Abstract
Adaptation to hypoxia is critical for survival and regulates multiple processes, including erythropoiesis and vasculogenesis. Chuvash polycythemia is a hypoxia-sensing disorder characterized by homozygous mutation (598C>T) of von Hippel-Lindau gene (VHL), a negative regulator of hypoxia sensing. Although endemic to the Chuvash population of Russia, this mutation occurs worldwide and originates from a single ancient event. That VHL 598C>T homozygosity causes elevated normoxic levels of the transcription factor hypoxia inducible factor-1α (HIF-1α), serum erythropoietin and hemoglobin is known, but the disease phenotype has not been documented in a controlled manner. In this matched cohort study, VHL 598C>T homozygosity was associated with vertebral hemangiomas, varicose veins, lower blood pressures, and elevated serum vascular endothelial growth factor (VEGF) concentrations (P < .0005), as well as premature mortality related to cerebral vascular events and peripheral thrombosis. Spinocerebellar hemangioblastomas, renal carcinomas, and pheochromocytomas typical of classical VHL syndrome were not found, suggesting that overexpression of HIF-1α and VEGF is not sufficient for tumorigenesis. Although hemoglobin-adjusted serum erythropoietin concentrations were approximately 10-fold higher in VHL 598C>T homozygotes than in controls, erythropoietin response to hypoxia was identical. Thus, Chuvash polycythemia is a distinct VHL syndrome manifested by thrombosis, vascular abnormalities, and intact hypoxic regulation despite increased basal expression of hypoxia-regulated genes.
Introduction
Chuvash polycythemia, the first hereditary condition of augmented hypoxia sensing to be recognized, is an autosomal recessive disorder with increased serum erythropoietin levels and hemoglobin concentrations in normoxia.1-5 Although congenital polycythemias are rare worldwide, hundreds of patients with Chuvash polycythemia are found in the Chuvash population of central Russia. We recently identified the mutation for Chuvash polycythemia to be 598C>T in the von Hippel-Lindau gene (VHL) on chromosome 3p25.4 This mutation was subsequently found in persons of European, African-American, and Pakistani/Bangladeshi ethnicities.6-8 Haplotype analysis indicates that the mutation originated from a single ancient event.9
VHL protein, the recognition component of an E3 ubiquitin-protein ligase complex, mediates proteosomal degradation of the α subunit of hypoxia inducible factor 1 (HIF-1) under normoxic conditions, whereas HIF-1α is stable during hypoxia.10 HIF-1 is the principal transcriptional activator in hypoxic cells, promoting or enhancing transcription of erythropoietin and a number of other genes.11 Patients with Chuvash polycythemia have increased cellular levels of HIF-1α during normoxia, and this accounts for the increased concentrations of serum erythropoietin and of hemoglobin.5
In classic VHL tumor predisposition syndrome, heterozygous germ line mutations of VHL are associated with the development of renal clear cell carcinoma, pheochromocytoma, pancreatic neuroendocrine tumors, central nervous system hemangioblastoma, and other vascular tumors. Tumors develop from cells that acquire a somatic mutation of the unaffected VHL gene in addition to the germ line mutation on the other allele. VHL codes for 213 amino acids, and more than 130 distinct intragenic germ line mutations associated with tumor predisposition have been identified, virtually all 5′ to the 598 position mutated in Chuvash polycythemia.12,13 Polycythemia is not a common manifestation of classic VHL syndrome, although increased hemoglobin concentrations can sometimes occur because of tumor production of erythropoietin.
Chuvash polycythemia differs from variants of VHL syndrome described thus far by the autosomal recessive rather than dominant pattern of inheritance, the relatively mild effect of the 598C>T mutation on the association of VHL with HIF-1α, and polycythemia as the presenting clinical manifestation.5 The natural history of Chuvash polycythemia has not been documented in a controlled study, and whether, as with other VHL mutations, there is an increased incidence of tumors is not known. The objective of this matched cohort study of patients with Chuvash polycythemia was to confirm our uncontrolled observation of premature mortality1-3 and to characterize the clinical effects of this inherited hypoxia-sensing defect.
Patients, materials, and methods
Study participants
We conducted a retrospective study of mortality and a cross-sectional study of morbidity from 2001 to 2003 in Chuvashia, Russia, with patients diagnosed to have Chuvash polycythemia before 1977 by Dr Lydia A. Polyakova, one of the authors of this report.1,2 The median age at diagnosis of Chuvash polycythemia was 16 years (interquartile range, 6-22 years), and patients typically presented with plethora, headache, and fatigue.1,2 We were able to visit or obtain information regarding 96 of 103 original patients. Two additional cohorts were studied: 65 spouses and 94 community members matched for age, sex, and village of birth who were identified through the regional Zapis Actov Grazhdanskovo Sostoyaniya (ZAGS; Registry of Citizen Status Events), a bureau that maintains records of births, deaths, and marriages. The Investigational Review Board of Howard University approved the investigations. Information was obtained from the research participant and/or medical records, death certificates, relatives, and neighbors. We recorded demographic and clinical characteristics and causes of death. If the participant was living and available, we obtained informed consent, performed a medical history and physical examination, and collected a venous blood sample.
Blood samples
Complete blood counts were performed with an automated method, and genomic DNA was isolated from frozen Buffy coats within 7 months of collection. Serum concentrations of erythropoietin were performed by enzyme-linked immunosorbent assay (ELISA) on an Immulite instrument (Diagnostic Products, Randolph, NJ), of vascular endothelial growth factor-165 (VEGF-165) by an enzyme immunometric assay (Assay Designs, Ann Arbor, MI), of total plasminogen activator inhibitor-1 (PAI-1) by ELISA (HYPEN BioMed, Andrésy, France), and of transferrin receptor and ferritin by ELISA (Ramco Laboratories, Stafford, TX). Serum concentrations of the stable nitric oxide breakdown products, nitrite and nitrate, were assayed on the basis of the conversion of nitrate to nitrite by the enzyme nitrate reductase (Assay Designs). Expected plasma ranges provided by the manufacturers are 5 to 35 IU/L for erythropoietin, 4 to 43 ng/mL for PAI-1, “around 26 pg/mL” for VEGF, 2.9 to 8.3 mg/L for transferrin receptor, and 20 to 300 μg/L for ferritin. Expected ranges for nitrite and nitrate were not provided.
PCR analysis for the VHL 598C>T mutation
Genomic DNA was isolated using a QIAGEN column (QIAGEN, Valencia, CA), and polymerase chain reactions (PCRs) were performed in 50-μL volumes containing 20 mM Tris (tris(hydroxymethyl)aminomethane)–HCl pH 8.4, 50 mM KCl, 1.5 mM MgCl2, 100 μM deoxynucleotide triphosphate (dNTP), 300 nM primers, and 2.5 U/reaction Taq DNA polymerase (Life Technologies, Grand Island, NY). The following primers were used for amplification of VHL exon 3: VHL3F, 5′-CCTTGTACTGAGACCCTAG, VHL3R, 5′-GCTGAGATGAAACAGTGTA. PCR product (10 μL) was incubated with 5 U Fnu4HI (New England Biolabs, Beverly, MA) for 2 hours to detect the mutation. The VHL 598C>T mutation abolishes restriction sites for Fnu4HI, resulting in an uncut 296–base pair (bp) band detected on 1.2% agarose gel.
Imaging and ophthalmologic studies in Chuvash polycythemia patients and control patients
Computed tomography (CT) of the abdomen and magnetic resonance imaging (MRI) of the spine were performed in 33 patients with Chuvash polycythemia (32 of them were documented to be VHL 598C>T homozygotes and 1 was not tested) and 34 Chuvash patients without the diagnosis of Chuvash polycythemia. These patients without the diagnosis of polycythemia were in addition to the matched cohorts described in “Study participants.” MRI of the brain and ophthalmologic studies were performed in 33 patients with Chuvash polycythemia but not the patients without the diagnosis of Chuvash polycythemia. CT was performed through the abdomen with 5-mm sections using helical mode. After obtaining preliminary nonenhanced scans, approximately 120 mL nonionic iodinated contrast agent was injected, and arterial and venous phase images were obtained. Routine clinical imaging of the brain and cervical and thoracic spine (including the entire spinal cord) was performed on a 1.5 T MRI system (GE Medical Systems, Milwaukee, WI) using T2 weighting and T1 weighting before and after intravenous injection of a Gadolinium chelate contrast agent. Indirect ophthalmoscopy and fluorescein angioscopy were performed by an ophthalmologist in Cheboksary, Russia.
Statistical analysis
Mortality was assessed with Kaplan-Meier analysis, the log-rank test, and Cox proportional hazards models. Clinical and laboratory measurements that were continuous variables were examined with linear regression, Spearman correlation, or analysis of variance models that adjusted for important covariates. Variables that followed a skewed distribution were log transformed. Because serum concentrations of PAI-1 and VEGF are influenced by release by platelets,14,15 we adjusted for peripheral blood platelet counts in the analysis of variance models. Proportions were examined with the Pearson chi-square test or the Fisher exact test. Logistic regression was used for certain statistical analyses.
Results
Demographic features of the patients with Chuvash polycythemia and the matched cohorts
There were approximately equal numbers of men and women among the patients with Chuvash polycythemia (Table 1), supporting the validity of using spouses as a matched cohort in addition to matched community members. More patients with Chuvash polycythemia died during the follow-up period encompassed by this study than spouses and community members. Histories of smoking and of diabetes mellitus were not overrepresented among the patients with Chuvash polycythemia. Not shown in Table 1 are observations made in surviving participants that we examined (43 patients with Chuvash polycythemia, 46 spouses, and 49 community members), suggesting that obesity and hypertension were not overrepresented in the polycythemia cohort. Obesity, defined as body mass index 30 kg/m2 or more, was found in 2.4% of surviving patients with Chuvash polycythemia compared with 13.3% of spouses and 12.2% of community members. Elevated blood pressure, defined as systolic pressure greater than 140 mm Hg and/or diastolic pressure greater than 90 mm Hg, was found in 17.1% of surviving patients with Chuvash polycythemia compared with 47.8% of spouses and 22.9% of matched community members.
Treatment of patients with Chuvash polycythemia
Of the 96 patients with Chuvash polycythemia (Table 1), we were able to obtain information on treatment for 87 (91%). Seventy-eight percent of these patients had been treated with some degree of phlebotomy. The median age of starting phlebotomy therapy was 23 years (range, 3-48 years). The median intensity of phlebotomy therapy was 1.4 phlebotomies per year (range, <1-5 per year). The median volume of blood removed per phlebotomy was 500 mL (range, 250-750 mL).
Among patients from whom we drew blood samples as part of the present study, the median hemoglobin concentration was 15.2 g/dL (152 g/L) (range, 9.9-23.8 g/dL [99-238 g/L]) in 8 participants who averaged 2 or more phlebotomies per year compared with 18.0 g/dL (180 g/L) (range, 12.7-25.4 g/dL [127-254 g/L]) in 34 participants who averaged less than 2 phlebotomies per year (P = .2). The median hemoglobin concentration was 19.0 g/dL (190 g/L) (range, 13.9-25.4 g/dL [139-254 g/L]) in 11 patients who gave a history of thrombosis (Table 2), and it was 16.8 g/dL (168 g/L) (range, 9.9-23.8 g/dL [99-238 g/L]) in 32 participants who did not give a history of thrombosis (P = .076). The median hemoglobin concentration was 18.0 g/dL (180 g/L) (range, 12.7-23.8 g/dL [127-238 g/L]) in 19 patients who had MRI or CT scan evidence of thrombosis (Table 3), and it was 16.6 g/dL (166 g/L) (range, 9.9-21.6 g/dL [99-216 g/L]) in 13 participants who did not have evidence of thrombosis (P = .2).
Forms of treatment other than phlebotomy included aspirin in 39% of the patients and busulfan in 17%. In a logistic regression model that examined the relationship of thrombosis history to a history of treatment with aspirin and whether the patient had averaged at least 2 phlebotomies per year, aspirin was associated with a 2.4-fold increase in the odds of thrombosis (95% confidence interval [CI], 0.7-7.7; P = .15) and phlebotomy with a 5.6-fold decrease (95% CI, 0.7-47.6; P = .12). Busulfan had been administered between the years of 1959 and 1971, before it was realized that Chuvash polycythemia was a distinct condition from polycythemia vera. The median age at the time of treatment with busulfan was 20 years (range, 12-46 years). One patient was treated with radioactive phosphorous in addition to busulfan.
Mortality
Cerebral vascular events were especially common as a cause of death among patients with Chuvash polycythemia (Table 1). Almost half of the cerebral vascular events in patients with Chuvash polycythemia were described as hemorrhagic, but this characterization may not have been confirmed radiographically or anatomically. The median age at death from a cerebral vascular event was 42 years (range, 26-70 years) in 11 patients with Chuvash polycythemia and 70 years (range, 58-81 years) in 3 control participants. Four of the patients but none of the spouse and community cohort members died of mesenteric thrombosis, and the median age of death was 49 years (range, 27-59 years).
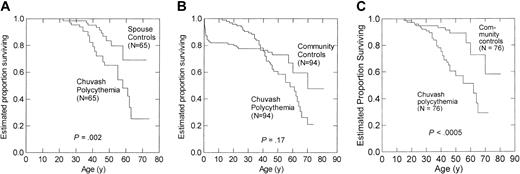
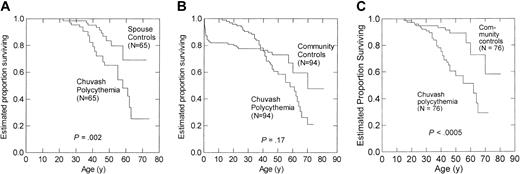
Among 65 patient–spouse pairs, estimated survival to age 65 years was 25% for patients with Chuvash polycythemia versus 69% for spouses (P = .002) (Figure 1A). Among all 94 patient–community member pairs, estimated survival to age 65 years was 31% for patients with Chuvash polycythemia versus 54% for community members (P = .17) (Figure 1B). Death from pneumonia, measles, and varicella was 16.0% by age 3 years in community members, reflecting high infant and childhood mortality in Chuvashia in the mid-20th century, and this high early mortality in community members appeared to bias the analysis in the 94 patient–community member pairs. Therefore, we also analyzed mortality for 76 patient–community member pairs in which the community member survived to at least age 16 years, the median age of diagnosis of Chuvash polycythemia, and found that estimated survival to age 65 years was 29% for patients with Chuvash polycythemia versus 64% for community members (P < .0005) (Figure 1C). After adjustments for histories of alcohol use and smoking, the hazards ratio of death for Chuvash polycythemia patients was 4.3 (95% CI, 2.0-9.3) in the 65 patient–spouse pairs and 3.8 (95%, 1.8-7.8) in the 76 patient–community member pairs. The adjusted hazards ratio of death from cerebral vascular events for patients with Chuvash polycythemia was 15.6 (95% CI, 1.5-160.9; P = .021) in the patient–spouse pairs and 17.3 (95% CI, 1.3-239.6; P = .033) in the patient–community member pairs. The hazards ratio of death from peripheral thrombosis was also significantly higher for the patients with Chuvash polycythemia in the analysis of the 65 patient–spouse pairs (P = .038) but not the patient–community member pairs. The hazards ratio for other causes of death (Table 1) did not reach statistical significance. In particular, we found no evidence of increased deaths from malignancy among the patients with Chuvash polycythemia, although some patients had received treatment with the alkylating agent, busulfan, an agent with leukemia- and other cancer-inducing properties.16
Kaplan-Meier survival curves for Chuvash polycythemia patients and spouses or community members matched for age, sex and place of birth. (A) Sixty-five patients with Chuvash polycythemia and 65 spouses. (B) Ninety-four patients with Chuvash polycythemia and 94 matched community members. There was high mortality for the community members in the first 3 years of life, whereas the median age of diagnosis of Chuvash polycythemia was 16 years. (C) Seventy-six patients with Chuvash polycythemia and 76 matched community members who survived to age 16 years.
Kaplan-Meier survival curves for Chuvash polycythemia patients and spouses or community members matched for age, sex and place of birth. (A) Sixty-five patients with Chuvash polycythemia and 65 spouses. (B) Ninety-four patients with Chuvash polycythemia and 94 matched community members. There was high mortality for the community members in the first 3 years of life, whereas the median age of diagnosis of Chuvash polycythemia was 16 years. (C) Seventy-six patients with Chuvash polycythemia and 76 matched community members who survived to age 16 years.
Among the 96 patients with Chuvash polycythemia, univariate analyses indicated that male sex was associated with a 5.3-fold increase in the hazards ratio of death (P < .0005), history of smoking with a 2.3-fold increase (P = .013), history of alcohol consumption with a 2.5-fold increase (P = .010), and receiving some degree of phlebotomy therapy with a 1.6-fold decrease (P = .2). In a multivariate analysis that included all of these variables, histories of smoking and alcohol consumption no longer had a significant influence on the hazards ratio of death (P > .6), but male sex was associated with a 4.6-fold increase in the ratio (95% CI, 1.6-12.9; P = .004) and history of phlebotomy therapy with a 1.7-fold decrease (95% CI, 0.8-3.7; P = .16). In similar multivariate analyses, only male sex had a trend of increasing the hazards ratio of death from cerebral vascular events (P = .16) and from peripheral thrombosis (P = .001).
Clinical findings in the living research participants according to VHL genotype
PCR analysis for the VHL 598C>T mutation revealed a perfect genotype-phenotype correlation for clinically diagnosed Chuvash polycythemia, with all 43 patients and none of 86 spouses or community members genotyped, proving to be homozygotes for the mutation (Table 2). Seven spouses (16%) and 2 community members (5%) were heterozygotes. Forty-one (95%) of the VHL 598C>T homozygotes gave a history of phlebotomy therapy, often for cosmetic reasons, with a frequency ranging from 0.3 to 5 phlebotomies per year and a median of 1.1 per year. In addition to higher hemoglobin concentrations, homozygotes for VHL 598C>T had more frequent thromboses by history, lower systolic and diastolic blood pressures, more venous varicosities, and lower white blood cell and platelet counts compared with unaffected research participants (Table 2; P < .0005). Although there were no significant differences between unaffected participants and the small number of VHL 598C>T heterozygotes for many of these clinical observations, adjusted systolic and diastolic blood pressures were significantly lower in the heterozygotes than in the unaffected participants and paralleled the findings for the homozygotes. We found no significant differences by genotype in the reported history of cancer, which was low, and in adjusted serum ferritin concentrations. Among the VHL 598C>T homozygotes, we did not observe significant differences in the variables listed in Table 2 according to whether the participants were treated with a phlebotomy intensity averaging 2 or more per year.
Circulating concentrations of proteins or metabolites of proteins encoded by HIF-1–regulated genes
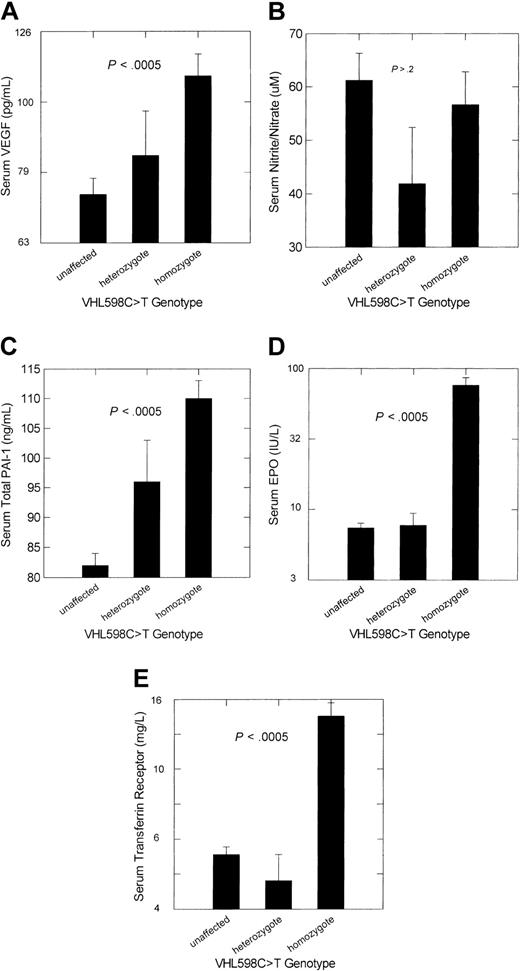
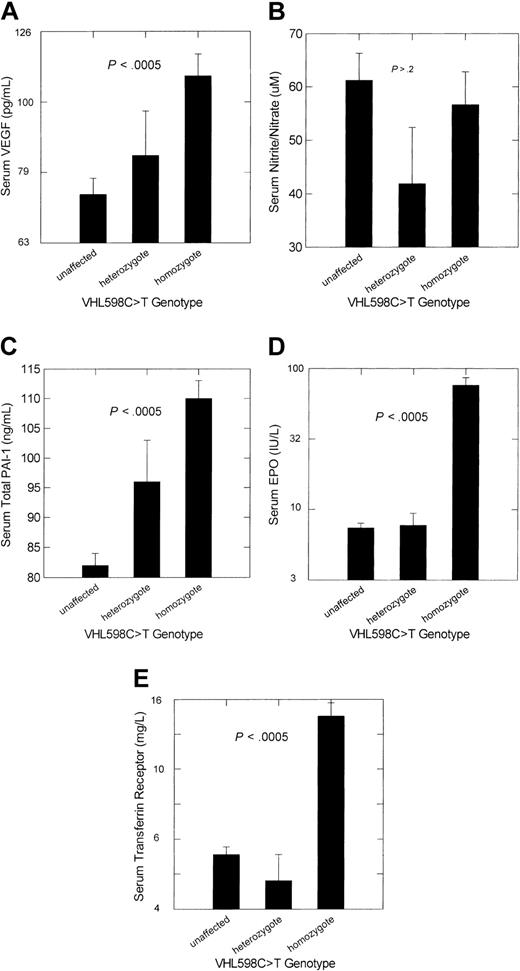
Adjusted mean serum concentrations of VEGF and total PAI-1 were significantly higher in VHL 598C>T homozygotes than in unaffected participants (P < .0005), but concentrations of the end products of nitric oxide breakdown, nitrite/nitrate, were not (Figure 2). Consistent with our previous studies,3-5 adjusted serum levels of erythropoietin and transferrin receptor were also significantly higher in homozygotes. Only adjusted serum total PAI-1 levels differed significantly between VHL 598C>T heterozygotes and unaffected participants (P = .032).
Adjusted mean ± SE concentrations of the products or metabolites of selected HIF-1–regulated genes according to VHL 598C>T genotype in persons of Chuvash ethnicity. (A) Serum VEGF analysis of variance (ANOVA) model with adjustment for peripheral blood platelet count. (B) Serum nitrite/nitrate, stable end products of nitric oxide breakdown. (C) Serum PAI-1 ANOVA model with adjustment for peripheral blood platelet count. (D) Serum erythropoietin (EPO) ANOVA model with adjustment for sex and hemoglobin concentration. (E) Serum transferrin receptor ANOVA model with adjustment for serum ferritin concentration. The numbers of subjects for each genotype category are as indicated in Table 2. The P value represents the significance level for the overall ANOVA and also for the comparison of unaffected participants with VHL 598C>T homozygotes. Only for PAI-1 was there a significant difference between unaffected participants and VHL 598C>T heterozygotes, and this was at the .03 significance level.
Adjusted mean ± SE concentrations of the products or metabolites of selected HIF-1–regulated genes according to VHL 598C>T genotype in persons of Chuvash ethnicity. (A) Serum VEGF analysis of variance (ANOVA) model with adjustment for peripheral blood platelet count. (B) Serum nitrite/nitrate, stable end products of nitric oxide breakdown. (C) Serum PAI-1 ANOVA model with adjustment for peripheral blood platelet count. (D) Serum erythropoietin (EPO) ANOVA model with adjustment for sex and hemoglobin concentration. (E) Serum transferrin receptor ANOVA model with adjustment for serum ferritin concentration. The numbers of subjects for each genotype category are as indicated in Table 2. The P value represents the significance level for the overall ANOVA and also for the comparison of unaffected participants with VHL 598C>T homozygotes. Only for PAI-1 was there a significant difference between unaffected participants and VHL 598C>T heterozygotes, and this was at the .03 significance level.
In 41 VHL 598C>T homozygotes, Spearman correlation revealed significant inverse correlations of serum erythropoietin concentration with number of months after last phlebotomy (R = –0.295, P < .05) and hemoglobin concentration (R =–0.519, P < .005). In a linear regression analysis that examined the relationship of serum erythropoietin concentration with both hemoglobin concentration and phlebotomy history in the same model, the significant negative correlation persisted with hemoglobin concentration (P = .001) but not with time since last phlebotomy (P = .5).
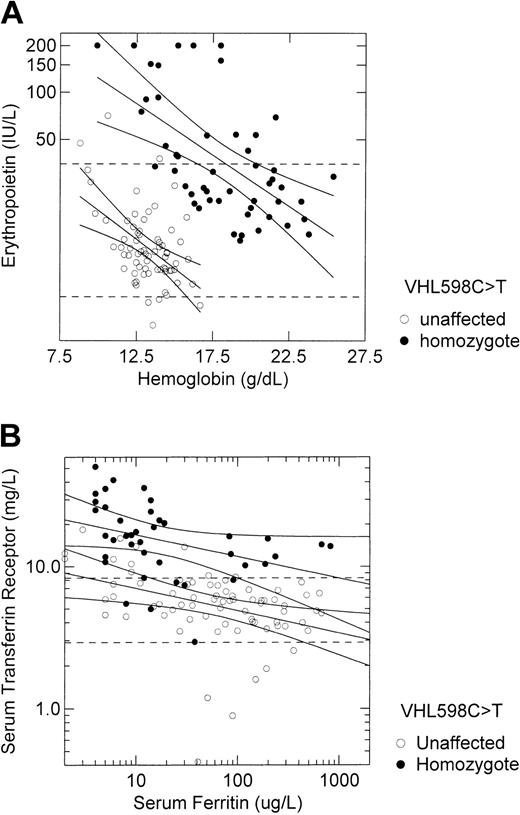
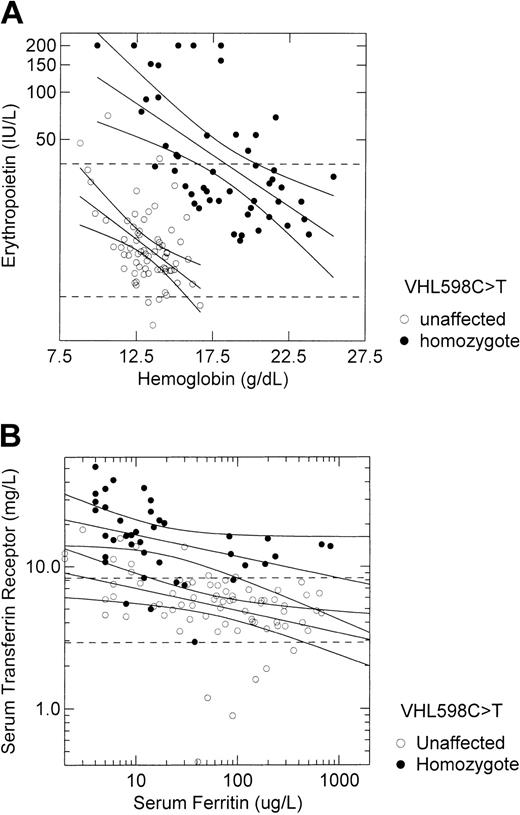
We contrasted the relationship of serum erythropoietin concentration with hemoglobin concentration and the relationship of serum transferrin receptor concentration with serum ferritin concentration among 41 VHL 598C>T homozygotes and 70 unaffected participants (Figure 3). Although the hemoglobin-adjusted erythropoietin concentration was approximately 10-fold higher in VHL 598C>T homozygotes than in unaffected participants (Figure 2D), the response of erythropoietin to hypoxia, as indicated by the slope of the regression line between log erythropoietin concentration and hemoglobin concentration, was identical between these 2 groups (–0.007 log erythropoietin units per 1 g/dL hemoglobin; Figure 3A). Similarly, although the serum ferritin-adjusted transferrin receptor concentration was approximately 3-fold higher in VHL 598C>T homozygotes than in unaffected participants (Figure 2E), the response of transferrin receptor to changing iron status, as indicated by the slope of the regression line between log transferrin receptor concentration and log ferritin concentration, was almost identical between these 2 groups (–0.154 log transferrin receptor unit per log ferritin unit in homozygotes versus–0.158 in unaffected subjects; Figure 3B).
Responses to hypoxia and to iron status are intact in VHL 598C>T homozygotes despite increased basal expression of hypoxia-regulated genes. (A) The relationship of serum erythropoietin concentration to hemoglobin concentration in 41 VHL 598C>T homozygotes and 70 unaffected participants, all of Chuvash ethnicity, is depicted. Regression lines and 95% confidence intervals are shown for each group. The dashed horizontal lines represent the lower (5 IU/L) and upper (35 IU/L) limits of the reference range for erythropoietin. The slope of the regression line is identical for each group (–0.007 log erythropoietin unit per 1 g/dL hemoglobin). (B) The relationship of serum transferrin receptor concentration to serum ferritin concentration in the same subjects is depicted. Regression lines and 95% confidence intervals are shown for each group. The dashed horizontal lines represent the lower (2.3 mg/L) and upper (8.9 mg/L) limits of the reference range for transferrin receptor. The slope of the regression line for the VHL 598C>T homozygotes (–0.154 log transferrin receptor unit per log ferritin unit) is almost identical to the slope for unaffected subjects (–0.158).
Responses to hypoxia and to iron status are intact in VHL 598C>T homozygotes despite increased basal expression of hypoxia-regulated genes. (A) The relationship of serum erythropoietin concentration to hemoglobin concentration in 41 VHL 598C>T homozygotes and 70 unaffected participants, all of Chuvash ethnicity, is depicted. Regression lines and 95% confidence intervals are shown for each group. The dashed horizontal lines represent the lower (5 IU/L) and upper (35 IU/L) limits of the reference range for erythropoietin. The slope of the regression line is identical for each group (–0.007 log erythropoietin unit per 1 g/dL hemoglobin). (B) The relationship of serum transferrin receptor concentration to serum ferritin concentration in the same subjects is depicted. Regression lines and 95% confidence intervals are shown for each group. The dashed horizontal lines represent the lower (2.3 mg/L) and upper (8.9 mg/L) limits of the reference range for transferrin receptor. The slope of the regression line for the VHL 598C>T homozygotes (–0.154 log transferrin receptor unit per log ferritin unit) is almost identical to the slope for unaffected subjects (–0.158).
Further analyses among the 129 research participants included in Table 2 revealed the following. In linear regression models that adjusted for VHL mutation status, age, body mass index, and history of smoking, a 10-fold increase in serum VEGF concentration was associated with an estimated 24–mm Hg decrease in systolic blood pressure (95% CI, 4-44 mm Hg; P = .021) and an estimated 24–mm Hg decrease in pulse pressure (95% CI, 6-42 mmHg; P = .008), but there was no significant correlation between diastolic blood pressure and serum VEGF concentration. Serum nitrite/nitrate concentrations did not correlate significantly with blood pressure. In an analysis of variance model that adjusted for VHL mutation status, there was no significant difference in serum VEGF concentrations according to the presence or absence of varicose veins on physical examination. However, among the subgroup of VHL 598C>T homozygotes, the geometric mean (SD range) serum VEGF concentration 113 (67-191 pg/mL in patients with varicose veins was significantly higher than the concentration of 81 (60-111) pg/mL in patients without varicose veins (P = .018). No significant correlation of serum PAI-1 concentrations with a history of thrombosis was found in analysis of variance models.
Imaging and ophthalmologic studies in patients with Chuvash polycythemia
We invited 44 living patients with Chuvash polycythemia, 43 of them documented to be VHL 598C>T homozygotes, to participate in imaging and ophthalmologic studies and 33 agreed, giving written informed consent. We also recorded the results of CT scans of the abdomen and MRI of the spinal cord in 34 Chuvash patients who did not have the diagnosis of Chuvash polycythemia; these patients were in addition to the matched community members described earlier. The patients were not acutely ill and were not hospitalized at the time of the studies. The results are summarized in Table 3.
Studies performed only in patients with Chuvash polycythemia. Magnetic resonance imaging of the brain of 33 patients with Chuvash polycythemia did not demonstrate cerebellar or supratentorial hemangioblastomas. Old infarcts or chronic ischemic lesions were identified in 15 (45.5%). Ophthalmologic examination in these patients showed no retinal hemangioblastomas. Serum PAI-1 concentrations did not differ significantly according to the presence or absence of cerebral ischemic lesions on MRI.
Studies performed in both patients with Chuvash polycythemia and Chuvash patients without polycythemia. MRI of the spine did not demonstrate direct or indirect evidence of spinal cord hemangioblastomas (ie, enhancing tumor or associated cord edema). However, vertebral body hemangiomas were identified in 18 (54.5%) of 33 patients with Chuvash polycythemia compared with 7 (20.6%) of 34 Chuvash patients without polycythemia (P = .006). In a logistic regression model, both presence of Chuvash polycythemia (P = .002) and age (P = .004) were associated with significant increases in the odds of having vertebral hemangiomas. Diagnosis of Chuvash polycythemia was associated with a 7.0-fold increase in the odds of finding vertebral hemangioma by MRI (95% CI, 2.0-24.5) and an increase in age by 10 years was associated with a 3.1-fold increase in the odds (95% CI, 1.4-6.7). Among the patients with Chuvash polycythemia, serum VEGF concentrations were not higher in patients with vertebral hemangiomas. CT scanning of the abdomen revealed no significant differences in the incidence of cystic lesions of the kidney or liver between Chuvash patients with and without polycythemia. None of the patients with Chuvash polycythemia had findings suggestive of renal cell carcinoma or pheochromocytoma.
Discussion
Our results indicate that Chuvash polycythemia is a unique VHL syndrome characterized by homozygous germ line mutation of VHL, increased mortality because of cerebral vascular events and peripheral thrombosis, distinct vascular abnormalities, intact hypoxia sensing despite increased systemic expression in normoxia of a broad range of HIF-1–regulated genes, and absence of a predisposition to develop tumors or malignancy. Limitations to our study include that: (1) data on mortality were obtained retrospectively from a variety of sources; (2) historical findings of the living participants were subject to recall bias; and (3) the sample size is rather small for an epidemiologic study. Nevertheless, that we used adefined set of subjects diagnosed before 1977 as patients, studied the patients and matched cohorts in a narrow time frame, and used both a spouse cohort and a rigorously matched community cohort to test study hypotheses strengthens the validity of our findings. Furthermore, the sample size is quite large for the study of a condition as rare as congenital polycythemia.
Premature mortality from cerebral vascular events and peripheral thrombosis
The peripheral and central nervous system thrombosis and the premature mortality we observed in Chuvash polycythemia have parallels with the complications of polycythemia vera,17-19 an acquired clonal disease as a result of a somatic hematopoietic stem cell mutation.20 A comparison of our follow-up of 96 patients with Chuvash polycythemia with the follow-up of 1213 Italian patients with polycythemia vera18 is instructive. Most patients in both cohorts received treatment, with the patients with Chuvash polycythemia predominantly receiving phlebotomy therapy alone and the Italian patients with polycythemia vera predominantly receiving myelosuppressive therapy; the degree of disease control for the Italian patients was not reported. The median age at diagnosis of Chuvash polycythemia was younger (16 years versus about 60 years), the period of follow-up was longer (more than 23 years in all patients with Chuvash polycythemia versus <20 years in 90% of patients with polycythemia vera), overall mortality was higher (47% versus 18.5%), age- and sex-standardized mortality rate was higher (3.8 times matched community members versus 1.7 times the general population), and proportion of deaths as a result of cerebral vascular events or peripheral thrombosis was higher (46.1% versus 21.9%) despite the younger age of the patients with Chuvash polycythemia. It is also of note that other polycythemic conditions, such as those associated with erythropoietin receptor mutations, high altitude, hemoglobin mutants with high oxygen affinity, and 2,3-biphosphoglyerate deficiency, do not seem to have similar high rates of thrombotic complications,20 and transgenic mice with constitutive overexpression of human erythropoietin have hematocrits up to 85% with no thrombotic tendency.21 Therefore, Chuvash polycythemia may be a polycythemic condition with an unusually high tendency for thrombosis, but any firm conclusions in this regard require further study. That we failed to find a statistically significant beneficial effect of phlebotomy therapy on mortality and thrombotic complications suggests that the thrombotic tendency may not correlate with hematocrit and may, therefore, be caused by a factor or factors yet to be identified. Further study is needed to definitively ascertain the potential benefit of phlebotomy therapy for this condition.
Vascular abnormalities
The increased prevalence of vertebral hemangiomas and varicose veins and the lower blood pressures that we observed in 598C>T VHL homozygotes in the present study are in contrast to the absence of recognized increased occurrence of hemangiomas and varicose veins and to the presence of a tendency for hypertension in patients with polycythemia vera17,19 and primary familial and congenital polycythemias as a result of gain of erythropoietin function.22 The background prevalence of 21% for vertebral hemangiomas in 34 Chuvash patients without polycythemia in the present study, which can be compared with the prevalence of 11% found at autopsy elsewhere,23 supports the conclusion that the finding of this benign vascular malformation in 55% of the 33 patients with Chuvash polycythemia imaged in this study represents a true disease association. The incidence of vertebral hemangiomas in classic VHL syndrome has not been documented. The lower blood pressures in patients with Chuvash polycythemia in the present study are in contrast to the tendency for hypertension observed in humans exposed to chronic hypoxia24 or recombinant erythropoietin therapy,25 suggesting the hypotensive tendency is the effect of another HIF-1 target gene or genes than erythropoietin. Our observation that blood pressures are significantly lower in VHL 598C>T heterozygotes than in unaffected participants, if confirmed in future studies, points to a possible survival advantage for carriers of this mutation in protection from hypertension. Another intriguing possibility is that VHL 598C>T heterozygosity might afford protection from preeclampsia,26 the leading cause of maternal and fetal mortality worldwide.
Serum levels of products of hypoxia-regulated genes
Our results provide further evidence that a broad range of hypoxia-regulated genes are up-regulated under normoxic conditions in patients with Chuvash polycythemia.
Erythropoietin and transferrin receptor. The identical increase in serum erythropoietin concentration per unit decrease in hemoglobin concentration that we observed in VHL 598C>T homozygotes and unaffected participants in the present study (Figure 3A) suggests that the hypoxia-sensing mechanism functions normally in VHL598C>Thomozygotes in a setting of marked basal up-regulation of HIF-1α–regulated genes. Also, given that low intracellular iron concentration enhances HIF-1α expression in a similar manner to hypoxia27 and that serum ferritin concentration reflects iron status,28 the virtually identical increase in serum transferrin receptor concentration per unit decrease in serum ferritin concentration in VHL 598C>T homozygotes and unaffected participants (Figure 3B) suggests that both the cellular iron-sensing and hypoxia-sensing mechanisms function normally in this setting.
VEGF. The serum VEGF levels we observed in VHL 598C>T homozygotes in the present study overlap those reported in patients with classic VHL syndrome in 2 studies,29,30 but our results contrast with the lack of finding significantly higher levels in patients with classic VHL syndrome compared with control subjects in one study.29 Other studies have shown that parenteral administration of VEGF lowers systemic blood pressure in experimental animals31 and humans.32 In our analyses that adjusted for VHL 598C>T mutation status, serum VEGF concentrations demonstrated a significant negative correlation with systolic blood pressure but not diastolic blood pressure. Although it is possible that increased expression of VEGF contributes to lower blood pressure, in patients with Chuvash polycythemia, a number of other genes up-regulated by HIF-1α whose products we did not measure, such as α1B-adrenergic receptor, adrenomedullin, endothelin-1, and heme oxygenase-1, could also plausibly contribute to blood pressure changes. Plasma VEGF levels have been reported to be increased in patients with varicose veins,33 and we observed an association between serum levels of VEGF and varicose veins among the VHL 598C>T homozygotes but not the unaffected subjects in the present study. Although increased expression of VEGF or mutations of its receptor have been associated with hemangiomas in mice34,35 and humans,36,37 we did not observe higher serum VEGF levels among the patients with Chuvash polycythemia with vertebral hemangiomas compared with those without vertebral hemangiomas in this study.
Nitrite/nitrate. Serum concentrations of nitrite/nitrate, the stable end products of nitric oxide metabolism, were not elevated in VHL 598C>T homozygotes compared with unaffected subjects and did not correlate significantly with either systolic or diastolic pressure in the present study. Nitric oxide is formed from l-arginine by nitric oxide synthase, and the endothelial form of nitric oxide synthase,38 rather than the hypoxia-regulated inducible form,39 seems to predominate in the vascular system.
PAI-1. Whether the increased circulating levels of total PAI-1 we observed in VHL 598C>T homozygotes in this study might have contributed to thrombogenesis is not clear. PAI-1 is the primary inhibitor in plasma of activators of plasminogen. Transgenic mice with increased expression of PAI-1 suffer spontaneous thromboses, and an association between elevated PAI-1 levels and thrombosis in humans has been observed in some but not all studies.40 In the present study, we did not find higher serum total PAI-1 levels in the living patients with Chuvash polycythemia according to a history of thrombosis or imaging evidence of thrombosis.
Absence of tumors of classic VHL syndrome
The failure to find VHL syndrome tumors in patients with Chuvash polycythemia is striking, for all cells are homozygous for a mutation that leads to increased expression of HIF-1α and VEGF in normoxia. The development of hemangioblastoma and renal cell carcinoma associated with other heterozygous VHL mutations in the context of VHL tumor predisposition syndrome seems to be related to increased expression of HIF41,42 and possibly VEGF.43 Our findings are consistent with the concept that deregulation of HIF-1 and VEGF may not be sufficient to cause tumorigenesis in VHL syndrome.41 VHL protein has other substrates than HIF, some yet unidentified, that may have roles in the growth and behavior of cells,12,41,42 and interaction with these substrates might not be affected by the Chuvash mutation. Also, the VHL 598C>T mutation results in a relatively mild increase in HIF-1α expression,5 whereas more severe dysregulation may be necessary to promote tumorigenesis.
Implications of early mortality for VHL 598C>T homozygotes but high gene frequency in the Chuvash population
Our demonstration of decreased survival for VHL 598C>T homozygotes indicates possible negative selection pressure for Chuvash polycythemia, especially because early mortality begins during childbearing age (Figure 1). In contrast, that this mutation has worldwide distribution, originated from a single founder a large number of generations past, and is found in various ethnicities9 suggests that there is no negative selection of the carrier state and possible survival advantage for heterozygotes. Considering the many generations since the occurrence of this mutation and the rarity of this disease outside the Chuvash ethnic isolate, any survival advantage is probably subtle. Whether the potential survival benefit of VHL 598C>T heterozygosity might be associated with up-regulation of iron absorption and erythropoiesis after blood loss, possible amelioration of preeclampsia, or other effects remains to be explored by future studies.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-07-2535.
Supported in part by a National Institutes of Health (NIH) research grant from the National Heart, Lung, and Blood Institute and the Office of Research on Minority Health (no. UH1-HL03679-05), Howard University General Clinical Research Center grant (no. MO1-RR10284), and NIH grants (nos. R01HL66333-01 [J.T.P., V.R.G.] and R01HL5007-09 [J.T.P.]).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.