Abstract
The antibody-targeted therapeutic, gemtuzumab ozogamicin (GO, Mylotarg), is approved for treatment of relapsed acute myeloid leukemia (AML). We previously showed that AML blasts from GO refractory patients frequently express the drug transporters P-glycoprotein (Pgp) and/or multidrug resistance protein (MRP). We also previously reported that inhibition of drug transport by the Pgp modulator, cyclosporine A (CSA), can increase GO sensitivity in Pgp+ AML cells and that the peripheral benzodiazepine receptor ligand, PK11195, sensitizes AML cells to standard chemotherapeutics both by inhibiting Pgp-mediated efflux and by promoting mitochondrial apoptosis. We now show that PK11195 also can overcome multiple resistance mechanisms to increase GO sensitivity in AML cells, including resistance associated with expression of drug transporters and/or antiapoptotic proteins. PK11195 substantially increases GO cytotoxicity in AML cells from many different cell lines and primary patient samples, often more effectively than CSA. We also show that PK11195 is nontoxic in NOD/SCID mice and can sensitize xenografted human AML cells to GO. Since PK11195 is well tolerated in humans as a single agent, its further study as a multifunctional chemosensitizer for anti-AML therapies, including GO-based therapies, is warranted. (Blood. 2004;103:4276-4284)
Introduction
Chemoresistance remains the major hurdle in curing acute myeloid leukemia (AML). Although most adult de novo AML patients obtain complete remission with conventional induction chemotherapies, the majority of responding patients relapse and ultimately die with treatment-refractory disease.1 Multidrug resistance (MDR) in AML classically involves drug efflux by permeability-glycoprotein (Pgp) and multidrug resistance protein 1 (MRP1) of the adenosine triphosphate (ATP)-binding cassette (ABC) superfamily, but it also involves apoptosis blockade by Bcl-2 and/or Bcl-xL proteins, for example.2-6 Expression of these chemoresistance mechanisms is associated with poor clinical outcomes in AML.2,3,5-10
Recently, gemtuzumab ozogamicin (GO; CMA-676, Mylotarg), a humanized anti-CD33 antibody conjugated to a calicheamicin toxin, was shown to induce remission in 26% of adults with CD33+, relapsed AML,11,12 and is now approved for treatment of relapsed AML. AML blasts from GO-refractory patients frequently show Pgp expression and drug efflux capacity, and Pgp+ AML blasts can be sensitized in vitro to GO by cyclosporine A (CSA),13 an established Pgp inhibitor.14-16 CSA also weakly inhibits MRP-mediated drug transport,17-19 but the MRP-selective inhibitor MK-571 less frequently increases GO-induced cytotoxicity in AML samples.20 In addition, neither CSA nor MK-571, nor CSA plus MK-571, enhance GO-induced cytotoxicity in about 30% of Pgp+ and/or MRP+ patient cell samples,20 suggesting that alternative mechanisms also contribute to GO resistance. Recently, a pilot study showed that Bcl-2 antisense oligonucleotides increase GO-induced cytotoxicity in HL-60 cells, suggesting that antiapoptotic proteins can limit GO sensitivity in CD33+ AML cells.21 Thus, mechanisms that protect AML cells from killing by standard chemotherapeutics apparently also protect AML cells from GO cytotoxicity.
CSA can improve clinical outcomes in Pgp+ AML patients treated with conventional chemotherapy,14 documenting the potential value of MDR reversal agents in antitumor regimens. However, CSA can cause significant toxicities that limit survival benefits.14 Proapoptotic agents, including antisense oligonucleotides,22-24 also are being clinically tested with standard anti-AML regimens, but suffer practical limitations, including stability and expense. Moreover, AMLs commonly express more than one chemoresistance mediator, especially at relapse.25 Therefore, a well-tolerated chemosensitizing agent that inhibits more than one resistance mechanism might afford therapeutic advantages, particularly when toxicities are limited by specifically targeting anti-cancer drug doses, as in GO-based anti-AML therapies.
Our recent in vitro studies suggest that the peripheral benzodiazepine receptor (pBzR) ligand, PK11195, is such an agent.26 pBzRs reside in multiprotein mitochondrial pore complexes that regulate mitochondrial membrane potential and thereby modulate apoptosis.27-29 Bcl-2 and related antiapoptotic proteins colocalize in these complexes and block apoptosis by keeping pores “closed,” while pBzR ligands promote “open” pore states and advance apoptosis even in cells expressing antiapoptotic proteins.30-37 Consistent with these findings, PK11195 antagonizes the cytoprotective effects of Bcl-2 and Bcl-xL and thereby sensitizes lymphoma and lymphocytic leukemia cell lines to various cytotoxic agents.30,31,34 In fact, PK11195 can promote mitochondrial pore-opening in purified mitochondria where high levels of Bcl-2 otherwise mediate the retention of apoptogenic factors, suggesting that pBzR ligands directly impact mitochondrial pore function rather than affecting the expression of Bcl-2 family members.31 In addition to this proapoptotic activity, PK11195 also potently reduces Pgp-mediated efflux by an as-yet-unidentified mechanism.26 Therefore, PK11195 sensitizes AML cell lines and AML patient samples to a range of standard chemotherapeutic agents, including the Pgp substrate drug daunorubicin (DNR) and the nonsubstrate drug cytarabine (ARAC). Importantly, PK11195 is well tolerated as a single agent and has good bioavailability after oral ingestion in healthy individuals.38,39
We now have investigated whether AML cells can be sensitized to GO by PK11195 and in which cases PK11195 might sensitize AMLs to GO more effectively than does CSA. To address these questions, we first confirmed that Pgp and MRP transporter expression associates with GO resistance in AML cells and asked whether Bcl-2 and Bcl-xL expression also associates with GO resistance in AML. Second, we asked whether PK11195 might increase GO sensitivity in AML cells expressing drug transporters and/or antiapoptotic proteins. Finally, we tested PK11195's ability to sensitize human AML cells to GO in a mouse xenograft model. Our positive findings suggest that PK11195 warrants further study as a clinical chemosensitizer in GO-based anti-AML regimens, and more generally, in therapies directed at cancers in which antiapoptotic protein and drug efflux protein expression is associated with refractory disease.
Materials and methods
Human cell lines
HL-60, HL-60/AR,40 HL-60/VCR,40 NB4, and TF1 cells were cultured as described.20 The Bcl-2-transfected HL-60 subline (HL-60/Bcl-2) and the Bcl-xL-transfected HL-60 subline (HL-60/Bcl-xL) were kindly provided by K. N. Bhalla (University of South Florida, Tampa, FL) and cultured in the presence of geneticin (1 mg/mL; Gibco Invitrogen, Carlsbad, CA), which was removed at least 2 days prior to each experiment, as previously described.40 ML-1 cells were maintained in Iscove modified Dulbecco medium (IMDM, Gibco) with 10% heat-inactivated fetal bovine serum (HyClone, Logan, UT).
Patient AML blast cell samples
Marrow samples were taken from adult patients in untreated first relapse of AML who participated in the phase 2 clinical trials with GO, as previously described.20 For acquisition of clinical samples, all patients signed informed consent, and the institutional review boards of the participating institutions approved all protocols.
Flow cytometry assays of protein expression
Flow cytometry was used to measure the expression of CD33, drug transporters, and antiapoptotic proteins.20,41 Monoclonal antibodies included L4F342 and P67.6 (Becton Dickinson, San Jose, CA) to recognize CD33; 4E3.16 for Pgp (provided by R. Arceci, John Hopkins University, Baltimore, MD); MRPm6 for MRP1 (Kamiya Biomedical, Seattle, WA); and Clone 124 for Bcl-2 (Dako; Carpinteria, CA). In addition, an unlabeled rabbit polyclonal anti-Bcl-xL serum (Santa Cruz Biotechnology, Santa Cruz, CA) was used. Appropriate monoclonal isotype-matched antibodies or unlabeled rabbit IgG were used as controls. MRP2 expression was not analyzed, as we previously showed that differential MRP2 expression is not relevant to variable GO sensitivity.20 At least 10 000 events were acquired; leukemic cells in primary cell samples were identified in light scatter plots,41 and propidium iodide (PI; Sigma; St Louis, MO)-negative cells (cell line samples) or CD33+/PI- cells (primary AML blast samples) were analyzed on a FACScan flow cytometer using Cellquest (Becton Dickinson) or MultiPlus (Phoenix Flow Systems, San Diego, CA) software. Expression levels were calculated by subtracting the mean fluorescence intensity of cells stained with the relevant isotype control antibody from the mean fluorescence intensity of cells stained with a particular specific antibody.
Determination of functional Pgp and MRP activity
Pgp function was measured as efflux of 3,3′-diethyloxacarbocyanine iodide (DiOC2(3), Aldrich, St Louis, MO) that was reduced by CSA (Novartis Pharma; Basel, Switzerland; Sigma). MRP function was measured as efflux of 5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate (CDCF; Molecular Probes, Eugene, OR) that was reduced by MK-571 (Biomol, Plymouth Meeting, PA).20 Results are expressed as the ratio of the mean fluorescence intensity in the presence of the inhibitor divided by the mean fluorescence intensity in the absence of the inhibitor, referred to as inhibitor-modulating factor.43 Of note, the inhibitor-modulating factor of HL-60/AR cells likely underestimated the true MRP activity because these cells did not load efficiently with CDCF.
Determination of pBzR expression/PK11195 binding
Cells were washed twice and resuspended in phosphate-buffered saline (PBS)/2% bovine calf serum (BCS, HyClone). Aliquoted in triplicate in round bottom 96-well plates were 2 × 105 cells/well (CoStar, Corning, NY). Competitors (10 μM) were added and allowed to prebind to cells for 30 minutes at 4°C with continuous rotation. 3 H-PK11195 (PerkinElmer, Torrance, CA) in PBS/2% BCS was added to cells and competitors for a final concentration of 10 nM. The plate was incubated for 2 hours at 4°C under continuous rotation. Cells were harvested onto 96-well Unifilter plates (Packard Bioscience, Meriden, CT) using PBS and then allowed to dry overnight before adding Microscint 0 (Packard Bioscience) to each well. The amount of radioactivity bound to filter plates was counted via TopCount (Packard Bioscience).
Assays for GO-induced cytotoxicity
For experiments with cell lines, 5 × 104 cells/well were taken during the log-phase of growth, distributed into 96-well round bottom plates (BD Biosciences, San Diego, CA), and incubated with various concentrations of GO. After 2-hour incubation at 37°C, cells were washed and resuspended in fresh medium without drug. Parallel cultures were incubated with GO (Wyeth-Ayerst, Radnor, PA) in the presence of various concentrations of PK11195 (Sigma), 2.5 μg/mL CSA, or 10 μM MK-571 prior to washing and resuspension in fresh medium containing the inhibitors without GO. Experiments with human blast cell samples were performed as described previously.20 Drug-induced cytotoxicity was determined by flow cytometry after 3 days of culture, based on our preliminary studies and observations by others44 that apoptotic changes are minimal after 24- to 48-hour exposures to GO but are significantly increased after 72- to 96-hour exposures. We previously showed that 75 μM PK11195 was not cytotoxic to AML cells during 24- to 48-hour exposures,26 and 75 μM PK11195 did not significantly increase dead TF1 cell fractions during 72-hour exposure. However, PI+ cell fractions did increase in other AML cell lines during 72-hour exposures to 75 μM PK11195, while 50 μM PK11195 was not cytotoxic during this longer period (data not shown). Therefore, 50 μM PK11195 was used in most assays. An annexin V (BD Biosciences)/PI apoptosis assay was used for primary AML cell assays,20 and a DiOC6(3)/PI staining assay was used for AML cell line assays.45 “Live” cell fractions are defined as annexin V-/PI- (patient AML samples) or high DiOC6(3)/low PI (AML cell lines). Treatment-specific live cell fractions were calculated by subtracting untreated live cell fractions from treated live cell fractions.
Assays for doxorubicin (DOX)- and ARA-C-induced cytotoxicity
Distributed into 96-well round bottom plates were 8 × 104 cells/well in 240 μL culture medium with various concentrations of DOX or cytosine β-D-arabinofuranoside (ARA-C, both from Sigma). Parallel cultures were incubated with cytotoxic drugs in the presence of various concentrations of PK11195, 2.5 μg/mL CSA, or 10 μM MK-571. Drug-induced cytotoxicity was determined after 24 hours using the DiOC6(3)/PI staining assay.
Western blot analyses
Protein extracts were prepared by cell lysis in 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (pH 7.2) containing 1% Triton X, 10% glycerol, 100 mM NaCl, 1 mM phenylmethylsulphonyl fluoride, 25 μg/mL leupeptin, and 10 μg/mL aprotinin, and protein concentrations were determined by DC Protein Assay (BioRad Laboratories, Hercules, CA). Boiled were 30 μg lysate in 0.06 M Tris (tris(hydroxymethyl)aminomethane)-HCl (pH 6.8), 2% sodium dodecyl sulfate (SDS), 10% glycerol, 2.5% β-mercaptoethanol, and 0.01% bromophenol blue, which were then loaded onto 12% SDS-polyacrylamide gels, electrophoresed, and transferred to a polyvinylidene difluoride membrane (BioRad) with a Mini Trans-Blot cell (BioRad) using a transfer buffer of 25 mM Tris, 192 mM glycine, and 20% methanol. Membranes were blocked overnight with 5% milk in TBST (25 mM Tris [pH 7.5], 150 mM NaCl, 0.5% Tween-20), washed with TBST, and incubated for 1 hour in TBST with 3% bovine serum albumin (BSA; Sigma) containing antibody mouse anti-Bcl-2 (Dako), antiactin (Santa Cruz Biotechnology), rabbit anti-Bcl-xL (BD Biosciences), and/or anti-Bax (Upstate, Charlottesville, VA). After washing, blots were incubated for 30 minutes with horseradish peroxidase conjugated secondary antibody (Amersham, Arlington Heights, IL) in TBST/BSA, washed, and immunological complexes visualized using enhanced chemiluminescence (Amersham).
Mouse model of human AML
NOD/SCID β2M mice were bred at the Fred Hutchinson Cancer Research Center and maintained in microisolator cages with full technical and veterinary support. After preconditioning with sublethal irradiation (350R) from a LINAC source (Linear accelerator [Clinac 4/100; Varian, Palo Alto, CA]), 3.5 × 107 HL-60 cells were subscapularly inoculated. Tumor-bearing mice were randomized to treatment groups 10 days later. GO was administered intraperitoneally in 300 μL PBS on days +11, +15, and +19, at 25 μg calicheamicin/kg = 27 μg GO/mouse, which reduced tumor growth by approximately 80% by day +28 in our previous study.46 We were unable to identify a maximally tolerated PK11195 dose, since pBzR ligands have very low aqueous solubility. PK11195 is soluble at 4 mg/mL in PBS containing 15% ethanol and 1.2% Tween 80, and repeated subcutaneous injections are well tolerated in NOD/SCID mice (data not shown), as previously shown for another pBzR ligand, RO5-4764, in SCID mice.47 GO was administered 2 hours after mice did or did not receive PK11195 injections at 1.2 mg/mouse in subscapular regions opposite sites of engraftment. Tumors were measured in 3 dimensions with calipers 3 times per week after first drug dosing. A second cycle of drug dosing was administered on day +31, +35, and +39. Mice were euthanized by halothane inhalation and cervical dislocation at day +45 or sooner if animals showed substantial weight loss, labored breathing, diarrhea, and/or righting difficulty.
Statistical analysis
Results are presented as mean ± SD from several independent experiments with single measurements for antibody staining ratios, inhibitor modulating factors, and data on apoptosis/necrosis in patient samples. For data on apoptosis and cell viability in cell lines, results are presented as mean ± SEM obtained from independent experiments performed in duplicate or triplicate wells. Continuous variables between groups were compared using a 2-tailed unpaired Student t test (Excel; Microsoft, Seattle, WA); a P value of less than .05 was considered significant.
Results
PK11195 can reduce GO resistance in AML cells
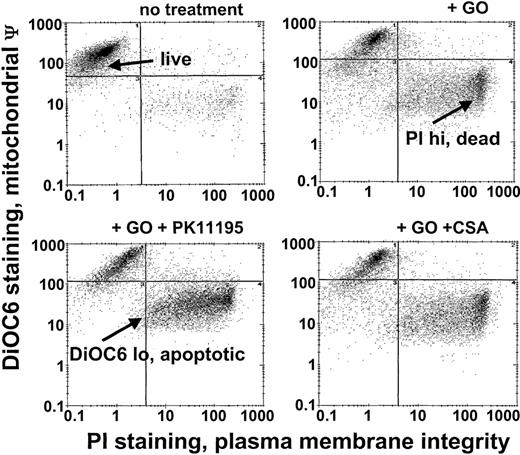
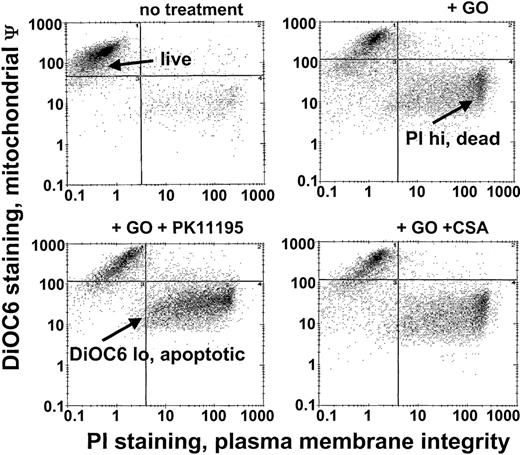
Since Pgp and MRP expression are frequently associated with low GO sensitivity in AML samples20 and PK11195 can sensitize Pgp+ and MRP+ cells to other therapeutic agents,30,31,34 we predicted that PK11195 would increase GO sensitivity in Pgp- and MRP-expressing AML cells. To begin testing this idea, we used different AML cell lines that we had previously characterized for Pgp and MRP expression and GO sensitivity.20 Based on these characteristics and demonstrations of specific PK11195 binding (Table 1), we analyzed PK11195 effects on GO cytotoxicity in Pgp+/MRP+ TF1 cells, Pgp-/MRP+ HL-60 cells, Pgp-/weakly MRP+ NB4 cells, and Pgp-/MRP- ML-1 cells, using multiparameter flow cytometry assays in which apoptotic and dead cells were distinguished from “live” cells by reduced DiOC6(3) staining (mitochondrial membrane potential) and increased PI staining (plasma membrane integrity) (Figure 1).
GO cytotoxicity can be measured in multiparameter flow cytometry assays. After drug treatments, TF1 cells were exposed to the potentiometric dye DiOC6(3) and the DNA dye propidium iodide (PI) that were used to identify apoptotic cells with reduced mitochondrial membrane potential (low DiOC6(3) staining) and dead cells with absent plasma membrane integrity (high PI staining), respectively, so that live (not apoptotic, not dead) cell fractions could be assessed. The GO-sensitizing activity of PK11195 was measured as further decreased live cell fractions in GO plus PK11195 cotreated cells. ψ indicates membrane potential.
GO cytotoxicity can be measured in multiparameter flow cytometry assays. After drug treatments, TF1 cells were exposed to the potentiometric dye DiOC6(3) and the DNA dye propidium iodide (PI) that were used to identify apoptotic cells with reduced mitochondrial membrane potential (low DiOC6(3) staining) and dead cells with absent plasma membrane integrity (high PI staining), respectively, so that live (not apoptotic, not dead) cell fractions could be assessed. The GO-sensitizing activity of PK11195 was measured as further decreased live cell fractions in GO plus PK11195 cotreated cells. ψ indicates membrane potential.
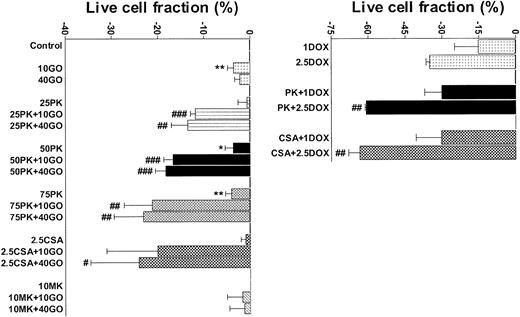
Pgp+/MRP+ TF1 cells were efflux competent and GO insensitive (Table 1; Figure 2), as we previously showed.20 As we previously showed for 24- to 48-hour assays for cells of other AML cell lines,26 25 μM, 50 μM, and 75 μM PK11195 minimally affected TF1 cells when used alone in 72-hour assays. Also consistent with our previous findings,20 a maximal nontoxic dose (2.5 μg/mL) of the Pgp-selective modulator CSA substantially blocked DiOC2(3) dye efflux and significantly increased the cytotoxicity of GO in TF1 cells. Like CSA, 75 μM PK11195 blocked DiOC2(3) dye efflux (data not shown) and increased TF1 cell killing by 1 and 2.5 μM DOX (Figure 2); 75 μM PK11195 also increased killing by 10 ng/mL GO (Figure 2; Table 1). The MRP modulator MK-571 blocked MRP-mediated CDCF dye efflux but did not increase GO cytotoxicity in TF1 cells (Figure 2; Table 1); 50 μM PK11195 and 25 μM PK11195 also significantly increased GO cytotoxicity, but to smaller degrees (Figure 2), documenting the dose dependence of PK11195's GO-sensitizing activity. In addition, we found that PK11195 additions less effectively enhanced GO cytotoxicity in TF1 cells when PK11195 was added 24 or 48 hours after GO exposure and were no more effective when added 8 or 24 hours before GO (n = 2; data not shown). These data suggest that PK11195 does not promote apoptosis by reducing antiapoptotic protein expression, for example. Rather, our data suggest that PK11195 must be present when apoptosis is initiated in order to enhance apoptosis and are consistent with published data showing that PK11195 amplifies the early apoptosis-inducing effects of cytotoxic agents by directly impacting mitochondrial pore function.31
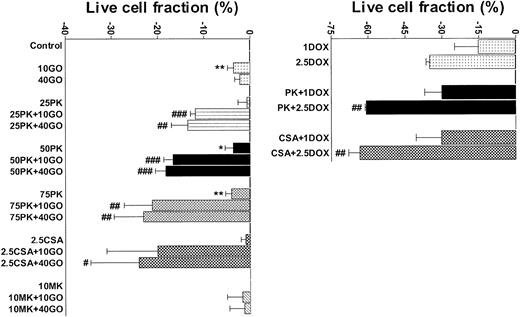
PK11195 significantly increases DOX and GO cytotoxicity in Pgp+- and MRP+- coexpressing AML cells. The cytotoxicities of DOX (1 μM and 2.5 μM) and GO (10 ng/mL and 40 ng/mL) were measured in TF1 AML cells and the treated live cell fractions displayed relative to untreated live cell fractions. Minimally toxic doses of both PK11195 (75 μM) and CSA (2.5 μg/mL) significantly increased DOX and GO cytotoxicity. *P < .05, **P < .01 compared to untreated cells; #P < .05, ##P < .01, ###P < .0001 compared to cells treated with DOX or GO alone. Data are shown as mean ± SEM from up to 5 independent experiments performed in duplicate or triplicate wells.
PK11195 significantly increases DOX and GO cytotoxicity in Pgp+- and MRP+- coexpressing AML cells. The cytotoxicities of DOX (1 μM and 2.5 μM) and GO (10 ng/mL and 40 ng/mL) were measured in TF1 AML cells and the treated live cell fractions displayed relative to untreated live cell fractions. Minimally toxic doses of both PK11195 (75 μM) and CSA (2.5 μg/mL) significantly increased DOX and GO cytotoxicity. *P < .05, **P < .01 compared to untreated cells; #P < .05, ##P < .01, ###P < .0001 compared to cells treated with DOX or GO alone. Data are shown as mean ± SEM from up to 5 independent experiments performed in duplicate or triplicate wells.
Although we previously reported that 75 μM PK11195 was not cytotoxic to AML cells during 24- to 48-hour exposures26 and 72-hour treatments with 75 μM PK11195 were minimally cytotoxic to TF1 cells, we found that 75 μM PK11195 treatments reduced the viable cell fraction in several AML cell lines by as much as 15%-20% during 72-hour assays, whereas 50 μM PK11195 was minimally cytotoxic in these cell lines. Therefore, 50 μM PK11195 was used in subsequent PK11195 plus GO combination treatments to begin testing how generally PK11195 increases GO cytotoxicity in AML cells. Efflux-deficient ML-1 cells were considerably more GO sensitive than TF-1 cells (Table 1). In fact, ML-1 cells were nearly as sensitive to 0.25 ng/mL GO as 10 ng/mL GO (-36% ± 2% vs -42% ± 1% reduced live, respectively), and PK11195, CSA, and MK-571 were unable to significantly increase GO cytotoxicity during 72-hour assay periods (Table 1). These data suggest that cytotoxicity was maximally induced in ML-1 cells by even the lowest tested GO dose such that chemosensitizing could not be measured.
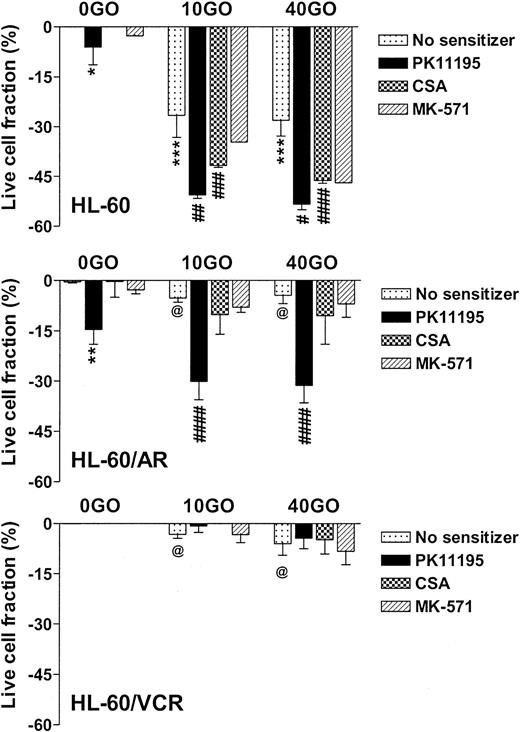
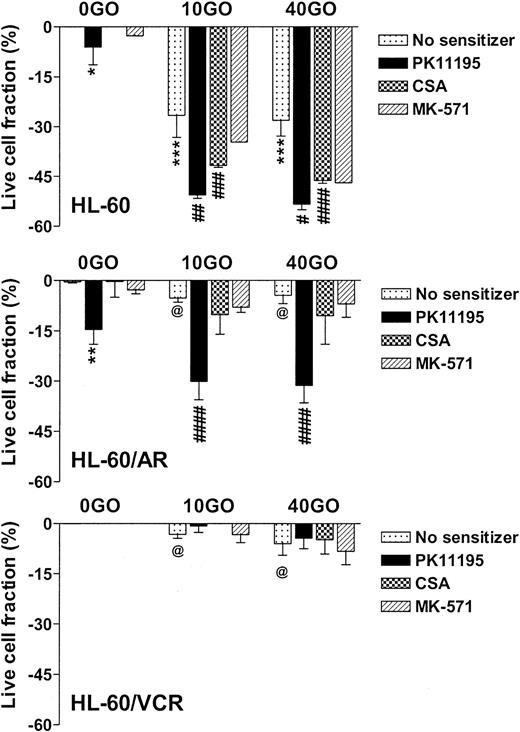
NB4 cells showed intermediate GO sensitivity, as compared with TF-1 and ML-1 cells, in association with intermediate MRP function (Table 1). Both PK11195 and CSA significantly increased GO cytotoxicity in NB4 cells. As in TF1 cells, MK-571 did not sensitize NB4 cells to GO cytotoxicity but did measurably block CDCF dye efflux. HL-60 cells showed even higher levels of MK-571-blocked dye efflux but were more sensitive to GO than NB4 cells (Table 1; Figure 3). PK11195 and CSA significantly sensitized HL-60 cells to GO, although CSA consistently sensitized HL-60 cells to a smaller degree than did PK11195 (Figure 3). To further test the ability of PK11195 to increase GO sensitivity in MRP+ cells, we assayed HL-60/AR cells in which we confirmed high-level functional MRP expression (Table 2) that was induced during repeated courses of selection in adriamycin.40 HL-60/AR cells were significantly resistant to GO relative to parental HL-60 cells and showed somewhat higher PK11195 sensitivity than HL-60 cells. PK11195 and GO consistently produced supra-additive cytotoxicity in HL-60/AR cells so that live cell fractions were more significantly reduced after GO plus PK11195 combination treatments than after treatments with GO or PK11195 alone, whereas CSA and MK-571 were ineffective GO-sensitizers in HL-60/AR cells (Table 2; Figure 3).
PK11195 significantly increases GO cytotoxicity in HL-60-derivative cells relatively overexpressing MRP or Pgp. The cytotoxicity of 40 ng/mL GO was measured in HL-60 AML cells, in HL-60/AR cells that relatively overexpress MRP due to repeated exposures to adriamycin, and in HL-60/VCR cells that relatively overexpress Pgp due to repeated exposures to vincristine. The treated live fractions are displayed relative to untreated live cell fractions, as in Figure 2. PK11195 alone (50 μM) measurably reduced live cell fractions but also supra-additively and significantly increased GO cytotoxicity in HL-60/AR cells, relative to treatments with GO alone or PK11195 alone. Neither 10 μM MK-571 nor 2.5 μg/mL CSA significantly increased GO cytotoxicity. Neither 50 μM PK11195 nor 2.5 μg/mL CSA nor 10 μM MK-571 increased GO cytotoxicity in HL-60/VCR cells, although both PK11195 and CSA increased DOX cytotoxicity in HL-60/VCR cells (data not shown). *P < .05, **P < .01, ***P < .0001 compared to untreated cells; #P < .05, ##P < .01, ###P < .001, ####P < .0001 compared to cells treated with GO alone; @P < .0001 compared to HL-60 cells treated with GO at the corresponding dose. Data are shown as mean ± SEM from up to 5 independent experiments performed in duplicate or triplicate wells.
PK11195 significantly increases GO cytotoxicity in HL-60-derivative cells relatively overexpressing MRP or Pgp. The cytotoxicity of 40 ng/mL GO was measured in HL-60 AML cells, in HL-60/AR cells that relatively overexpress MRP due to repeated exposures to adriamycin, and in HL-60/VCR cells that relatively overexpress Pgp due to repeated exposures to vincristine. The treated live fractions are displayed relative to untreated live cell fractions, as in Figure 2. PK11195 alone (50 μM) measurably reduced live cell fractions but also supra-additively and significantly increased GO cytotoxicity in HL-60/AR cells, relative to treatments with GO alone or PK11195 alone. Neither 10 μM MK-571 nor 2.5 μg/mL CSA significantly increased GO cytotoxicity. Neither 50 μM PK11195 nor 2.5 μg/mL CSA nor 10 μM MK-571 increased GO cytotoxicity in HL-60/VCR cells, although both PK11195 and CSA increased DOX cytotoxicity in HL-60/VCR cells (data not shown). *P < .05, **P < .01, ***P < .0001 compared to untreated cells; #P < .05, ##P < .01, ###P < .001, ####P < .0001 compared to cells treated with GO alone; @P < .0001 compared to HL-60 cells treated with GO at the corresponding dose. Data are shown as mean ± SEM from up to 5 independent experiments performed in duplicate or triplicate wells.
Pgp-overexpressing HL-60/VCR cells were similarly derived from HL-60 cells by repeated courses of selection in vincristine40 and were also GO resistant relative to parental HL-60 cells (Table 2). Like CSA, PK11195 substantially blocked Pgp-associated DiOC6(3) dye efflux (Table 2; data not shown) and significantly increased the cytotoxicity of DOX in HL-60/VCR cells (eg, P = .008 with CSA and P = .003 with PK11195 added to 1 μM DOX). However, neither CSA nor PK11195 increased GO cytotoxicity in these highly Pgp+ cells, when added as single agents (Figure 3), or when HL-60/VCR cells were exposed to GO with both CSA and PK11195 added (n = 2; data not shown). Levels of CD33 immunostaining and radioactive PK11195 binding were similar in HL-60, HL-60/AR, and HL-60/VCR cells, suggesting that lower GO cytotoxicity in HL-60/VCR cells was not due to lower GO binding and that PK11195 insensitivity was not due to lower PK11195 binding (Table 2).Together, the AML cell line data demonstrate that PK11195-insensitive (and CSA-insensitive) GO resistance can develop in AML cells but that PK11195 can sensitize highly Pgp-expressing (TF1) and variably MRP-expressing (TF1, NB4, HL-60, HL-60/AR) AML cells to GO cytotoxicity and can sensitize particular AML cells to GO cytotoxicity more effectively than CSA, as we previously showed for DNR cytotoxicity in Pgp- and MRP+ AML cells.26
Enforced Bcl-2 and Bcl-xL expression is associated with GO resistance that is reversed by PK11195
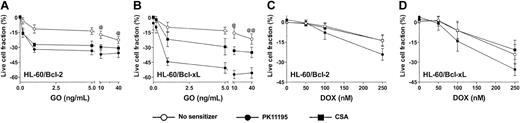
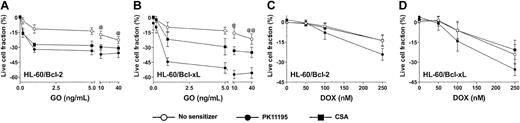
Since antisense Bcl-2 oligonucleotides reduced Bcl-2 expression and enhanced apoptosis in GO-treated HL-60 cells21 and PK11195 has well-established drug-sensitizing activity in cells expressing antiapoptotic proteins,30,31,34 we hypothesized that PK11195 would increase GO sensitivity in AML cells expressing antiapoptotic proteins. To begin testing this idea, we first used HL-60/Bcl-2 and HL-60/Bcl-xL cells40 in which we confirmed stable overexpression of Bcl-2 and Bcl-xL, respectively, using Western blot assays (Figure 4) and confirmed relative DOX insensitivity relative to parental HL-60 cells (data not shown). Both HL-60/Bcl-2 and HL-60/Bcl-xL cells were significantly resistant to GO relative to parental HL-60 cells (Figure 4). PK11195 and CSA both increased GO cytotoxicity in HL-60/Bcl-2 and HL-60/Bcl-xL cells, as in parental MRP+ HL-60 cells, but PK11195 sensitized HL-60/Bcl-xL cells to GO significantly more effectively than CSA (Figure 4). By comparison, CSA was ineffective in increasing DOX-induced cytotoxicity in HL-60/Bcl-2 and HL-60/Bcl-xL cells, whereas PK11195 significantly increased DOX-induced cytotoxicity in HL-60/Bcl-2 cells and tended to increase DOX-induced cytotoxicity in HL-60/Bcl-xL cells (Figure 4). Of note, neither PK11195 nor CSA reduced Bcl-2 or Bcl-xL protein expression in these cells (data not shown), consistent with previous reports suggesting that pBzR ligands interfere with the antiapoptotic activities of these mitochondrial proteins rather than reducing their expression.31
Bcl-2 or Bcl-xL overexpression is associated with relative GO resistance in HL-60-derivative cells, and PK11195 significantly increases GO cytotoxicity in these AML cells. Relative Bcl-2 and Bcl-xL overexpression in HL-60/Bcl-2 and HL-60/Bcl-xL cells was confirmed in Western blot analyses, in which we also found that NB4 and HL-60/AR cells relatively overexpress Bcl-2, TF1 cells relatively overexpress Bcl-xL, and ML-1 cells relatively overexpress proapoptotic Bax. The cytotoxicity of 0.2-40 ng/mL GO and 50-250 nM DOX was measured in HL-60/Bcl-2 and HL-60/Bcl-xL cells, and treated live cell fractions are displayed relative to untreated live cell fractions. While parental HL-60 cells were maximally killed by 10 ng/mL GO (Figure 3), statistically higher fractions of HL-60/Bcl-2 or HL-60/Bcl-xL cells survived at 10 and 40 ng/mL GO (@P < .01, @@P < .001), compared to HL-60 cells treated with GO at the same doses (HL-60 data presented in Figure 3). PK11195 (50 μM) and CSA (2.5 μg/mL) were minimally cytotoxic in HL-60/Bcl-2 and HL-60/Bcl-xL cells and significantly (P < .01) increased cytotoxicity of GO at all doses tested in these cells. PK11195 significantly increased GO cytotoxicity (eg, P < .001 with 10 ng/mL GO) in HL-60/Bcl-xL cells to a greater degree than did CSA (eg, P < .01 with 10 ng/mL GO). CSA was ineffective in increasing DOX-induced cytotoxicity in HL-60/Bcl-2 and HL-60/Bcl-xL cells, whereas PK11195 significantly increased DOX-induced cytotoxicity in HL-60/Bcl-2 cells (P < .05) and tended to increase DOX-induced cytotoxicity in HL-60/Bcl-xL cells (P = .07). Data are shown as mean ± SEM from up to 3 independent experiments performed in duplicate or triplicate wells.
Bcl-2 or Bcl-xL overexpression is associated with relative GO resistance in HL-60-derivative cells, and PK11195 significantly increases GO cytotoxicity in these AML cells. Relative Bcl-2 and Bcl-xL overexpression in HL-60/Bcl-2 and HL-60/Bcl-xL cells was confirmed in Western blot analyses, in which we also found that NB4 and HL-60/AR cells relatively overexpress Bcl-2, TF1 cells relatively overexpress Bcl-xL, and ML-1 cells relatively overexpress proapoptotic Bax. The cytotoxicity of 0.2-40 ng/mL GO and 50-250 nM DOX was measured in HL-60/Bcl-2 and HL-60/Bcl-xL cells, and treated live cell fractions are displayed relative to untreated live cell fractions. While parental HL-60 cells were maximally killed by 10 ng/mL GO (Figure 3), statistically higher fractions of HL-60/Bcl-2 or HL-60/Bcl-xL cells survived at 10 and 40 ng/mL GO (@P < .01, @@P < .001), compared to HL-60 cells treated with GO at the same doses (HL-60 data presented in Figure 3). PK11195 (50 μM) and CSA (2.5 μg/mL) were minimally cytotoxic in HL-60/Bcl-2 and HL-60/Bcl-xL cells and significantly (P < .01) increased cytotoxicity of GO at all doses tested in these cells. PK11195 significantly increased GO cytotoxicity (eg, P < .001 with 10 ng/mL GO) in HL-60/Bcl-xL cells to a greater degree than did CSA (eg, P < .01 with 10 ng/mL GO). CSA was ineffective in increasing DOX-induced cytotoxicity in HL-60/Bcl-2 and HL-60/Bcl-xL cells, whereas PK11195 significantly increased DOX-induced cytotoxicity in HL-60/Bcl-2 cells (P < .05) and tended to increase DOX-induced cytotoxicity in HL-60/Bcl-xL cells (P = .07). Data are shown as mean ± SEM from up to 3 independent experiments performed in duplicate or triplicate wells.
To further address the impact of antiapoptotic protein expression on the ability of PK11195 to sensitize AML cells to GO, we analyzed Bcl-2 and Bcl-xL protein expression in Western blot analyses of the AML cell lines in which we had already measured PK11195 activity in GO cytotoxicity assays. We found that MRP-expressing NB4 and HL-60/AR cells also relatively overexpressed Bcl-2, while Pgp-expressing TF1 cells also relatively overexpressed Bcl-xL (Figure 4). Since PK11195 sensitized TF1, HL-60/AR, and NB4 cells to GO cytotoxicity, these data support the idea that PK11195 can overcome GO resistance, even in cells that express drug efflux and antiapoptotic chemoresistance mechanisms. Of note, the proapoptotic protein, Bax, was expressed in all AML cells analyzed, with highest expression in ML-1 cells that were also most highly GO sensitive.
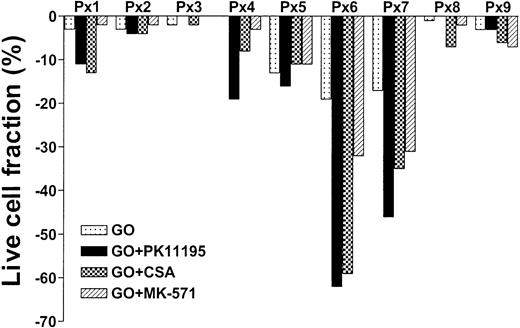
PK11195 increases GO sensitivity in primary AML cell samples, often more effectively than CSA or MK-571
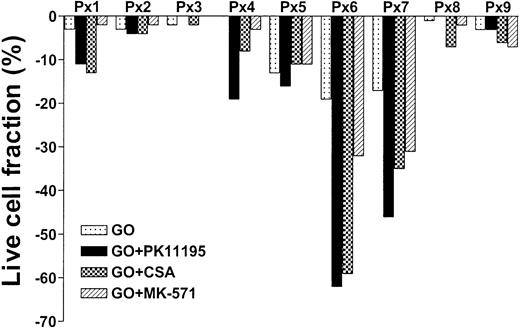
To test whether PK11195 can increase GO cytotoxicity in primary AML cells, we compared the in vitro effects of PK11195, CSA, and MK-571 on GO cytotoxicity in 9 bone marrow samples that were collected from AML patients prior to the administration of GO therapy.20 We also measured Pgp and MRP expression and function and CD33 and pBzR expression, as in cell line analyses, and measured Bcl-2 and Bcl-xL expression in flow cytometry assays. We found that live cell fractions were reduced as much as 19% when AML blast populations were exposed to 10 ng/mL GO alone (Table 3; Figure 5). PK11195 increased GO-induced cytotoxicity in 6 (66%) of 9 samples, was more than supra-additive with GO in 5 samples (55%: sample nos. 1, 4, 5, 6, 7), and more effective than CSA or MK-571 in 4 of these (44%), including highly Pgp+ (no. 5), highly MRP+ (no. 7), Bcl-2 overexpressing (no. 6) samples, and sample no. 4. Thus, PK11195 can effectively incapacitate both drug efflux and antiapoptotic resistance mechanisms and sensitize more effectively than established MDR modulators in primary AML cells.
PK11195 can increase GO cytotoxicity in primary AML cell samples. Nine primary cell samples from AML patients who had not yet received GO therapy were exposed to 10 ng/mL GO with or without 50 μM PK11195, 2.5 μg/mL CSA, or 20 μM MK-571. The treated live cell fractions are displayed relative to untreated live cell fractions, as in Figure 2. PK11195 specifically increased GO-induced cytotoxicity in 6 samples, was more than supra-additive with GO in 5 samples, and was more effective than CSA or MK-571 in 4 of these samples. Each primary cell sample was exposed to each treatment condition in triplicate wells.
PK11195 can increase GO cytotoxicity in primary AML cell samples. Nine primary cell samples from AML patients who had not yet received GO therapy were exposed to 10 ng/mL GO with or without 50 μM PK11195, 2.5 μg/mL CSA, or 20 μM MK-571. The treated live cell fractions are displayed relative to untreated live cell fractions, as in Figure 2. PK11195 specifically increased GO-induced cytotoxicity in 6 samples, was more than supra-additive with GO in 5 samples, and was more effective than CSA or MK-571 in 4 of these samples. Each primary cell sample was exposed to each treatment condition in triplicate wells.
PK11195 improves human AML tumor regression in NOD/SCID mice treated with subcurative GO doses
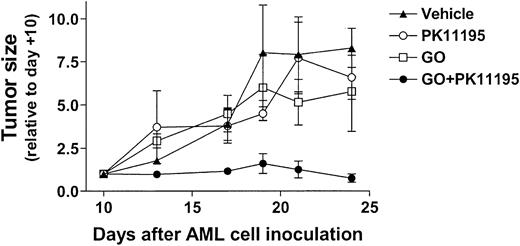
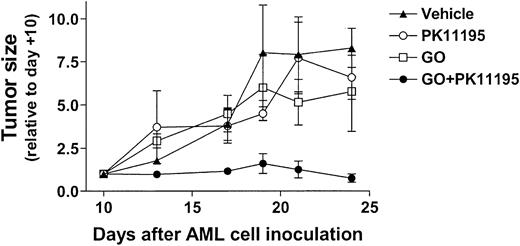
Based on our in vitro findings, we hypothesized that PK11195 would sensitize AML cells to GO in vivo. To begin testing this idea, 30 NOD/SCID β2M mice were inoculated with HL-60 cells. Subcutaneous nodules were palpable in 29 mice by 10 days after inoculation (day +10), and 6 tumor-bearing mice were randomly assigned to each treatment group: (1) vehicle (day +10 tumors = 84 ± 42 mm3), (2) PK11195 only (66 ± 15 mm3), (3) GO only (56 ± 12 mm3), or (4) GO+PK11195 (116 ± 36 mm3). Subcurative GO doses were administered on days 11, 15, and 19, based on our previous experience with GO in nude mice.46 At -2 hours relative to GO administrations, we subcutaneously delivered 4 mg/mL PK11195 in a vehicle that was previously used in SCID mice to document the tumor chemosensitizing activity of another pBzR ligand, RO5-4764, with ifosfamide or etoposide administrations.47 In our study, one mouse each in the vehicle treatment, GO treatment, and combination treatment groups died by the end of dosing and were censored from further analyses, leaving at least 5 mice in each treatment group. Tumor measurements were made 3 times per week and are presented relative to day +10 tumor measurements.
As shown in Figure 6, vehicle-treated HL-60 tumors substantially increased in size across the 24-day experimental period, becoming more than 7-fold larger on day +24 relative to day +10. GO treatments reduced tumor growth relative to vehicle controls, to approximately 5-fold larger than on day +10, but this difference was not significant. PK11195 alone had little effect on HL-60 tumor growth, but PK11195 plus GO combination treatments substantially reduced HL-60 tumor growth to an average of 0.6 ± 0.1-fold relative to day +10 tumors. In fact, tumor growth was significantly less in mice treated with PK11195 plus GO than in vehicle controls on days +13 (P < .01), +17 (P < .05), +21 (P < .05), and +24 (P < .01), and significantly less in mice treated with PK11195 plus GO than in GO-treated mice on days +13 (P < .01), +17 (P < .05), +21 (P < .05) (Figure 6). Thus, PK11195 GO-sensitizing effects can be measured in vivo.
PK11195 cotreatments significantly increase the AML growth-inhibiting effects of subcurative GO doses in NOD/SCID mice bearing HL-60 xenografts. HL-60 tumors substantially increased in size across the 24-day experimental period, and GO treatments reduced tumor growth relative to vehicle controls, but this effect was not significant. PK11195 alone had little effect on HL-60 tumor growth, but tumor growth was significantly less in mice treated with PK11195 plus GO than in vehicle controls on days +13 (P < .01), +17 (P < .05), +21 (P < .05), and +24 (P < .01), and significantly less than in GO-treated mice on days +13 (P < .01), +17 (P < .05), and +21 (P < .05), as denoted. Data are shown as means ± SEM for 5 to 6 mice per treatment group.
PK11195 cotreatments significantly increase the AML growth-inhibiting effects of subcurative GO doses in NOD/SCID mice bearing HL-60 xenografts. HL-60 tumors substantially increased in size across the 24-day experimental period, and GO treatments reduced tumor growth relative to vehicle controls, but this effect was not significant. PK11195 alone had little effect on HL-60 tumor growth, but tumor growth was significantly less in mice treated with PK11195 plus GO than in vehicle controls on days +13 (P < .01), +17 (P < .05), +21 (P < .05), and +24 (P < .01), and significantly less than in GO-treated mice on days +13 (P < .01), +17 (P < .05), and +21 (P < .05), as denoted. Data are shown as means ± SEM for 5 to 6 mice per treatment group.
Discussion
The data presented in this report support 3 general conclusions. First, we confirmed that functional Pgp and MRP expression associate with GO resistance in AML and showed that cotreatments with the pBzR ligand, PK11195, can increase GO sensitivity in AML cells expressing these drug transporters. Second, we found that Bcl-2 and Bcl-xL overexpression also associate with relative GO resistance in AML and showed that PK11195 can increase GO cytotoxicity in AML cells expressing these antiapoptotic proteins and in AML cells expressing both antiapoptotic proteins and drug transporters. Finally, we found that PK11195 can safely increase the antileukemia activity of GO in a mouse model. Together, these data support further investigations of PK11195 as a clinical chemosensitizer.
We previously reported that blasts from GO-refractoryAML patients frequently express functional ABC transporters and that the Pgp modulator, CSA, and the MRP modulator, MK-571, can increase GO cytotoxicity in some of these AML samples.13,20 We confirmed these findings in our current study. LikeABC transporter expression, antiapoptotic protein expression associates in AML with in vitro and clinical resistance to standard chemotherapeutics,2,3,5-10 and Bcl-2 antisense oligonucleotides can increase GO-induced cytotoxicity in HL-60 cells,21 suggesting that antiapoptotic proteins may also limit GO efficacy. In a direct test of this possibility, we found that Bcl-2 and Bcl-xL overexpression is indeed associated with GO-resistance in HL-60 cells that stably overexpress these antiapoptotic proteins. In analyses of other AML cell lines and primary patient samples, we found that Bcl-2 and Bcl-xL also can be relatively overexpressed in GO-resistant AML cells that coexpress functional drug transporters. In contrast, highly GO-sensitive MDR- ML-1 cells express high levels of Bax, a proapoptotic Bcl-2 relative that has been associated in AML with high sensitivity to standard chemotherapeutics.48,49 A recent report shows that free calicheamicin induces apoptosis in a Bax-dependent manner.50 Thus, apoptosis-regulating proteins of the Bcl-2 family can apparently regulate GO sensitivity in AML, and effective GO-sensitizing agents may need to overcome more than one mechanism of GO resistance, including apoptosis blockade and drug efflux.
We previously showed that CSA can increase GO cytotoxicity in certain MDR+ AML cells and suggested that CSA might be used to improve clinical outcomes for patients treated with GO-based therapies.13 However, CSA can produce dose-limiting toxicities in AML patients and is most likely to benefit only AML patients whose leukemic blasts express functional Pgp.14 We have now shown that PK11195 can increase GO sensitivity in AML cell lines and primary cells from AML patients, including AML cells overexpressing Pgp, MRP, Bcl-2, and/or Bcl-xL. PK11195 and CSA apparently can increase GO cytotoxicity to similar degrees in certain AML cells, as in TF1, NB4, and HL-60/Bcl-2 cells, as well as in primary cells from one AML patient. However, PK11195 apparently can sensitize other AMLs to GO more effectively than CSA or MK-571, as in HL-60/AR and HL-60/Bcl-xL cells and in primary AML cells from 4 different patients. In our study of AML cell lines and primary AML cell samples, CSA never GO sensitized AML cells to a significantly greater degree than did PK11195. These findings are consistent with our previous demonstration that PK11195 increases the cytotoxicity of the efflux substrate drug DOX and increases the cytotoxicity of the nonsubstrate drug ARA-C more frequently in primary AML cell samples than does CSA.26 Together, our data strongly support the idea that PK11195 might be a particularly effective clinical chemosensitizer because it can overcome more than one chemoresistance mechanism, even when they coexist in tumor cells.
Nonetheless, PK11195-insensitive mechanisms of GO resistance apparently exist. As an example, neither CSA nor PK11195, nor CSA in combination with PK11195, substantially increases GO cytotoxicity in HL-60/VCR cells, although both CSA and PK11195 reduce efflux and increase DOX cytotoxicity in these highly Pgp+ cells. Reduced CD33 expression would reduce GO binding and contribute to GO resistance that PK11195 might not reduce, but CD33-specific immunostaining is similar in GO-resistant HL-60/VCR cells and parental HL-60 cells and variable CD33 expression was not associated with clinical GO responses in phase 2 trials.11 Specific PK11195 binding varied more than 500-fold in our analyses of AML cell lines and primary AML cell samples, and lower pBzR expression might reduce PK11195's chemosensitizing activity, but our data show that relative levels of PK11195 binding also are not directly correlated with PK11195's drug-sensitizing or efflux-blocking activities. Perhaps levels of drug efflux that remain after CSA or PK11195 exposures protect AML cells from GO more efficiently than from DOX. Ongoing analyses are aimed at defining cellular parameters that associate with PK11195 chemosensitizing efficacy, as these might be used to identify AML patients who would most benefit from PK11195 addition to standard and/or targeted anti-AML regimens. However, our findings reported here and in our previous publication26 suggest that PK11195 could improve clinical responses for many AML patients treated with various therapeutic regimens, including patients in whom CSA would be less effective.
Finally, we have shown in preliminary AML xenograft analyses that PK11195 can be safely administered to mice and can enhance GO-mediated AML tumor regression. HL-60 cells engrafted NOD/SCID mice with high efficiency, and tumor growth in mice that received only PK11195 was statistically indistinguishable from tumor growth in vehicle-treated mice. GO dosing that we previously showed was subcurative in nude mice46 also did not significantly reduce HL-60 tumor growth in NOD/SCID mice. However, PK11195 plus GO cotreatments significantly decreased tumor growth relative to vehicle controls and relative to tumors treated with GO as a single agent. Although more extensive investigations are required to elucidate the most effective dose and schedule with which to administer PK11195 in GO regimens—and to begin characterizing the range of AMLs that can be sensitized to GO by PK11195 in animal models—our data provide evidence that PK11195 can improve GO antileukemia efficacy in vivo.
In summary, we have shown that antiapoptotic protein expression as well as MDR protein expression is associated with reduced GO sensitivity in AML cells and that additional resistance mechanisms also may play roles in GO-refractory leukemias. The pBzR ligand, PK11195, frequently increases GO sensitivity in AML cell lines and primary AML cell samples and is often more effective than the established Pgp modulator, CSA. PK11195 also can enhance GO's anti-AML activity in a mouse model without significant toxicity. Given that PK11195 cotreatments did not produce any additional toxicities in our study and that PK11195 has been safely administered to patients as a single agent and has good bioavailability,38,39 our data support additional preclinical and clinical studies pursuing PK11195 as a chemosensitizer in GO-based and other anti-AML therapies.
Prepublished online as Blood First Edition Paper, February 12, 2004; DOI 10.1182/blood-2003-11-3825.
Supported in part by research funding from the Leukemia and Lymphoma Society (#7040-03) and the National Institutes of Health (#CA92316, #CA89491, #P30-DK56465). R.B.W. is a recipient of a fellowship from the Swiss Foundation for Medical-Biological Grants (no. 1098). I.D.B. is a recipient of an FM Kirby/American Cancer Society Clinical Research Professorship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to acknowledge the excellent technical assistance of Cyd Nourigat and other technical staff in the NOD/SCID core facility at the Fred Hutchinson Cancer Research Center, a National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-supported “Core Center of Excellence in Molecular Hematology.”