GENE THERAPY
For the past decade, gene therapy for hemophilia, the X-linked bleeding disorder caused by mutations in the factor VIII (F8 in hemophilia A) or factor IX gene (F9 in hemophilia B), has been at the center of the efforts of many gene transfer laboratories. Several clinical trials have been carried out or are under way, and sustained, nearly curative correction of canine hemophilias A and B have been reported using viral vectors.1 However, clear clinical success has not yet been achieved, and continuous development of novel gene transfer vectors and an improved understanding of existing vector systems are prudent.
Just a few years ago, lentiviral vectors were developed, and they have since emerged as powerful tools for gene transfer to dividing and nondividing target cells.2 In particular, transduction of hematopoietic stem cells in murine models of β-thalassemia and sickle cell disease was achieved with spectacular efficiencies.3,4 The latest generation of vectors are devoid of genes from the HIV parent virus and are produced using protocols with minimal potential for accidental generation of wild-type HIV through recombination.
Lentiviral vectors can potentially be engineered to circumvent pre-existing antibody or memory lymphocyte responses to vector antigens in the human population. Thus, it was rationalized that intravenous infusion of such a vector carrying a F8 or F9 gene should represent an ideal strategy to transduce hepatocytes in vivo and thereby provide a continuous supply of functional coagulation factor. However, efficiency of transduction of hepatocytes was variable, and sustained expression was mostly confined to immune-deficient animals. It is now clear that lentiviral vectors share a feature with adenoviral vectors that is undesirable in treatment of genetic disease, namely, transduction of professional antigen-presenting cells (APCs).
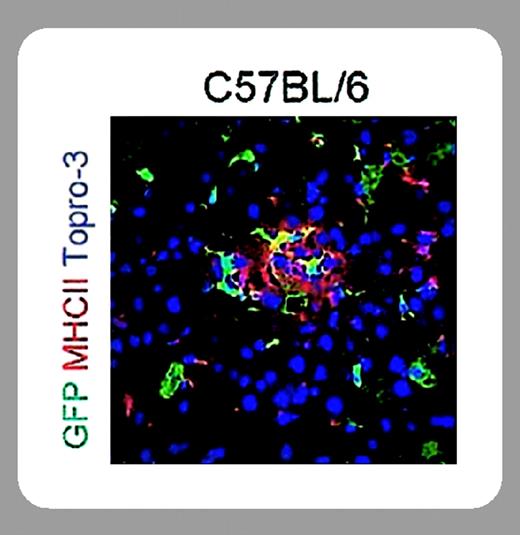
In this issue of Blood, Follenzi and colleagues (page 3700) demonstrate sustained therapeutic levels of human Factor IX expression in mice following lentiviral hepatic gene transfer. The authors were able to accomplish this by paying careful attention to the immunobiology of their vector system. Use of a green fluorescent protein (GFP) reporter gene and of a human F9 therapeutic transgene revealed induction of CD8+ T-cell (cytotoxic T lymphocyte) responses and antibody formation to the transgene product. These immune responses caused inflammation of the liver and ultimately eliminated or neutralized transgene expression. However, immune responses to the transgene product were mostly observed if a nonspecific promoter was chosen, which apparently drove expression in splenic APCs, much as seen before with adenoviral vectors. In contrast, sustained expression of 2% to 4% of normal human F9 levels was achieved by restricting expression to hepatocytes (using a hepatocyte-specific promoter, thereby eliminating expression in APCs).
Future studies should show whether this sustained expression is associated with induction of immune tolerance to Factor IX, as has been shown for adeno-associated vector, and whether success can be repeated in a large-animal model such as hemophilia B dogs.5 Furthermore, applicability of this approach to treatment of hemophilia A deserves further study. Design of the viral envelope could be further improved by avoiding viral glycoproteins that may promote inflammatory responses. Nonetheless, the study by Follenzi et al represents a significant step toward in vivo application of lentiviral gene transfer.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal