Abstract
Stable gene replacement by in vivo administration of lentiviral vectors (LVs) has therapeutic potential for metabolic disorders and other systemic diseases. We studied the expression of intracellular and secreted proteins by LVs in immunocompetent mice. Liver, spleen, and bone marrow cells were efficiently transduced. However, transgene expression, driven by a ubiquitous promoter, was limited by transgene-specific cellular and humoral immune responses, leading to the clearance of transduced cells. After green fluorescent protein (GFP) gene transfer, the liver showed infiltration of CD8+ cytotoxic T cells, and GFP-specific CD8+ T cells were isolated from the spleen. After human factor IX (hF.IX) gene transfer, anti-hF.IX antibodies were induced. These immune responses were not detected in mice injected with heat-inactivated or genome-lacking LVs or in GFP-transgenic mice, indicating that they were specifically triggered by transgene expression in vivo. Intriguingly, selective targeting of LV expression to hepatocytes limited the immune responses to the transgenes. By this approach, high levels of hF.IX, potentially in the therapeutic range, were reached and maintained long term in immunocompetent mice, without inducing antibody formation. These results prompt further studies in relevant animal models to explore the potential of in vivo LV administration for the gene therapy of hemophilias and other liver-based diseases.
Introduction
Stable gene replacement by direct in vivo administration of gene transfer vectors has tremendous potential as an effective, long-lasting, and relatively easy way to administer therapy for several inherited disorders. Stable expression of a functional enzyme or secreted factor in a fraction of liver cells could counteract most manifestations of a metabolic deficiency or a systemic disorder, such as familial hypercholesterolemia, Wilson and Fabry diseases, and hemophilia.1 In the latter case, although protein replacement therapy is effective, it fails to provide stable, long-term plasma levels of the coagulation factor, is costly, not always available, and not without risks for the patients. Even a small but sustained rise in circulating factor VIII or IX, obtained by gene-based delivery, would have significant therapeutic effects, protecting against spontaneous bleeding and potentially transforming the lives of patients with severe hemophilia.2,3
To these aims, several gene transfer vectors have been developed, and, although most of them lead to significant levels of gene transfer in the liver of experimental animals, major challenges remain to safely reach high-level and long-term expression of the therapeutic gene product. In addition, the risk of inducing an immune response against the therapeutic protein is a major concern for the gene therapy of genetic deficiencies. The de novo expression of a protein, which is lacking in the patient because of deletions or other mutations in the genes, may result in immune responses leading to the clearance of the transduced cells and to the formation of antibodies that inhibit the activity of secreted factors.4 Intracellular proteins are continuously sampled in most cell types and exposed to the cell surface as peptides bound to major histocompatibility complex (MHC) class I molecules. Secreted proteins are taken up by antigen-presenting cells (APCs), processed, and presented in the context of MHC class II molecules. Depending on the gene delivery method, antigen presentation by professional or nonprofessional APCs, such as tissue cells, and by the direct or indirect pathway, may be favored.5 Thus, the immunologic consequences of gene transfer are expected to vary with the type of transgene, vector, and target cells; the average level of transgene expression; and the genetic and immunologic background of the host.6-10
Lentiviral vectors (LVs) are well suited for gene replacement therapy because they can be delivered in vivo, integrate efficiently into the genome of nondividing cells, and provide stable long-term expression of the transgene.1 On intravenous administration of late-generation LVs into immunodeficient mice, we and others have shown high levels of transduction of parenchymal and nonparenchymal cells of the liver, spleen, and bone marrow and stable expression of intracellular marker genes, such as green fluorescent protein (GFP), without signs of toxicity.11-13 On delivery of the cDNA for the human clotting factors IX or VIII, we and others detected significant levels of the factors in the mice plasma that would be therapeutically useful if achieved in patients. Notably, human factor IX (hF.IX) expression was sustained for the lifetime of the mice after a single intravenous injection of LVs.11,14,15 However, initial attempts to reproduce these results in immunocompetent mice failed.14,16 In some of these studies, antibodies against the clotting factors were detected in the treated mice and were considered responsible for the failure to obtain long-term expression. However, no further analyses of the immune response were performed. The widespread transduction of nonparenchymal liver and spleen cells by LVs raises the concern that APCs are efficiently transduced by LVs administered into the blood-stream.13 Although LVs do not transfer viral genes, the potential of pseudotype LV particles to induce inflammatory cytokines on delivery into different tissues remains to be established. In addition, suboptimal vector manufacturing or excessive dose escalation may trigger toxic and inflammatory responses on intravenous administration. These nonspecific effects must be distinguished from effects dependent on the intrinsic features of the vector system and consequent to the integration and expression of an exogenous gene.
We studied the efficiency and time course of expression of both an intracellular marker (GFP) and a secreted therapeutic molecule (hF.IX) after systemic delivery of late-generation LVs into immunocompetent mice. We report that transgene-specific immune responses were induced on LV-mediated in vivo gene transfer and led to the clearance of the transduced cells and the transgene product. However, selective targeting of LV expression to hepatocytes limited these responses and allowed robust, long-term vector expression. Remarkably, by this approach, high levels of hF.IX, potentially in the therapeutic range, were reached and maintained long term in immunocompetent mice without inducing antibody formation.
Materials and methods
Vectors
The self-inactivating pRRLsin.cPPT.CMV.Wpre and pRRLsin.cPPT.ALB. Wpre constructs were used to generate vesicular stomatitis virus (VSV)–pseudotyped LV stocks as described.11 Titers, determined on HeLa and mouse liver (MLP29) cells for GFP vectors, were 5 × 109 to 2 × 1010 transducing units/mL with 100 to 400 μg HIV-1 p24/mL. Transduced cells were analyzed by flow cytometry.
Animal experiments
SCID (severe combined immunodeficient), C57BL/6, BALB/c, FVB/N, FVB/N-TgN (TIE2GFP) 287Sato17 mice were purchased from Charles River Laboratories (Calco, Milan, Italy) and maintained in germ-free conditions. Vector or phosphate-buffered saline (PBS) was injected into the tail vein (final volume 0.3-0.5 mL). LV-hF.IX vector-injected mice were bled weekly or monthly. Serum alanine aminotransferase (ALT) activity was measured by standard clinical test. All mice procedures were done according to protocols approved by the Torino University Bioethics Committee and the Italian Ministry of Health.
Tissue analysis
Mice were killed by CO2 inhalation. The organs were removed and cut in several pieces for different analyses. For immunofluorescence, tissues were fixed in 4% paraformaldehyde, embedded in optimal cutting temperature (OCT), and frozen. Cryostate sections (5-μm thick) were postfixed with paraformaldehyde, blocked in 5% goat serum (Vector Laboratories, Burlingame, CA), 1% bovine serum albumin (BSA), 0.1% Triton in PBS, and incubated with rabbit anti-GFP (Molecular Probes, Leiden, the Netherlands) and with rat anti-mouse F4/80 (Serotec, Raleigh, NC) or antimouse MHCII or CD8 (BD Pharmingen, San Diego, CA). Sections were washed and incubated with fluorescein isothiocyanate (FITC)–conjugated goat α-rabbit immunoglobulin G (IgG) and with tetramethyl rhodamine isothiocyanate (TRITC)–conjugated goat antirat IgG (Molecular Probes). Nuclei were stained with TOPRO-3 (Molecular Probes). Fluorescent signals from single optical sections were acquired by 3-laser confocal microscope (Radiance 2100; Bio-Rad, Hercules, CA), as decribed.18 For histology, livers were formalin-fixed and paraffin-embedded. Sections (5-μm thick) were stained with hematoxylin-eosin. For flow cytometry, spleen and liver tissues were rinsed in PBS before mechanical disruption and red cells lysis. Cell suspensions were labeled with anti-CD4, anti-CD8, and anti-CD11b phycoerythrin (PE)–conjugated antibodies (Becton Dickinson [BD], Heidelberg, Germany) and analyzed with a FACScan flow cytometer equipped with CellQuest software (BD).
T-cell lines and IFN-γ detection
Splenocytes were collected as described earlier and cultured at a concentration of 1 × 106/mL with 1 μg/mL r-GFP (Clontech, Palo Alto, CA). After 7 days, live cells were recovered by Fico/Lite (Atlanta Biologicals, Norcross, GA) and left resting for an additional 7 days with 50 U/mL interleukin 2 (IL-2; BD). For interferon γ (IFN-γ) detection, 1 × 105 anti-GFP T-cell lines were stimulated with 1 μg/mL r-GFP and 4 × 105 irradiated self-splenic APCs depleted of T cells by magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany). IFN-γ in the supernatants collected 48 hours after culture was quantified by sandwich enzyme-linked immunosorbent assay (ELISA) by using commercially available kits (BD).
IFN-γ ELISPOT assay
GFP-specific IFN-γ–secreting cells were enumerated by enzyme-linked immunospot (ELISPOT) assay as described.19 Briefly, 1 × 105 sorted splenic T cells were plated in ELISPOT plates (Nunc, Rochester, NY) coated with α–IFN-γ capture antibody (clone R46A2; BD). IL-2 (50 U/mL) was added to the culture together with 2 μg/mL r-GFP and 0.5 × 105 irradiated self-dendritic cells (DCs) differentiated in vitro as described.20 Wells containing IL-2 and DCs without the protein were added as controls. After incubation at 37° C for 42 hours, plates were washed, and IFN-γ–producing cells were detected by the α–IFN-γ detection antibody (clone XMG 1.2; BD). Spots were counted by KS ELISPOT system (Zeiss Vision, Göttingen, Germany). The number of spots in control wells was subtracted from the test samples.
Quantification of vector DNA by real-time PCR
Vector copies per genome were quantified by real-time polymerase chain reaction (PCR) from 300 ng template DNA extracted from the tissues by a commercial kit (Qiagen, Hilden, Germany), using 2 sets of primers and probe to detect the LV backbone: LV forward primer, 5′-TGAAAGCGAAAGGGAAACCA-3′; LV reverse primer, 5′-CCGTGCGCGCTTCAG-3′; LV probe, 5′-(VIC)-CTCTCTCGACGCAGGACT-(TAMRA)-3′; and to detect the mouse β-actin gene: β-actin forward, 5′-AGAGGGAAATCGTGCGTGAC-3′; β-actin reverse, 5′-CAATAGTGACCTGGCCGT-3′; β-actin probe, 5′-(VIC)-CACTGCCGCATCCTCTTCCTCCC-(TAMRA)-3′. Reactions were carried out according to manufacturer's instructions and analyzed by using the ABI Prism 7700 sequence detection system (PE-Applied Biosystem, Weiterstadt, Germany) as described.21
hF.IX immunoassays
hF.IX concentration was determined in mouse citrated plasma by an enzyme-immunoassay for the factor IX:Ag (Roche, Milan, Italy). Plasma samples were tested for the presence of hF.IX antibodies by ELISA. Microtiter plates were coated with purified hF.IX from plasma (0.2 μg/well in 0.1 M carbonate buffer, pH 9.6; Sigma, St Louis, MO). Ten-fold dilutions of mouse plasma (1:20 to 1:2000) were added, and hF.IX antibodies were detected with peroxidase-conjugated rabbit antimouse immunoglobulin (DAKO, Glostrup, Denmark). Plates were reacted with H2O2 and orthophenylenediamine and read at 490 nm. Negative controls included PBS and GFP LV–injected mice.
Results
LV expression after systemic delivery into immunocompetent mice
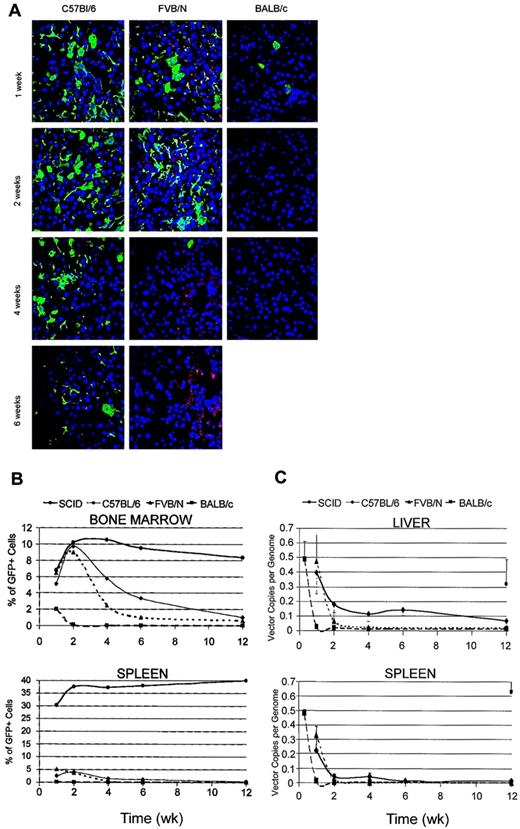
To study the biodistribution and time course of LV expression after systemic delivery in vivo, we injected purified high-titer stocks of LVs expressing the GFP marker from the immediate early enhancer/promoter of the human cytomegalovirus (CMV) into the tail vein of adult C57BL/6, FVB/N, and BALB/c mice. On the basis of our previous dose-response studies in SCID mice, we injected 7 to 10 μg HIV-1 Gag p24 equivalents of LVs, corresponding to 0.7 to 1 × 109 TUHeLa. At different times after injection we analyzed GFP expression in the liver, spleen, and bone marrow of vector- and vehicle-injected mice by fluorescence microscopy of cryostatic sections and fluorescence-activated cell sorting (FACS) analysis of single cell suspensions obtained by mechanical destruction of the tissue (Figure 1A-B). As previously reported,11 these tissues represent the major sites of transduction after LV administration into the peripheral circulation.
LV expression after systemic administration into immunocompetent mice. (A) Confocal microscopy of liver sections from the indicated mice injected into the tail vein with LVs expressing GFP from the human cytomegalovirus promoter (CMV) and analyzed at the indicated time after injection. Immunostaining for GFP (green) and DNA staining by TOPRO3 (blue). Representative sections are from 3 injected mice per time point per mouse strain. Original magnification, × 400. (B) FACS analysis of GFP expression in bone marrow and spleen of vector-injected mice at different times after injection. Single cell suspensions were obtained by mechanical disruption of the tissue. Mean frequency of GFP+ cells in 3 mice analyzed per time point per strain. (C) Quantitative real-time PCR of vector DNA in liver and spleen DNA of injected mice at different times after injection. Means ± SD, n = 3, are shown.
LV expression after systemic administration into immunocompetent mice. (A) Confocal microscopy of liver sections from the indicated mice injected into the tail vein with LVs expressing GFP from the human cytomegalovirus promoter (CMV) and analyzed at the indicated time after injection. Immunostaining for GFP (green) and DNA staining by TOPRO3 (blue). Representative sections are from 3 injected mice per time point per mouse strain. Original magnification, × 400. (B) FACS analysis of GFP expression in bone marrow and spleen of vector-injected mice at different times after injection. Single cell suspensions were obtained by mechanical disruption of the tissue. Mean frequency of GFP+ cells in 3 mice analyzed per time point per strain. (C) Quantitative real-time PCR of vector DNA in liver and spleen DNA of injected mice at different times after injection. Means ± SD, n = 3, are shown.
We detected GFP expression in the tissues of all mice 1 week after injection. The frequency of GFP+ cells was highest in the liver and bone marrow of C57BL/6 mice, in which it approached the values previously obtained in SCID mice injected with comparable amounts of vector (17% ± 3% liver cells and 5% ± 2% bone marrow [BM] cells).11 It was slightly lower in FVB/N mice and significantly lower in BALB/c mice. In the liver, GFP expression was observed in large cells with the features of hepatocytes and in smaller, branched or thin elongated cells with the features of Kupffer and endothelial cells, respectively. The identity of the different cell types transduced by LV was confirmed by costaining for cell type–specific markers (cytokeratine 18 for hepatocytes, F4/80 for Kupffer cells, platelet endothelial cell adhesion molecule 1 [PECAM-1] for endothelial cells; data not shown). In the spleen, the frequency of GFP+ cells appeared lower than observed in SCID mice. In contrast with the stable expression observed in SCID mice, we found a progressively lower GFP expression in the tissues examined later after injection. GFP+ cells decreased in frequency but remained detectable in the liver of C57BL/6 mice, and they disappeared completely in FVB/N and BALB/c mice 2 weeks after injection. Interestingly, the GFP+ cells that remained detectable in C57BL/6 mice appeared to be those with lower intensity of fluorescence by direct microscopy (not shown).
To distinguish between shut-off of vector transcription and clearance of transduced cells as the mechanism underlying the loss of GFP expression, we measured the vector DNA content in the liver and spleen at different times after injection by quantitative real-time PCR (Figure 1C). In C57BL/6 mice, the vector DNA showed a faster rate of clearance in the initial weeks after injection, then stabilized at an average level of 0.14 ± 0.04 copy per genome in the liver, whereas it disappeared almost completely in the spleen after 6 weeks. In FVB/N mice, the vector DNA content, measured at 1 week after injection, was similar to that found in C57BL/6, but it later dropped with a faster clearance rate and became undetectable. In BALB/c mice, the vector DNA content was much lower than that observed in the other mice already 1 week after injection and became undetectable later. However, analysis of BALB/c tissues 48 hours after injection showed similar vector DNA content as that detected in the other mice. Because the DNA sequence probed by the real-time PCR is generated only in transduced target cells when reverse transcription is nearly completed, this finding indicated that transduction occurred with similar efficiency in all the mice. A similar clearance of the vector DNA was observed in the spleen. In SCID mice injected with comparable amounts of LV, the vector DNA remained stable at an average level of 0.3 ± 0.16 copy per mouse cell genome in the liver and 0.6 copy per genome in the spleen, in good agreement with the observed frequency of GFP+ cells. In summary, the vector DNA content within the tissues of the injected mice varied with time concordantly with the observed changes in GFP expression, suggesting that clearance of transduced cells was mainly responsible for the loss of vector expression in immunocompetent mice.
Liver histology and cytotoxicity in LV-injected mice
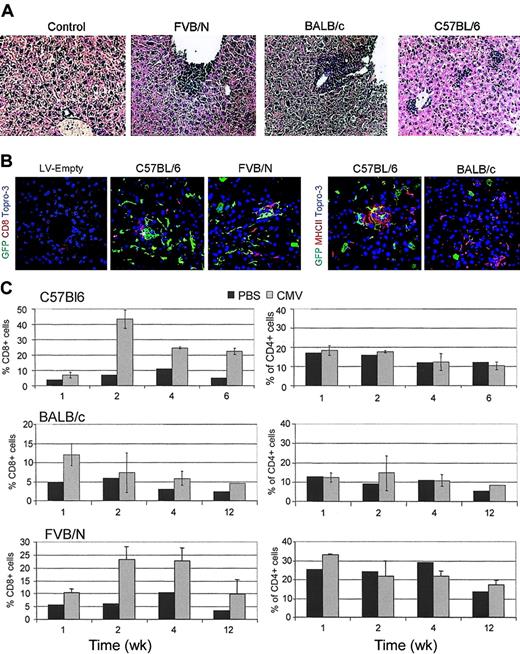
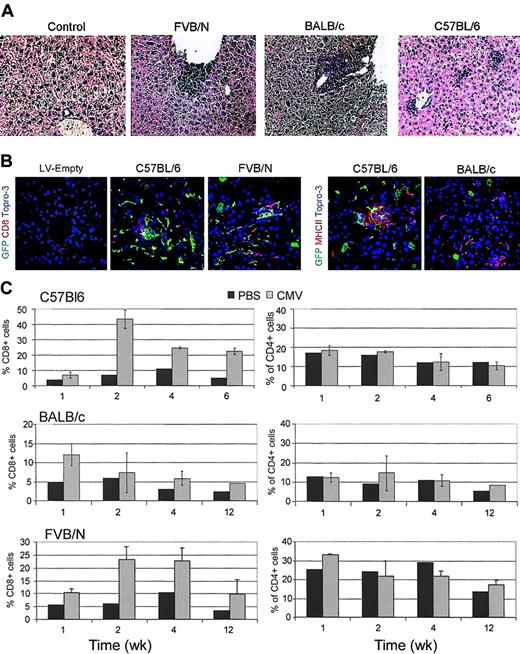
Histologic analysis of paraffin-embedded liver sections showed foci of mononuclear cell infiltration near the vasculature and throughout the lobules of vector-injected, but not vehicle-injected, mice (Figure 2A). In C57BL/6 mice, infiltrating cells were barely detectable 1 week after injection and well detectable 2 weeks after. In FVB/N and BALB/c mice, the infiltrate was already well established 1 week after injection and decreased 2 weeks later. FACS analysis of liver cell suspension immunostained for CD8, CD4, and CD11b antigens (Figure 2C and not shown) showed significantly more cells in the mononuclear cell gate of vector-injected than vehicle-injected mice. CD8+ T cells and CD11b+ cells, but not CD4+ cells, were present at the same times at which peak infiltration was observed by histology. Confocal microscopy of immunostained liver sections showed a significant proportion of CD8+ T cells infiltrating the tissue and clusters of mononuclear cells strongly staining for MHC class II molecules, typically found in close proximity with GFP+ cells (Figure 2B). Notably, liver infiltration was not observed by histology or FACS analysis in C57BL/6 and Balb/c mice injected with equal doses of LV particles (by HIV-1 Gag p24 equivalents) that lacked the vector genome and did not express the transgene.
Lymphomonocytic liver infiltration in GFP LV-injected mice. (A) Histologic analysis of paraffin-embedded liver sections of the indicated mice injected 2 weeks before with CMV-GFP LV or vehicle after hematoxylin and eosin staining. Representative pictures from 3 mice analyzed per strain. Original magnification, × 200. (B) Confocal microscopy analysis of liver sections of mice injected 2 weeks before with LV particles with or without (LV-Empty) the vector genome and immunostained for GFP (green), MHCII, or CD8 (red), and DNA by TOPRO3 (blue). Representative pictures are from 3 mice analyzed per strain. Original magnification, × 400. (C) FACS analysis of liver cell suspensions obtained from LV and vehicle (PBS) injected mice at the indicated time after injection and labeled with CD4 and CD8 antibodies. The percentage of positive cells in the mononuclear cell gate (mean ± SD, n = 3) is shown.
Lymphomonocytic liver infiltration in GFP LV-injected mice. (A) Histologic analysis of paraffin-embedded liver sections of the indicated mice injected 2 weeks before with CMV-GFP LV or vehicle after hematoxylin and eosin staining. Representative pictures from 3 mice analyzed per strain. Original magnification, × 200. (B) Confocal microscopy analysis of liver sections of mice injected 2 weeks before with LV particles with or without (LV-Empty) the vector genome and immunostained for GFP (green), MHCII, or CD8 (red), and DNA by TOPRO3 (blue). Representative pictures are from 3 mice analyzed per strain. Original magnification, × 400. (C) FACS analysis of liver cell suspensions obtained from LV and vehicle (PBS) injected mice at the indicated time after injection and labeled with CD4 and CD8 antibodies. The percentage of positive cells in the mononuclear cell gate (mean ± SD, n = 3) is shown.
The serum levels of ALT were within the normal range in all mice 48 hours after injection, indicating the absence of acute liver toxicity. However, 1 week after injection, ALT levels increased in Balb/c and FVB/N mice, but not in C57BL/6 mice, indicating hepatotoxicity. ALT levels returned to the normal range in all mice 4 weeks after injection, concomitantly with the disappearance of liver infiltration and GFP expression (Table 1). We also found that the spleen of vector-injected BALB/c mice was significantly enlarged compared with that of vehicle-injected mice 1 and 2 weeks after injection, whereas only a mild enlargement was observed in FVB/N and C57BL/6 mice (not shown). In accordance with the lack of liver infiltration, no spleen enlargement was observed in mice injected with vector particles without genome.
These data indicate that systemic administration of LV induced a lymphomonocytic infiltration to the liver. The delayed timing, mouse strain dependence, and self-limiting course of this response, as well as its absence in mice injected with LV lacking the genome, suggested that it was an immune reaction to transgene expression.
Induction of transgene-specific immune responses in LV-injected mice
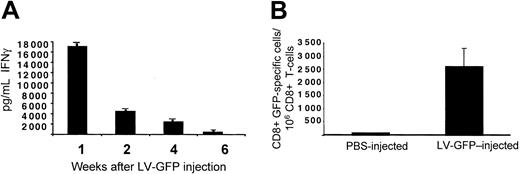
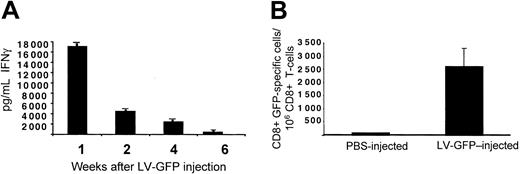
To determine whether the lymphomonocytic infiltration and the loss of LV expression correlated with the induction of antigen-specific T cells, we developed GFP-specific T-cell lines in vitro from the splenocytes of vector- and vehicle-injected C57BL/6 mice. After 1 week of stimulation with recombinant GFP, the T-cell lines were highly enriched in CD8+ T cells (data not shown). To assess the presence of CD8+ T cells specific for GFP, we evaluated IFN-γ produced by the T-cell lines isolated at different times. We observed high IFN-γ production in response to GFP as soon as 1 week after vector injection (Figure 3A). IFN-γ production decreased in T-cell lines isolated 2 weeks after injection and disappeared by 6 weeks after injection. To eliminate any bias possibly introduced by the in vitro expansion of GFP-specific T cells, we evaluated directly the number of cells, which produced IFN-γ in response to GFP without in vitro restimulation. ELISPOT assay performed on freshly isolated splenic T cells from C57BL/6 mice 1 week after injection showed the presence of an average number of 2500 CD8+ T cells of 1 × 106 that specifically produced IFN-γ in response to GFP (Figure 3B). Considering that the average precursor frequency of Ag-specific T cells in a naive spleen is 10 of 1 × 106 T cells, a significant frequency of GFP-specific T cells was observed in vector-injected mice.22 This result strongly supports the notion that a cellular response mediated by CD8+ T cells and directed against GFP was responsible for the clearance of transduced cells.
Induction of GFP-specific immune response in GFP LV-injected mice. (A) T-cell lines derived from splenocytes of C57BL/6 mice killed at different times after injection of CMV-GFP LV were tested for GFP-specific IFN-γ production by ELISA. IFN-γ produced in control experiment performed in the absence of GFP was subtracted from that produced in the presence of GFP. (B) Freshly isolated T cells 1 week after injection from PBS- (white bar, n = 3) and LV-GFP–injected mice (black bar, n = 3) were directly tested in ELISPOT assay for IFN-γ production in response to GFP. The percentage of CD8+ T cells present during the ELISPOT assay was determined by FACS analysis. One representative experiment of 3 performed is shown. Means ± SD are shown.
Induction of GFP-specific immune response in GFP LV-injected mice. (A) T-cell lines derived from splenocytes of C57BL/6 mice killed at different times after injection of CMV-GFP LV were tested for GFP-specific IFN-γ production by ELISA. IFN-γ produced in control experiment performed in the absence of GFP was subtracted from that produced in the presence of GFP. (B) Freshly isolated T cells 1 week after injection from PBS- (white bar, n = 3) and LV-GFP–injected mice (black bar, n = 3) were directly tested in ELISPOT assay for IFN-γ production in response to GFP. The percentage of CD8+ T cells present during the ELISPOT assay was determined by FACS analysis. One representative experiment of 3 performed is shown. Means ± SD are shown.
LV expression and liver histology in GFP-transgenic mice
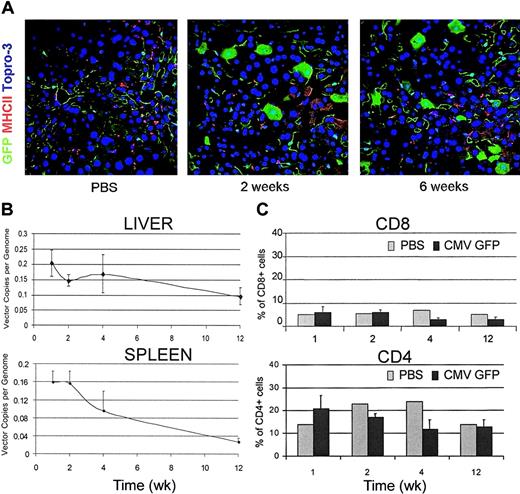
To evaluate whether the immune response triggered by LV-transduced cells was specific only for the foreign gene product, we performed the same experiments described earlier in FVB/N TgN(Tie2GFP) transgenic mice. These mice express GFP selectively in endothelial cells because of a germ line GFP transgene driven by the promoter and enhancer of the endothelial cell–specific Tie2 gene. Thus, they are expected to be nonresponsive to GFP. Confocal microscopy of liver sections from LV-injected GFP transgenic mice showed efficient GFP expression in all liver cell types, without clearance over time (Figure 4A). LV-mediated GFP expression could be easily distinguished from that of the germ line transgene because the first occurred in hepatocytes and Kupffer cells. In sharp contrast with our findings in wild-type FVB/N mice, no liver infiltration by mononuclear cells was observed either by immunostaining of sections for CD8 and MHC II antigens or by FACS analysis of liver cell suspension (Figure 4A,C). Moreover, real-time PCR demonstrated the persistence of vector DNA at high levels in the liver and spleen of LV-injected transgenic mice (Figure 4B) as compared with the clearance observed in the parental mice injected with the same dose of LV (Figure 1C). Thus, the tissue reactions and the clearance of transduced cells observed in LV-injected wild-type mice were not innate responses or acquired responses against vector components other than the transgene.
LV-mediated GFP expression in GFP transgenic mice. (A) Confocal immunofluorescence analyses of liver sections immunostained for GFP and MHC II from CMV-GFP LV-injected FVB/N TgN (Tie2GFP) transgenic mice that express GFP in endothelial cells. Representative sections from 3 mice were analyzed per time point. Original magnification, × 400. (B) Quantitative real-time PCR of vector DNA from the liver and spleen DNA of LV-injected transgenic mice at different times after injection. Means ± SD, n = 3, are shown. (C) FACS analysis of liver cell suspensions from PBS- and LV-injected transgenic mice labeled with CD4 and CD8 antibodies. The percentage of positive cells in the mononuclear cell gate (mean ± SD, n = 3) is shown.
LV-mediated GFP expression in GFP transgenic mice. (A) Confocal immunofluorescence analyses of liver sections immunostained for GFP and MHC II from CMV-GFP LV-injected FVB/N TgN (Tie2GFP) transgenic mice that express GFP in endothelial cells. Representative sections from 3 mice were analyzed per time point. Original magnification, × 400. (B) Quantitative real-time PCR of vector DNA from the liver and spleen DNA of LV-injected transgenic mice at different times after injection. Means ± SD, n = 3, are shown. (C) FACS analysis of liver cell suspensions from PBS- and LV-injected transgenic mice labeled with CD4 and CD8 antibodies. The percentage of positive cells in the mononuclear cell gate (mean ± SD, n = 3) is shown.
Prolonged in vivo expression of LV carrying a hepatocyte-specific promoter
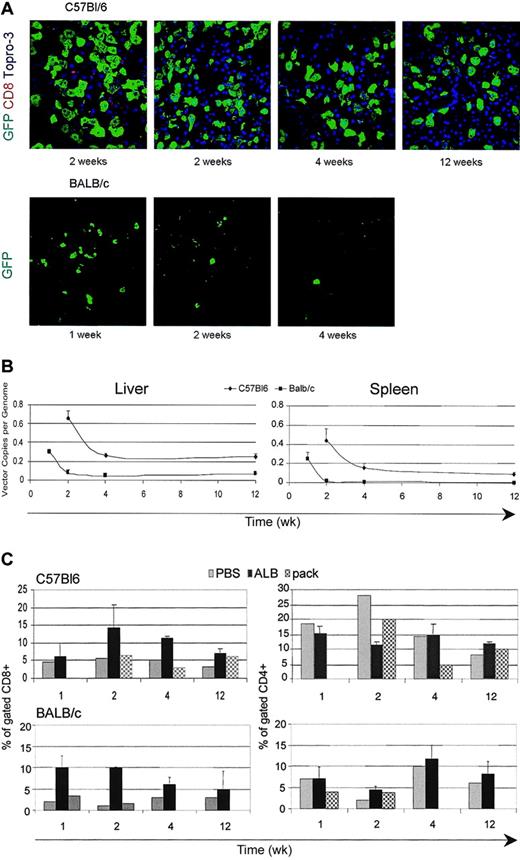
We previously showed that LVs carrying the albumin gene promoter (ALB) were selectively expressed in hepatocytes on systemic delivery into SCID mice.11 In such conditions transgene expression in liver and spleen APCs could be largely avoided; therefore, we tested whether ALB LV injection allowed longer GFP expression. We injected ALB-GFP LV, in amounts matching the CMV-GFP LV, into C57BL/6 and BALB/c mice as described earlier. As expected, GFP expression was restricted to hepatocytes (Figure 5A). Remarkably, GFP expression was maintained long term and to high frequency in C57BL/6 mice without significant signs of CD8+ T-cell infiltration (Figure 5C). In contrast, GFP expression was transient and cleared by 2 weeks after injection in BALB/c mice (Figure 5A). Real-time PCR confirmed the long-term maintenance of vector DNA in the liver and in the spleen of C57BL/6 mice to a relatively high level of 0.3 ± 0.16 copy per mouse cell genome, although a significant fraction of vector DNA was lost between 2 and 4 weeks after injection (Figure 5B). In BALB/c mice, there was a delayed but still effective clearance of vector DNA as compared with that observed after administration of CMV-GFP LV. A fraction of vector DNA, however, remained stably associated with liver DNA, which was most likely due to transduced nonparenchymal cells that did not express the transgene, because we could not detect any GFP+ cells at these longer times (Figure 5B). Thus, selective LV expression in hepatocytes allowed stable expression of a foreign transgene in a mouse strain–dependent manner.
Expression of LV carrying a hepatocyte-specific promoter. (A) Confocal immunofluorescence analyses of liver sections immunostained for GFP, CD8, and TOPRO3 from C57BL/6 and BALB/c mice injected into the tail vein with LVs expressing the GFP marker from the ALB promoter; representative sections from 2 mice analyzed per time point per strain. Original magnification, × 400. (B) Quantitative real-time PCR of vector DNA from the liver and spleen DNA of injected mice at different times after injection. Means ± SD, n = 3, are shown. (C) FACS analysis of liver cell suspensions labeled with CD4 and CD8 antibodies from vector-injected mice and mice injected with vector particles without genome (pack) at different times after injection. The percentage of positive cells in the mononuclear cell gate (mean ± SD, n = 3) is shown.
Expression of LV carrying a hepatocyte-specific promoter. (A) Confocal immunofluorescence analyses of liver sections immunostained for GFP, CD8, and TOPRO3 from C57BL/6 and BALB/c mice injected into the tail vein with LVs expressing the GFP marker from the ALB promoter; representative sections from 2 mice analyzed per time point per strain. Original magnification, × 400. (B) Quantitative real-time PCR of vector DNA from the liver and spleen DNA of injected mice at different times after injection. Means ± SD, n = 3, are shown. (C) FACS analysis of liver cell suspensions labeled with CD4 and CD8 antibodies from vector-injected mice and mice injected with vector particles without genome (pack) at different times after injection. The percentage of positive cells in the mononuclear cell gate (mean ± SD, n = 3) is shown.
Expression of human clotting factor IX by systemic delivery of LV
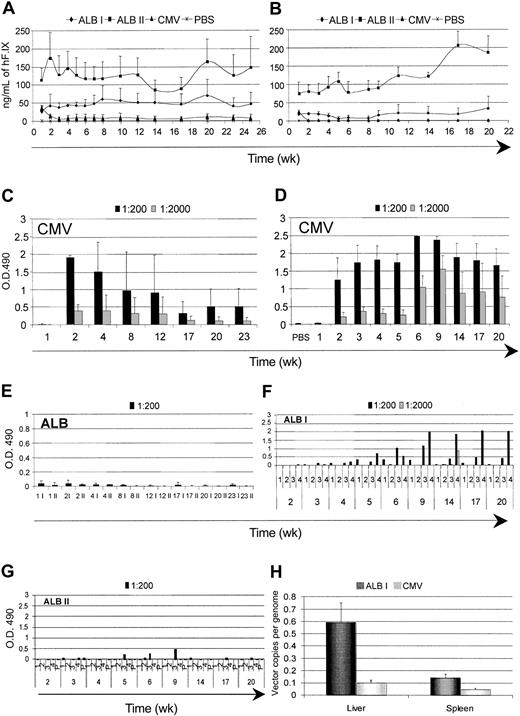
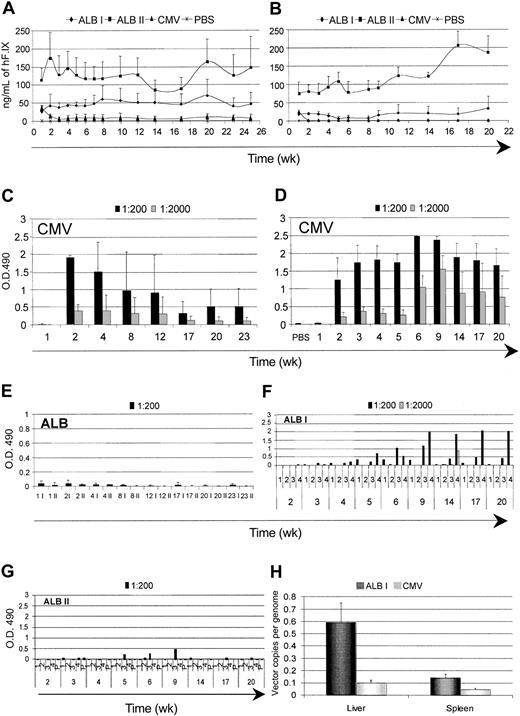
The results described so far were obtained on gene-based delivery of an intracellular protein. Gene therapy applications aimed at the replacement of a secreted molecule may face similar as well as distinct issues. We investigated the time course of expression and the humoral immune response against hF.IX. We injected purified high-titer stocks of LV expressing hF.IX from the CMV and ALB promoters into the tail vein of adult C57BL/6 and BALB/c mice. We matched the doses of the 2 vector types on the basis of the particle amount, measured by p24 antigen and on the basis of expression activity, measured by the yield of hF.IX secreted by transduced human hepatocarcinoma cells (HepG2). In the first case, we compared mice injected with 12 to 20 μg of both vectors (dose I), whereas, in the second case, we compared mice injected with 12 μg CMV LV and 40 μg ALB LV (dose II). In both strains of mice injected with CMV LV, we initially detected hF.IX to similar levels by immunocapture assay, but it became undetectable 2 weeks after (Figure 6A-B). The failure to detect hF.IX correlated with the induction of high-titer anti-hF.IX antibodies, as measured by ELISA (Figure 6C-D). In C57BL/6 mice injected with ALB LV, however, we observed dose-dependent stable long-term expression (up to 25 weeks, the longest time tested) of hF.IX to plasma levels that would be therapeutic if achieved in patients, without induction of antibodies (Figure 6A,E). Remarkably, BALB/c mice injected with ALB LV also reached similar levels of hF.IX expression, and the majority of them stably maintained it over time (up to 22 weeks, the longest time tested) without induction of antibodies (Figure 6B,F,G). A fraction of BALB/c mice injected with ALB-hF.IX (dose I), however, developed low-titer hF.IX antibodies, although the biologic significance of this response remains to be determined.
Time course of expression and immune response against hF.IX after systemic administration of hF.IX LVs. The plasma levels of human clotting factor IX were monitored after tail-vein injection of LVs expressing hF.IX from the CMV and ALB promoters and vehicle (PBS) into adult C57BL/6 (A) and BALB/c (B) mice. For the ALB LVs, 2 different amounts of vector were injected: 20 μg (ALB I) and 40 μg (ALB II) p24 vector equivalents. Means + SD, n = 4, are shown. (C-G) ELISA immunoassay was performed on the plasma to detect anti-hF.IX antibodies. Absorbance of the indicated dilutions are shown as mean ± SD, n = 4, for panels C-E, and for individual mice for panels F-G. (H) The vector DNA content was measured in the liver and spleen of C57BL/6 LV-injected mice 25 weeks after injection. Means + SD, n = 3, are shown.
Time course of expression and immune response against hF.IX after systemic administration of hF.IX LVs. The plasma levels of human clotting factor IX were monitored after tail-vein injection of LVs expressing hF.IX from the CMV and ALB promoters and vehicle (PBS) into adult C57BL/6 (A) and BALB/c (B) mice. For the ALB LVs, 2 different amounts of vector were injected: 20 μg (ALB I) and 40 μg (ALB II) p24 vector equivalents. Means + SD, n = 4, are shown. (C-G) ELISA immunoassay was performed on the plasma to detect anti-hF.IX antibodies. Absorbance of the indicated dilutions are shown as mean ± SD, n = 4, for panels C-E, and for individual mice for panels F-G. (H) The vector DNA content was measured in the liver and spleen of C57BL/6 LV-injected mice 25 weeks after injection. Means + SD, n = 3, are shown.
To determine whether, in addition to the induction of hF.IX antibodies, injection of CMV-hF.IX LV also induced clearance of the transduced cells, we measured the vector DNA content in the liver and spleen of C57BL/6 mice 25 weeks after (dose I, Figure 6H). The vector DNA was much lower in CMV LV-injected mice than in the tissues of ALB LV-injected mice. In addition, the content of ALB-hF.IX LV long term after injection was similar to that observed in SCID or GFP-transgenic mice after LV-GFP injection (Figures 1C and 4B). These results indicated long-term maintenance of ALB-hF.IX LV-transduced cells in vivo and clearance of CMV-hF.IX LV-transduced cells.
We then investigated whether the lack of a humoral response to hF.IX after delivery by ALB LV was dependent on the selective in vivo expression of this vector. Anti-hF.IX antibodies could have been induced in the mice by the injection of hF.IX protein copurified with the vector from the conditioned medium of producer cells. Because hF.IX was secreted to higher levels by CMV LV producer cells than ALB LV producer cells (not shown), this difference could be responsible for the absence of antibody induction in ALB LV-injected mice. We injected 2 groups of C57BL/6 mice with the same amount of CMV-hF.IX LV, either with or without heat inactivation at 60° C for 15 minutes and monitored hF.IX expression and antibodies formation. As shown in Figure 7A, heat inactivation of LV completely prevented hF.IX expression and antibody formation (Figure 7B).
LV inactivation prevented hF.IX expression and antibody formation. hF.IX expression (A) and antibody formation (B) in C57BL/6 mice injected with the same amount of CMV-hF.IX LV, either with (HI-LV) or without (LV) heat inactivation at 60° C for 15 minutes. Mean ± SD, n = 4.
LV inactivation prevented hF.IX expression and antibody formation. hF.IX expression (A) and antibody formation (B) in C57BL/6 mice injected with the same amount of CMV-hF.IX LV, either with (HI-LV) or without (LV) heat inactivation at 60° C for 15 minutes. Mean ± SD, n = 4.
Overall, these results indicate that systemic delivery and selective expression in hepatocytes of LV allowed long-term expression of hF.IX in immunocompetent mice of different strains.
Discussion
We showed that LV efficiently transduced liver cells on in vivo administration to immunocompetent mice. However, transgene expression, when driven by a ubiquitous promoter, was limited by transgene-specific cellular and humoral immune responses leading to the clearance of transduced cells and/or the transgene product, respectively. As expected from the different genetic background and previous immunologic studies, the immune response was more vigorous in BALB/c and FVB/N mice than in C57BL/6 mice.10,23-26 The low level of transgene expression found in BALB/c mice 1 week after injection was not due to a low efficiency of gene transfer, as compared with the other mice, but to a faster and more effective clearance of transduced cells. Indeed, when we prevented the immune response by expressing hF.IX from the ALB promoter, BALB/c and C57BL/6 mice showed similar levels of hF.IX in the plasma. LVs were not inactivated by incubation with sera from either immunocompetent or SCID mice, suggesting that the in vivo half-life of the vector was similar in all mouse strains tested (not shown). This notion was further supported by high levels of reverse-transcribed vector DNA detected in the tissues of BALB/c mice shortly after vector injection. However, C57BL/6 mice failed to clear GFP-expressing cells completely, despite the fact that they generated GFP-specific CD8+ T cells and induced a lymphomonocytic infiltration in the liver. It is possible that a self-limited immune response allowed cells expressing low levels of antigen to escape immune clearance.
We and others previously showed that intravenously administered LVs transduce splenocytes expressing high levels of MHC class II, the costimulatory molecule B7-2, and the intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and PECAM-1, which are all characteristics of APCs.13 Direct transgene expression within APCs may result in more efficient antigen processing and presentation than selective expression in parenchymal cells.27,28 Our findings show that LVs are proficient at inducing an immune response against the transgene product, as GFP-specific immune responses were not found previously in C57BL/6 mice challenged with GFP-expressing tumor cells.24,25,29 It is possible that LV-mediated gene delivery acted as an adjuvant in this respect.30 We did not detect signs of acute toxicity in any of the LV-injected mice and did not detect tissue reactions in mice injected with LVs lacking the genome or tolerant to the transgene. Thus, it is unlikely that injection of vector contaminants, or direct particle toxicity, contributed to trigger the immune response. Rather, efficient transgene expression, most likely within APCs, may be responsible for the efficient induction of the T-cell response. Although the LV proficiency at inducing immune responses could be exploited for vaccine development and immunotherapy approaches,31 it represents a hurdle when attempting in vivo gene replacement.
This hurdle also applied to gene-based delivery of a secreted factor. Administration of LVs expressing hF.IX from a ubiquitous promoter yielded only a transient expression of free factor in the mouse plasma, which was followed by the appearance of specific antibodies14 and the clearance of transduced cells. As observed with GFP-transduced cells, clearance of hF.IX-transduced cells was not complete in C57BL/6 mice. Our findings confirm and extend previous reports of antibody formation on LV-mediated factor IX and factor VIII gene delivery to healthy mice14,15 and are consistent with the reported failure to correct mice with hemophilia A by LV-mediated gene therapy.16
Remarkably, hepatocyte-specific LV expression limited transgene-specific immune responses both in the case of GFP and in the case of the secreted hF.IX, allowing stable long-term expression. Recent studies with adenoviral and adeno-associated viral (AAV) vectors also indicated prolonged expression and mitigated immune response to secreted transgene products, including hF.IX, when hepatocyte-specific promoters were used.32-37 The mechanism of this effect is under active investigation. Among the proposed explanations are the low to absent antigen expression induced directly within APCs and an active, antigen-specific tolerogenic effect of hepatic gene expression.6,34 Further immunologic studies in the mice showing long-term transgene expression will help to clarify this important issue.
The protection from immune clearance afforded by hepatocyte-selective LV expression was not complete. We observed both mouse strain dependence, in the case of GFP, and the occurrence of few low-titer antibody responses against hF.IX among similarly treated syngenic mice. The lack of GFP protection in BALB/C mice may be due to a higher immunogenicity of GFP as compared with hF.IX and to the more vigorous response against GFP found in BALB/C versus C57BL/6 mice in this and previous studies.10,24-26 A more reactive immune system in BALB/C mice may be more easily triggered by rare, low-level GFP expression in nonhepatocytes. However, the successful establishment of stable, high-level expression of hF.IX in vigorously immunoreactive mice such as BALB/c by LVs had not been reported yet, to our knowledge. It is likely that more sophisticated vector engineering and/or a combined pharmacologic or genetic manipulation of the immune system may be needed for gene transfer to fully escape immune recognition. However, the approach developed here may suffice when a low frequency of antigen-specific lymphocyte precursors is expected, such as in the case of healthy mice expressing allogenic F.IX and, in the future, in hemophilia B patients at low risk of inhibitor formation. Whether such favorable outcome also applies to the hemophilia B mouse models, lacking endogenous F.IX expression, remains to be established.
Several gene transfer vectors have shown stable F.IX expression and phenotypic disease correction on delivery to the muscle or liver of hemophilia B animal models.3,38 The encouraging results obtained with AAV-based vectors led to the first human clinical trials using these approaches.37,39-44 Until now, these studies have proved the safety but also the relatively low efficacy of AAV-mediated hF.IX gene transfer to the human muscle.45 High-dose intramuscular administration of AAV vectors induced F.IX antibodies in hemophilic mice and dogs, raising concerns about dose escalation in humans.23,46 In addition, the limited amount of DNA carried by this type of vector may hinder efficient delivery of a factor VIII transgene to treat the more common A form of hemophilia. More efficient liver gene transfer was achieved by large-capacity adenoviral vectors.36,47 These vectors deliver genome-size transcription units and persist in the transduced muscle or liver cells for prolonged time. However, the inflammatory properties of adenoviral particles remain a concern for the systemic administration of these late-generation adenoviral vectors.48
LV-mediated gene delivery has the potential of lifelong expression because of efficient integration in target cells, and significant DNA capacity, as required for factor VIII gene transfer. Although the long-term safety of in vivo administration of LV needs to be further investigated, the findings reported here prompt studies in the relevant animal models to verify their therapeutic potential for hemophilias and other liver-based diseases.
Prepublished online as Blood First Edition Paper, December 30, 2003; DOI 10.1182/blood-2003-09-3217.
Supported by grants from Telethon (A143 and TIGET), Italian Association for Cancer Research (AIRC), and the Italian Ministry of Scientific Research (MIUR) to L.N. and M.G.R.
A.F. and M.B. contributed equally to this work.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank L. Sergi Sergi, F. Benedicenti, R. Albano, and R. Lonoce for excellent technical assistance; A. Sottile for the ALT assay; and C. Pisacane for pathology.