither pathway by itself is essential for target cell death. Perforin and FasL are principle effector mechanisms of CD8+ CTLs; because Fas-mediated killing is not required, it is likely that perforin/granzyme-mediated killing alone is sufficient. Alternatively, or in addition, TRAIL could be playing a role given recent data showing TRAIL-mediated alloreactive CTL killing of leukemic cell lines in vivo.60 We hope to address the role of TRAIL in future experiments. FasL and perforin are also thought to be the principle effector mechanisms for CD4 CTLs.57,58,65,66 That CD4-mediated GVL was intact against Faslpr mCP-CML demonstrates that FasL-mediated killing is not required and that the perforin/granzyme pathway is likely to be sufficient. As with CD8-mediated GVL, TRAIL may also play a role in CD4-mediated GVL, and this too needs to be examined in the future.
Both FasL and TNF-α are important pathogenic mechanisms in murine GVHD models,42,43,86-90 and anti-TNF-α therapy already has a role in treating human GVHD. Our results suggest that blockade of FasL/Fas interactions is not likely to affect adversely GVL against CP-CML and may provide a means to deliver GVL with reduced GVHD. TNF-α blockade is similarly unlikely to impair CTL effector function; however, because TNF-α promotes T-cell activation, TNF-α blockade may still weaken GVL.
In summary, our results suggest that CP-CML sensitivity is at least in part explained by the multiple effector mechanisms sufficient for GVL. GVL against mCP-CML could be mediated by either CD4 or CD8 cells and was independent of Fas or TNFR expression, and CD4 cells could kill without directly contacting mCP-CML targets. Thus, GVL might still be effective even if multiple potential effector mechanisms fail because of either properties of the immune response itself (eg, no CD4 alloimmunity) or the absence of major apoptotic pathways in targets. However, unlike CP-CML, some leukemias such as BC-CML are generically GVL resistant, even in the face of GVHD. Alloreactive T cells could be ineffective for multiple reasons. They may fail to traffic to sites of disease, which could play a role in central nervous system relapses. However, GVL sensitive and resistant leukemias are found in blood, bone marrow, and spleen, sites to which T cells normally have access. Loss of a critical immunodominant miHA is unlikely to explain resistance either. We found effective GVL in 3 different MHC identical strain pairings in which the immunodominant antigens are likely to be different; thus, there is a high degree of plasticity in the T-cell response. However, we found that a single mutation—loss of MHC II expression—resulted in substantial GVL resistance of an otherwise GVL-sensitive neoplasm. Thus, genes that affect sensitivity to killing, like MHC II, could be responsible for de novo resistance to GVL or might be major targets for mutations that render leukemias resistant to GVL. In future studies, by way of generation of additional gene-deficient mCP-CMLs, we plan to investigate these and other potential mechanisms of GVL resistance. We hope that these studies will identify pathways that, if augmented, will overcome GVL resistance and could identify rational ways to maximize GVL without undue GVHD.
Prepublished online as Blood First Edition Paper, February 24, 2004; DOI 10.1182/blood-2003-10-3735.
Supported by grants (R01 HL66279, R01-CA-96943, K08-HL03979) (W.D.S).
C.C.M. and J.C. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.