Abstract
Macrophages are called upon to ingest both IgG-coated targets and apoptotic cells. Important roles for tyrosine kinase Syk and leukotriene B4 (LTB4) are recognized in FcγR-mediated phagocytosis. Here we evaluated the roles of Syk and LTB4 in macrophage phagocytosis of apoptotic thymocytes versus IgG-coated erythrocytes. Macrophage ingestion of apoptotic thymocytes was not influenced by exogenous or endogenous LTB4 nor associated with Syk activation (phosphorylation). By contrast, LTB4 dose-dependently amplified FcγR-mediated phagocytosis as well as Syk activation. Furthermore, a role for endogenous LTB4 in Syk activation during FcγR-mediated phagocytosis was demonstrated using pharmacologic and genetic abrogation of 5-lipoxygenase. LTB4 was unique among 5-lipoxygenase products in this regard, since LTD4 and 5-hydroxyeicosatetraenoic acid (HETE) were unable to amplify Syk activation in response to FcγR engagement. Ca2+ chelation studies revealed that FcγR-mediated Syk activation as well as LTB4 amplification thereof was Ca2+ regulated. These 2 parallel phagocytic processes therefore exhibit initial divergence in signal transduction events, with Syk activation being an LTB4-regulated event in FcγR-mediated but not apoptotic cell ingestion. As LTB4 is an important proinflammatory product of macrophages, we speculate that this divergence evolved to permit FcγR-mediated phagocytosis to proceed in an inflammatory milieu, while apoptotic cell clearance is noninflammatory.
Introduction
As professional phagocytes, macrophages are called upon to ingest and eliminate various targets. Microbial pathogens and apoptotic cells are 2 of the most critical targets. The former is essential in innate immune responses, and the latter in ontogeny, tissue remodeling, and the resolution of inflammation. In order to recognize and discriminate between these particular targets, macrophages express a number of phagocytic cell surface receptors.1 Receptors for the constant region of IgG, the Fcγ receptors (FcγRs), enable these cells to detect and destroy IgG-coated microorganisms during infection and IgG-coated blood cells in autoimmune disorders.2,3 A variety of surface receptors have been implicated in recognition and phagocytosis of apoptotic cells,1 with the specific receptors used depending both on the apoptotic cell target and on the activation state of the phagocyte. Recognition of exposed phosphatidylserine (PS) on the surface of apoptotic cells is an important mechanism for initiating the ingestion of many apoptotic targets, including lymphocytes.4,5 Fadok and colleagues have recently described a specific macrophage receptor for PS, the PSR.6
The signal transduction events triggered by IgG-FcγR interaction and their role in the phagocytic process have been extensively studied (reviewed in Greenberg and Grinstein1 and Daeron7 ). By contrast, the signals generated during the process of apoptotic cell ingestion are much less well understood. Both types of phagocytosis appear to share certain signals, including the activation of phosphatidylinositol 3-kinase (PI-3K), protein tyrosine kinases (PTK), and protein kinase C (PKC).8 However, since FcγR-mediated phagocytosis promotes inflammation9 while PSR-mediated phagocytosis is anti-inflammatory,10 it is likely that divergent features must distinguish these 2 parallel processes. The activation of Syk and of 5-lipoxygenase (5-LO) are 2 events that have been shown to be critical for optimal ingestion of IgG-coated targets but have not been investigated in the context of apoptotic cell phagocytosis.
Syk is a nonreceptor PTK whose activation is a key proximal step in the ingestion of IgG-coated targets. Following FcγR engagement, the 2 N-terminal SH2 domains of Syk bind to the immunoreceptor tyrosine–based activation motifs of the γ-chain of FcγRI and FcγRIII and with the cytoplasmatic domain of FcγRIIA. Following this interaction, Syk becomes phosphorylated on tyrosine and is itself activated. A requirement for Syk in FcγR-mediated phagocytosis was demonstrated using both antisense oligodeoxynucleotide11 and gene knockout approaches.12
Leukotrienes (LTs) are lipid mediators of inflammation derived from the 5-LO pathway of arachidonic acid metabolism. The enzyme 5-LO, in conjunction with its helper protein 5-LO activating protein (FLAP), oxygenates arachidonic acid to form LTA4. This intermediate can be hydrolyzed to form the potent leukocyte activator and chemoattractant LTB4 or conjugated with glutathione to form cysteinyl-LTs (LTC4,LTD4, and LTE4), which elicit smooth muscle contraction and microvascular permeability.13 An important role for LTs in host defense was suggested by our previous report that 5-LO knockout mice exhibited enhanced lethality and reduced bacterial clearance, as compared with their wild-type (WT) counterparts, after intratracheal administration of Klebsiella pneumoniae.14 Subsequent studies demonstrated that genetic or pharmacologic inhibition/antagonism of LTs impaired, while exogenous addition of LTs augmented, FcγR-mediated phagocytosis by macrophages and neutrophils.14-16 However, the mechanisms by which 5-LO products enhance phagocytosis remain to be clarified.
In the present study, we sought to evaluate the role of Syk activation and of LTs in apoptotic cell phagocytosis. We also examined the effects of LTs on Syk activation in FcγR-mediated phagocytosis. Our findings demonstrate that these 2 parallel phagocytic processes exhibit divergence in their dependence on Syk activation and its regulation by LTB4.
Materials and methods
Animals
5-LO KO (129-Alox5tm1Fun)17 and strain-matched WT mice were bred in the University of Michigan Unit for Laboratory Animal Medicine (Ann Arbor) from breeders obtained from Jackson Laboratories (Bar Harbor, ME). C57BL/6 mice also were purchased from Jackson Laboratories, and Wistar rats were obtained from Charles Rivers Laboratories (Portage, MI). Animal protocols were approved by the University Committee on Use and Care of Animals.
Cell isolation and culture
Resident alveolar macrophages were obtained by lung lavage from rats and mice as previously described.14,18 Resident peritoneal macrophages were harvested by peritoneal lavage of mice with 5 mL Dulbecco modified Eagle medium (DMEM) (Gibco, Grand Island, NY). The cell suspensions were enumerated using a hemocytometer, adhered in flat bottom 6-well plates (Becton Dickinson, Franklin Lakes, NJ) for 1 hour at 37°C in a 5% CO2 atmosphere, and nonadherent cells were removed by washing. After adherence, the cultures were composed of more than 98% macrophages, as assessed by a modified Wright-Giemsa stain (Diff-Quik; American Scientific Products, McGaw Park, IL). Macrophage monolayers were cultured overnight in DMEM with 10% fetal bovine serum (FBS; HyClone Laboratories, Logan, UT). The cells were washed and the medium changed to DMEM without serum 20 minutes before the challenge with phagocytic targets.
Preparation of erythrocytes and thymocytes
Sheep red blood cells (SRBCs; ICN, Costa Mesa, CA) were opsonized with a subagglutinating concentration of IgG rabbit antisheep erythrocyte antibody (Cappel Organon Teknika, Durham, NC) as previously described.19 Thymuses were harvested from normal mice and rats and minced to yield a single-cell suspension. To induce apoptosis, thymocytes were resuspended at 1 × 106/mL DMEM containing 10% FBS and incubated for 6 hours with a final concentration of 1 μM dexamethasone (Sigma, St Louis, MO). Apoptosis was assessed by flow cytometric analysis of the cells staining simultaneously with annexin V–fluorescein isothiocyanate (FITC) and propidium iodide. This assay shows that dexamethasone treatment results in a large percentage of the cells exhibiting early apoptosis (42%-54% annexin V positive) with little secondary necrosis (9%-13% propidium iodide positive).8
Phagocytosis assays and experimental incubations
Phagocytosis of apoptotic thymocytes and IgG-coated SRBCs (IgG-SRBCs) was assayed using adherent macrophage monolayers in DMEM medium as described previously.20 Briefly, macrophage monolayers were cultured in 8-well slides and co-incubated with SRBCs or apoptotic thymocytes (1:10 ratio for both) for 1 hour at 37°C in a 5% CO2 atmosphere. At the end of the incubation period, the cells were washed 5 times and stained with the modified hematoxylin/eosin stain Hema 3 (Biochemical Sciences, Swedesboro, NJ). Results were expressed as phagocytic index, which was derived by multiplying the percent of macrophages containing at least one ingested target by the mean number of phagocytosed targets per positive macrophage.
In some experiments, cells were pretreated prior to the addition of LTB4 or IgG-SRBCs with LY 292476 (Eli Lilly, Indianapolis, IN), zileuton (Abbott Laboratories, Chicago, IL), or MK 886 (Merck-Frosst, Montreal, QC, Canada) for 10 minutes, or with EGTA (ethylene glycol tetraacetic acid) (Sigma) or BAPTA-AM (1,2-bis(2-aminophenoxy)ethane-N, N, N', N'-tetraacetic acid tetra(acetoxymethyl)ester), (Calbiochem, San Diego, CA) for 30 minutes.
Immunoprecipitation
The macrophage monolayers were lysed in buffer containing 1% Triton X-100 containing 50 mM Tris [tris(hydroxymethyl)aminomethane] (pH 8.0), 100 mM NaCl, 1 mM Na3VO4, 1 mM PMSF (phenylmethylsulfonyl fluoride), 50 mM NaF, and 1 μg/mL leupeptin. Lysates were precleared with protein A-Sepharose for 30 minutes and incubated overnight at 4°C with anti-Syk (1:80; Santa Cruz Biotechnology, Santa Cruz, CA). Protein A-Sepharose was added to each sample and incubated for 3 hours with rotation at 4°C. The beads were washed briefly 3 times with lysis buffer without Triton X-100 and separated on 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels. The entire volume recovered after boiling the beads was loaded onto the gel. Protein concentration of lysates could not be accurately determined because of interference with the Coomassie protein assay by SDS contained in loading buffer. Thus, lysates are derived from equal numbers of cells, but total Syk per lane was subject to variation. The proteins were transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, NH) overnight at 100 amps (A) and for 3 hours at 200 A.
Immunoblotting
The membrane was blocked with 5% fat-free milk in Tris-buffered saline (TBS) containing 0.1% Tween 20 for 1 hour, washed 3 times, and then probed with antiphosphotyrosine (1:900; PY20; Transduction Laboratories, Lexington, KY) for 1.5 hours. After that, the membrane was washed and incubated with a horseradish-peroxidase (HRP)–conjugated sheep anti–mouse secondary Ab (1:15 000; Amersham Pharmacia Biotech, Piscataway, NJ). Phosphorylated bands were visualized using the enhanced chemiluminescence system (ECL, Amersham; Arlington Heights, IL). The membranes were then stripped, blocked, and reprobed with anti-Syk (1:800) for 1 hour, followed by an incubation with HRP-conjugated donkey anti–rabbit secondary Ab (1:20 000; Amersham Pharmacia Biotech). The bands were visualized using the ECL system. Relative band densities were determined by densitometric analysis using National Institutes of Health Image Software, and the ratios calculated. The results were expressed as normalized Syk-PY/Syk, which represents the value of density obtained with the anti-PY blot divided by the value obtained with the anti-Syk blot. In all instances, density values of bands were corrected by subtraction of the background values.
Statistical analysis
The data are reported as a representative blot from 2 or 3 different experiments. Graphs represent the mean ± SEM from 2 or 3 different experiments. The means from different treatments were compared by ANOVA. When significant differences were identified, individual comparisons were subsequently made with the Bonferroni t test for unpaired values. Statistical significance was set at a P value less than .05.
Results
LTB4 modulates phagocytosis of IgG-SRBC but not apoptotic cells
In our previous reports, we demonstrated that phagocytosis of IgG-coated particles by alveolar macrophages was modulated by 5-LO products.14,15,21 The same pattern of response was observed here using mouse peritoneal macrophages, as WT cells treated with a FLAP inhibitor (MK 886; 1 μM) as well as 5-LO KO cells showed a significant reduction in phagocytosis of IgG-SRBCs (Figure 1A-B). In addition, preincubation of WT cells with LTB4 (10 and 100 nM) increased the phagocytic index (Figure 1C). On the other hand, none of these interventions modified the phagocytosis of apoptotic thymocytes by peritoneal macrophages, indicating that 5-LO–derived products are not involved in this process (Figure 1D-F). The dependence of FcγR-mediated, but not apoptotic cell phagocytosis, on 5-LO products was further corroborated with the specific 5-LO inhibitor zileuton (10 and 50 μM; data not shown).
LTB4 modulates phagocytosis of IgG-SRBCs but not apoptotic cells. Mouse peritoneal macrophages were pretreated in the absence (open bars) or presence of MK 886 (1 μM; 10 minutes) or LTB4 (10 and 100 nM; 2 minutes) prior to the addition of IgG-SRBCs (panels A and C) or apoptotic thymocytes (panels D and F), respectively. Peritoneal macrophages harvested from WT or 5-LO gene knockout mice (5-LO–/–) were challenged with IgG-SRBCs (B) or apoptotic thymocytes (E). *P < .05 compared with respective controls (ANOVA followed by Bonferroni t test). Results are the mean ± SEM of triplicate determinations from n = 2-3 separate experiments.
LTB4 modulates phagocytosis of IgG-SRBCs but not apoptotic cells. Mouse peritoneal macrophages were pretreated in the absence (open bars) or presence of MK 886 (1 μM; 10 minutes) or LTB4 (10 and 100 nM; 2 minutes) prior to the addition of IgG-SRBCs (panels A and C) or apoptotic thymocytes (panels D and F), respectively. Peritoneal macrophages harvested from WT or 5-LO gene knockout mice (5-LO–/–) were challenged with IgG-SRBCs (B) or apoptotic thymocytes (E). *P < .05 compared with respective controls (ANOVA followed by Bonferroni t test). Results are the mean ± SEM of triplicate determinations from n = 2-3 separate experiments.
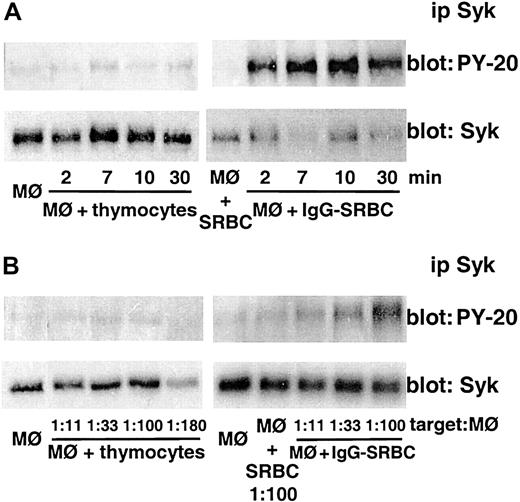
Apoptotic cell phagocytosis is not associated with Syk activation
In order to determine if Syk protein is activated during the phagocytosis of apoptotic cells, mouse peritoneal macrophages were co-incubated with apoptotic thymocytes (1:20 ratio) for time intervals ranging from 2-30 minutes and Syk phosphorylation was evaluated. Syk immunoprecipitated from these cells was not activated at any time point analyzed. By contrast, challenge of macrophages with IgG-SRBCs rapidly induced the expected activation of Syk, which peaked within 7 minutes (Figure 2A). Because of the greater importance of the lung as a target organ for microbial infection and the greater cell numbers obtainable from rats, subsequent studies of Syk activation were performed in rat alveolar macrophages. To ensure that the failure of Syk phosphorylation during apoptotic cell ingestion was not a function of an inadequate number of thymocytes in the assay, we examined increased thymocyte-macrophage ratios. As can be seen in Figure 2B, even with ratios of apoptotic thymocytes–macrophage higher than 150:1, we were unable to detect Syk activation. Under parallel conditions in the same experiment, a target dose–dependent response was clearly observed in macrophages challenged with IgG-SRBCs (Figure 2B). It is also seen in Figure 2 that neither macrophages alone nor macrophages incubated with unopsonized SRBCs exhibited significant Syk phosphorylation.
Apoptotic cell phagocytosis is not associated with Syk activation. (A) Mouse peritoneal macrophages were incubated with apoptotic thymocytes (left) or IgG-SRBCs (right) for the indicated times at 37°C at a macrophage-target ratio of 1:20. (B) Rat alveolar macrophages were incubated with increasing amounts of apoptotic thymocytes (left) or IgG-SRBCs (right) for 7 minutes at 37°C. Incubations were terminated by addition of lysis buffer and lysates were subjected to immunoprecipitation and immunoblotting as described in “Materials and methods.” In panels A and B, immunoblots in upper panels represent phosphorylated Syk detected with antiphosphotyrosine antibody, and those in lower panels, the amounts of Syk protein evaluated with anti-Syk antibody. Results are representative of 3 separate experiments.
Apoptotic cell phagocytosis is not associated with Syk activation. (A) Mouse peritoneal macrophages were incubated with apoptotic thymocytes (left) or IgG-SRBCs (right) for the indicated times at 37°C at a macrophage-target ratio of 1:20. (B) Rat alveolar macrophages were incubated with increasing amounts of apoptotic thymocytes (left) or IgG-SRBCs (right) for 7 minutes at 37°C. Incubations were terminated by addition of lysis buffer and lysates were subjected to immunoprecipitation and immunoblotting as described in “Materials and methods.” In panels A and B, immunoblots in upper panels represent phosphorylated Syk detected with antiphosphotyrosine antibody, and those in lower panels, the amounts of Syk protein evaluated with anti-Syk antibody. Results are representative of 3 separate experiments.
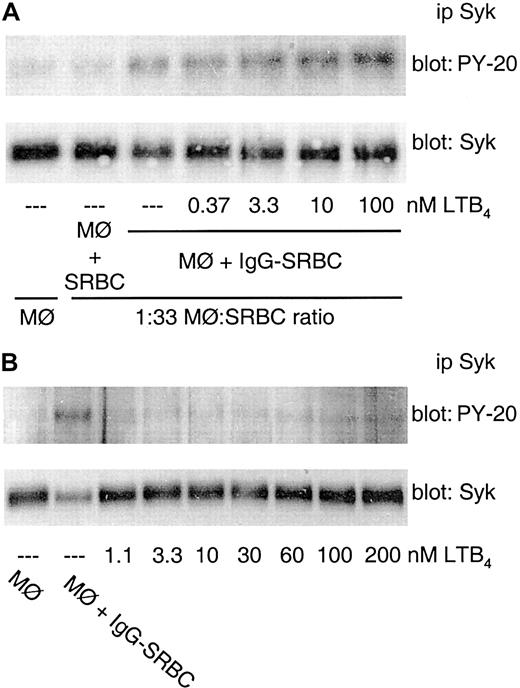
FcγR-mediated Syk activation is enhanced by exogenous LTB4 but not by other 5-LO–derived products
The treatment of rat alveolar macrophages with LTB4 2 minutes before IgG-SRBC challenge dose dependently augmented the degree of Syk phosphorylation evoked by FcγR engagement (Figure 3A). LTB4 enhancement was optimal with pretreatment intervals of 2-5 minutes prior to IgG-SRBC addition (data not shown). However, in the absence of IgG-SRBC challenge, LTB4 was incapable of inducing Syk activation by itself, even at high concentrations (Figure 3B).
FcγR-mediated Syk activation is enhanced by exogenous LTB4. (A) Rat alveolar macrophages were pretreated with the indicated concentrations of LTB4 for 2 minutes prior to the addition of IgG-SRBCs (1:33 ratio) and then incubated for 7 minutes at 37°C. (B) Rat alveolar macrophages were incubated for 9 minutes at 37°C with the indicated concentrations of LTB4. The cells also were incubated in absence (negative control) or in presence of IgG-SRBCs (1:33 ratio; positive control). Incubations were terminated by addition of lysis buffer, and lysates were subjected to immunoprecipitation and immunoblotting as described in “Materials and methods.” In panels A and B, immunoblots in upper panels represent phosphorylated Syk detected with antiphosphotyrosine antibody, and those in lower panels, the amounts of Syk protein evaluated with anti-Syk antibody. Results are representative of 3 separate experiments.
FcγR-mediated Syk activation is enhanced by exogenous LTB4. (A) Rat alveolar macrophages were pretreated with the indicated concentrations of LTB4 for 2 minutes prior to the addition of IgG-SRBCs (1:33 ratio) and then incubated for 7 minutes at 37°C. (B) Rat alveolar macrophages were incubated for 9 minutes at 37°C with the indicated concentrations of LTB4. The cells also were incubated in absence (negative control) or in presence of IgG-SRBCs (1:33 ratio; positive control). Incubations were terminated by addition of lysis buffer, and lysates were subjected to immunoprecipitation and immunoblotting as described in “Materials and methods.” In panels A and B, immunoblots in upper panels represent phosphorylated Syk detected with antiphosphotyrosine antibody, and those in lower panels, the amounts of Syk protein evaluated with anti-Syk antibody. Results are representative of 3 separate experiments.
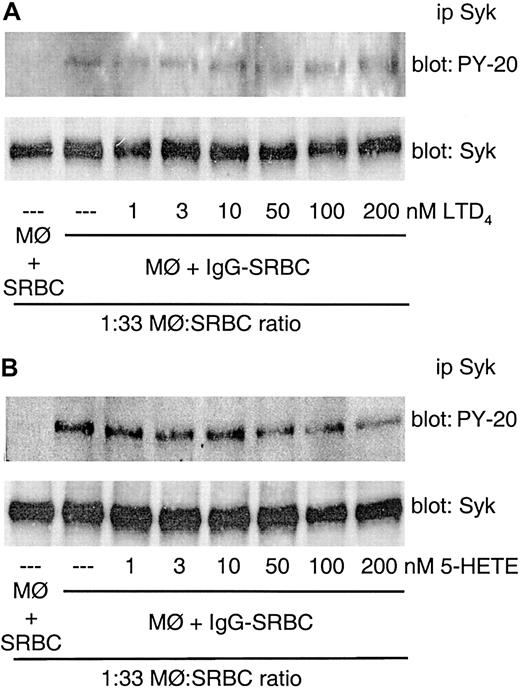
Our group has previously demonstrated that 5-LO products other than LTB4, including LTD4 and 5-hydroxyeicosatetraenoic acid (HETE), also were able to increase FcγR-mediated phagocytosis.15 We therefore tested the effects of LTD4 and 5-HETE on FcγR-induced Syk activation. Neither LTD4 nor 5-HETE influenced Syk phosphorylation induced by FcγR engagement in alveolar macrophages (Figure 4A-B).
FcγR-mediated Syk activation is not enhanced by other 5-LO–derived products. Rat alveolar macrophages were pretreated with the indicated concentrations of LTD4 (A) or 5-HETE (B) for 2 minutes prior to the addition of IgG-SRBCs (1:33 ratio) and then incubated for 7 minutes at 37°C. Incubations were terminated by addition of lysis buffer, and lysates were subjected to immunoprecipitation and immunoblotting as described in “Materials and methods.” In panels A and B, immunoblots in upper panels represent phosphorylated Syk detected with antiphosphotyrosine antibody, and those in lower panels, the amounts of Syk protein evaluated with anti-Syk antibody. Results are representative of 2 separate experiments.
FcγR-mediated Syk activation is not enhanced by other 5-LO–derived products. Rat alveolar macrophages were pretreated with the indicated concentrations of LTD4 (A) or 5-HETE (B) for 2 minutes prior to the addition of IgG-SRBCs (1:33 ratio) and then incubated for 7 minutes at 37°C. Incubations were terminated by addition of lysis buffer, and lysates were subjected to immunoprecipitation and immunoblotting as described in “Materials and methods.” In panels A and B, immunoblots in upper panels represent phosphorylated Syk detected with antiphosphotyrosine antibody, and those in lower panels, the amounts of Syk protein evaluated with anti-Syk antibody. Results are representative of 2 separate experiments.
Amplification of FcγR-induced Syk activation by LTB4 is receptor mediated
In order to verify that LTB4 action was mediated by binding to its receptors (BLT), we pretreated the cells with a specific LTB4 receptor antagonist (LTB4RA; LY 292476) for 10 minutes before addition of LTB4. As shown in Figure 5, treatment with LTB4RA completely blocked the amplification of Syk activation evoked by exogenous LTB4.
Amplification of FcγR-induced Syk activation by LTB4 is receptor mediated. Rat alveolar macrophages were pretreated with LTB4RA (LY 292476) at 1 μM for 10 minutes prior to the addition of LTB4 (10 nM). Two minutes after LTB4 treatment, the cells were challenged with IgG-SRBCs (1:33 ratio) and then incubated for 7 minutes at 37°C. Incubations were terminated by addition of lysis buffer, and lysates were subjected to immunoprecipitation and immunoblotting as described in “Materials and methods.” Immunoblots in upper panels represent phosphorylated Syk detected with antiphosphotyrosine antibody, and those in lower panels, the amounts of Syk protein evaluated with anti-Syk antibody. Results are representative of 2 separate experiments.
Amplification of FcγR-induced Syk activation by LTB4 is receptor mediated. Rat alveolar macrophages were pretreated with LTB4RA (LY 292476) at 1 μM for 10 minutes prior to the addition of LTB4 (10 nM). Two minutes after LTB4 treatment, the cells were challenged with IgG-SRBCs (1:33 ratio) and then incubated for 7 minutes at 37°C. Incubations were terminated by addition of lysis buffer, and lysates were subjected to immunoprecipitation and immunoblotting as described in “Materials and methods.” Immunoblots in upper panels represent phosphorylated Syk detected with antiphosphotyrosine antibody, and those in lower panels, the amounts of Syk protein evaluated with anti-Syk antibody. Results are representative of 2 separate experiments.
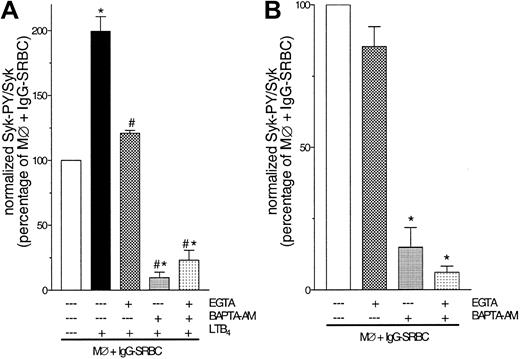
FcγR-mediated Syk activation as well as LTB4 amplification is Ca2+ regulated
Having established that LTB4 action was mediated by its receptors, and knowing that such interaction increases intracellular Ca2+ concentrations,22 we considered the possibility that the amplification of Syk activation by LTB4 is a Ca2+-dependent process. Rat alveolar macrophages were pretreated with the extracellular Ca2+ chelator EGTA and/or the intracellular chelator BAPTA-AM for 30 minutes before addition of LTB4 and subsequent challenge with IgG-SRBCs. As observed in Figure 6A, LTB4-mediated amplification of Syk activation was abolished by EGTA treatment. Pretreatment with BAPTA-AM also abolished the incremental activation elicited by LTB4 but had the additional effect of suppressing Syk activation to the level observed in macrophages challenged with unopsonized SRBCs. In order to further investigate the possibility that Ca2+ participates in Syk activation evoked by FcγR engagement itself, we again examined the effect of the 2 chelators, but now in the absence of LTB4. Treatment with EGTA was unable to modify FcγR-evoked Syk activation, but BAPTA-AM treatment reduced it by ∼90% (Figure 6B). In the presence of BAPTA-AM, EGTA caused no further abrogation of FcγR-induced Syk activation (Figure 6B) or in its amplification by LTB4 (Figure 6A).
FcγR-mediated Syk activation as well as LTB4 amplification are Ca2+ regulated. (A) Rat alveolar macrophages were pretreated with EGTA (10 mM), BAPTA-am (50 μM), or both for 30 minutes prior to addition of LTB4 (10 nM). Two minutes after LTB4 treatment, the cells were challenged with IgG-SRBCs (1:33 ratio) and then incubated for 7 minutes at 37°C. *P < .05 compared with IgG-SRBC group and #P < .05 compared with IgG-SRBC plus LTB4 group (ANOVA followed by Bonferroni t test). (B) Rat alveolar macrophages were pretreated with EGTA (10 mM), BAPTA-am (50 μM), or both for 30 minutes prior to IgG-SRBC challenge. Incubations were terminated by addition of lysis buffer, and lysates were subjected to immunoprecipitation and immunoblotting as described in “Materials and methods.” Data are given as mean ± SEM. *P < .05 compared with IgG-SRBC group (ANOVA followed by Bonferroni t test). Results are representative of 3 separate experiments.
FcγR-mediated Syk activation as well as LTB4 amplification are Ca2+ regulated. (A) Rat alveolar macrophages were pretreated with EGTA (10 mM), BAPTA-am (50 μM), or both for 30 minutes prior to addition of LTB4 (10 nM). Two minutes after LTB4 treatment, the cells were challenged with IgG-SRBCs (1:33 ratio) and then incubated for 7 minutes at 37°C. *P < .05 compared with IgG-SRBC group and #P < .05 compared with IgG-SRBC plus LTB4 group (ANOVA followed by Bonferroni t test). (B) Rat alveolar macrophages were pretreated with EGTA (10 mM), BAPTA-am (50 μM), or both for 30 minutes prior to IgG-SRBC challenge. Incubations were terminated by addition of lysis buffer, and lysates were subjected to immunoprecipitation and immunoblotting as described in “Materials and methods.” Data are given as mean ± SEM. *P < .05 compared with IgG-SRBC group (ANOVA followed by Bonferroni t test). Results are representative of 3 separate experiments.
Role of endogenous LTs in FcγR-induced Syk activation
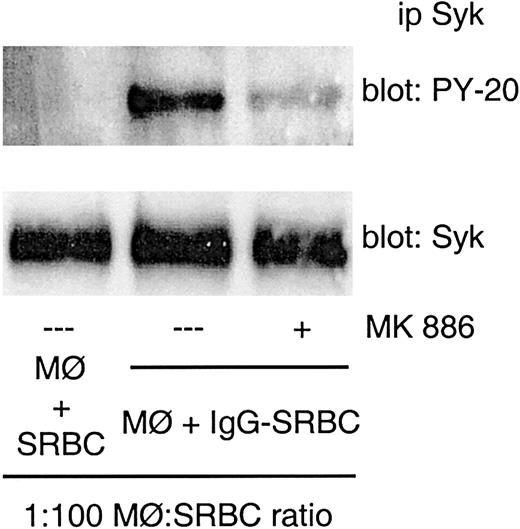
In order to test the involvement of endogenous LTs in Syk activation evoked by IgG-FcR binding, we first used a pharmacological approach. Rat alveolar macrophages were pretreated with a FLAP inhibitor for 10 minutes before IgG-SRBC challenge, and Syk phosphorylation was assessed. MK 886 at 1 μM inhibited Syk activation evoked by FcγR engagement (Figure 7), suggesting an endogenous role for LTs.
Effect of MK 886 on FcγR-induced Syk activation. Rat alveolar macrophages were pretreated with MK 886 at 1 μM for 10 minutes prior to IgG-SRBC challenge (1:100 ratio) and then incubated for 7 minutes at 37°C. Incubations were terminated by addition of lysis buffer, and lysates were subjected to immunoprecipitation and immunoblotting as described in “Materials and methods.” Immunoblots in upper panels represent phosphorylated Syk detected with antiphosphotyrosine antibody, and those in lower panels, the amounts of Syk protein evaluated with anti-Syk antibody. Results are representative of 3 separate experiments.
Effect of MK 886 on FcγR-induced Syk activation. Rat alveolar macrophages were pretreated with MK 886 at 1 μM for 10 minutes prior to IgG-SRBC challenge (1:100 ratio) and then incubated for 7 minutes at 37°C. Incubations were terminated by addition of lysis buffer, and lysates were subjected to immunoprecipitation and immunoblotting as described in “Materials and methods.” Immunoblots in upper panels represent phosphorylated Syk detected with antiphosphotyrosine antibody, and those in lower panels, the amounts of Syk protein evaluated with anti-Syk antibody. Results are representative of 3 separate experiments.
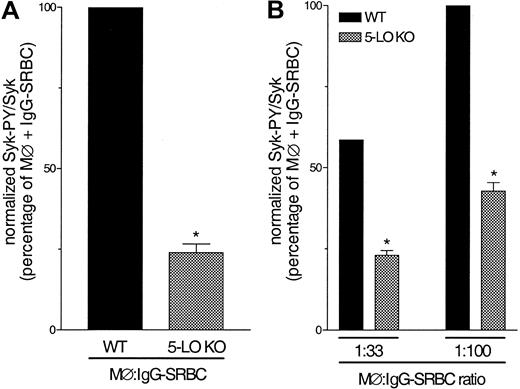
Using alveolar (Figure 8A) and peritoneal (Figure 8B) macrophages obtained from 5-LO KO mice, we were able to confirm our pharmacological data. Both cell populations harvested from 5-LO KO animals showed a significant decrease in Syk activation in response to IgG-SRBC, compared with that observed in WT cells.
Effect of 5-LO gene knockout in FcγR-mediated Syk activation. (A) Alveolar macrophages harvested from WT and 5-LO KO mice were challenged with IgG-SRBC (1:100 ratio) and then incubated for 7 minutes at 37°C. (B) Peritoneal macrophages harvested from WT and 5-LO KO mice were challenged with IgG-SRBCs (1:33 and 1:100 ratio) and then incubated for 7 minutes at 37°C. Incubations were terminated by addition of lysis buffer, and lysates were subjected to immunoprecipitation and immunoblotting as described in “Materials and methods.” Data are given as means ± SEMs. *P < .05 compared with WT group (ANOVA followed by Bonferroni t test). Results are representative of 3 separate experiments.
Effect of 5-LO gene knockout in FcγR-mediated Syk activation. (A) Alveolar macrophages harvested from WT and 5-LO KO mice were challenged with IgG-SRBC (1:100 ratio) and then incubated for 7 minutes at 37°C. (B) Peritoneal macrophages harvested from WT and 5-LO KO mice were challenged with IgG-SRBCs (1:33 and 1:100 ratio) and then incubated for 7 minutes at 37°C. Incubations were terminated by addition of lysis buffer, and lysates were subjected to immunoprecipitation and immunoblotting as described in “Materials and methods.” Data are given as means ± SEMs. *P < .05 compared with WT group (ANOVA followed by Bonferroni t test). Results are representative of 3 separate experiments.
Discussion
In the present study, we extended our exploration of the mechanisms by which LTs modulate macrophage phagocytosis by focusing on the nonreceptor PTK Syk. We used both genetic and pharmacologic approaches to assess the role of LTs in Syk activation in 2 distinct types of phagocytosis–ingestion of IgG-coated targets via the FcγR and of apoptotic thymocytes, which proceeds, in part, via the PS receptor. Our data provide 3 novel insights. First, FcγR-mediated Syk activation is enhanced by exogenous and endogenous LTB4. Second, FcγR-mediated Syk activation as well as its amplification by LTB4 is Ca2+ regulated. Third, unlike FcγR-mediated phagocytosis, apoptotic cell ingestion is not associated with Syk activation nor influenced by LTB4. The proposed mechanisms by which LTB4, Ca2+, and Syk inter-relate in FcγR-mediated phagocytosis are depicted in the model illustrated in Figure 9.
Schematic representation of proposed mechanism by which LTB4 amplifies FcγR-mediated phagocytosis. (A) Resting cell is characterized by unoccupied FcγR and BLT, low [Ca2+], and inactive Syk and 5-LO. (B) Upon FcγR cross-linking by an IgG-coated particle, tyrosine residues in the cytoplasmatic tail of the FcγR become phosphorylated, creating a docking site for Syk, which in turn becomes tyrosine phosphorylated (activated). Syk then initiates a variety of other signaling events, culminating in increase of [Ca2+], 5-LO activation with LTB4 synthesis, and phagocytosis. Endogenously produced or exogenously added LTB4 interacts with BLT receptors. This further increases [Ca2+], which amplifies Syk activation as well as phagocytosis.
Schematic representation of proposed mechanism by which LTB4 amplifies FcγR-mediated phagocytosis. (A) Resting cell is characterized by unoccupied FcγR and BLT, low [Ca2+], and inactive Syk and 5-LO. (B) Upon FcγR cross-linking by an IgG-coated particle, tyrosine residues in the cytoplasmatic tail of the FcγR become phosphorylated, creating a docking site for Syk, which in turn becomes tyrosine phosphorylated (activated). Syk then initiates a variety of other signaling events, culminating in increase of [Ca2+], 5-LO activation with LTB4 synthesis, and phagocytosis. Endogenously produced or exogenously added LTB4 interacts with BLT receptors. This further increases [Ca2+], which amplifies Syk activation as well as phagocytosis.
Because activation of Syk is an essential proximal event in FcγR-mediated ingestion,23 it was a logical potential target for the stimulatory effects of LTs. Indeed, Western blotting of immunoprecipitated Syk showed that LTB4 induced a dose-(Figure 3A) and time-dependent increase in tyrosine phosphorylation of Syk evoked by IgG-FcγR interaction. LTB4 amplification of FcγR-induced Syk phosphorylation was observed in both alveolar and peritoneal macrophages. The amplifying effect of LTB4 on Syk activation is directly dependent on the engagement of FcγR, since no Syk activation occurred after treatment of the cells with LTB4 in the absence of the IgG-coated target. This finding suggests that Syk activation requires interaction with the immunoreceptor tyrosine–based activation motifs in the γ-chain of FcγRI and FcγRIII or in the cytoplasmatic domain of FcγRIIA. Thus, the amplifying effect of LTB4 requires that Syk be recruited from the cytoplasm to the membrane.
The pharmacological treatment of WT cells with an LT synthesis inhibitor (the FLAP24 inhibitor MK 886) (Figure 7) or the use of 5-LO KO cells (Figure 8) showed less Syk activation upon IgG-SRBC challenge, suggesting that endogenous LTs contribute to the observed degree of Syk activation. This same effect was observed in phagocytosis assays, where we observed a substantial but incomplete inhibition with MK 886 or in 5-LO KO cells. Taken together, these results implicate a 5-LO metabolite as an amplifier of FcγR-mediated phagocytosis through a mechanism that involves Syk activation. It is very likely that LTB4 is the endogenous 5-LO product responsible for amplification of Syk activation, since LTB4 was the only 5-LO product able to augment Syk activation when added to the cells. The identification of LTB4 as an endogenous metabolite responsible for Syk phosphorylation is also suggested by results with LTB4RA (data not shown). That LTD4 and 5-HETE also up-regulate FcγR-dependent phagocytosis by macrophages15 yet fail to enhance Syk activation indicates that these alternative 5-LO products must act on molecular targets other than Syk. Taken together, the results of these experiments demonstrate for the first time that LTB4, which is known to be produced upon FcγR engagement,15,25 acts as an autocrine/paracrine stimulus for enhanced phagocytosis by enhancing activation of the proximal signal, Syk.
LTB4 interaction with its receptor leads to the generation of various intracellular signals, such as Ca2+ and activation of various kinases, including PKC, mitogen-activated protein kinases (MAPK), PI-3K, and PTK.26-29 Because Ca2+ flux occurs within 1 minute of LTB4 receptor binding,26 we evaluated the role of Ca2+ in LTB4-induced Syk activation using both intracellular and extracellular chelators. In this context, we demonstrated that Ca2+ plays a pivotal role in Syk activation evoked by IgG-FcγR interaction, as well as in LTB4-mediated amplification of Syk activation (Figure 6). Interestingly, Syk activation induced by IgG-FcγR interaction was exclusively dependent on intracellular Ca2+, while in LTB4-mediated amplification of Syk activation we observed the involvement of both extracellular and intracellular Ca2+. It is well known that increased intracellular Ca2+ is a downstream consequence of Syk activation.1,30 By contrast, we are aware of only a single report showing that increases in intracellular free calcium are necessary for Syk activation—in particular, in platelet-activating factor-induced activation of Syk in human B lymphoblastoid cells.31 Our results establish for the first time that activation of Syk in macrophages is calcium dependent.
In contrast to FcγR-mediated phagocytosis, it had been unknown whether Syk is activated during macrophage phagocytosis of apoptotic cells. In order to answer this question, we incubated macrophages with apoptotic thymocytes and analyzed Syk phosphorylation. Interestingly, no Syk activation was observed under any of the conditions tested (Figure 2). In view of the importance of Syk as a target for LTB4 amplification of FcγR-mediated phagocytosis, the divergent results during apoptotic cell phagocytosis provide an explanation for the LT independence of this process, since no difference in apoptotic cell phagocytosis was observed with LTB4 treatment, with LT synthesis inhibitors, or with 5-LO KO cells (Figure 1). Although the MAPK pathway contributes to signaling in response to both FcγR and LTB4,1,7,28 one possible explanation for the LT independence of apoptotic cell ingestion is that this process is independent of MAPK activation.8 This possibility requires direct investigation.
Phagocytosis of apoptotic cells is not associated with LT biosynthesis; in fact, this process has been reported to inhibit LT synthesis.32 Our results therefore allow us to speculate that the divergence between these 2 phagocytic pathways in their dependence on Syk and LTs may reflect the fundamentally different degrees of inflammation associated with FcR-dependent clearance and apoptotic cell ingestion. The fact that FcγR-mediated phagocytosis depends on Syk activation, an LTB4-regulated event, is logical in the context of the intense inflammation associated with this process. By contrast, the Syk- and LTB4 independence during apoptotic cell ingestion is logical in view of the noninflammatory nature of apoptotic cell clearance.
Prepublished online as Blood First Edition Paper, May 1, 2003; DOI 10.1182/blood-2003-02-0534.
Supported by Conselho Nacional de Pesquisa (CNPq–Brazil), HL 58897, HL 56309, and HL 6157 from the United States Public Health Service; by Merit Review funding and a Research Enhancement Award Program (REAP) grant from the Department of Veterans Affairs; and funding from the Michigan Life Sciences Initiative.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Teresa Marshall and Susan Phare for technical assistance.



![Figure 9. Schematic representation of proposed mechanism by which LTB4 amplifies FcγR-mediated phagocytosis. (A) Resting cell is characterized by unoccupied FcγR and BLT, low [Ca2+], and inactive Syk and 5-LO. (B) Upon FcγR cross-linking by an IgG-coated particle, tyrosine residues in the cytoplasmatic tail of the FcγR become phosphorylated, creating a docking site for Syk, which in turn becomes tyrosine phosphorylated (activated). Syk then initiates a variety of other signaling events, culminating in increase of [Ca2+], 5-LO activation with LTB4 synthesis, and phagocytosis. Endogenously produced or exogenously added LTB4 interacts with BLT receptors. This further increases [Ca2+], which amplifies Syk activation as well as phagocytosis.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/5/10.1182_blood-2003-02-0534/6/m_h81734879009.jpeg?Expires=1765083377&Signature=GrYfDOx-QHxS4gV3MVvdIbWC~P0MEo-RhO6-WAZLSgxxPskozykJ61ptu74yWyVu7O0~NG5vfgsX0yapP02rCOvbAP4PMcQnjnDJ2zyRR0vQW2~DBJ4z4q18-alB~fGc8FVktJicrG~siuCE7JKiwsafUjV882v8Gs9HG2Eqq8JkPpwjGTK3y~AISGA1Zc6m8FVeaYG2vhg97TPMFp6ORIYWObgeSijBm8UdHAWiq5TR1wP8Ix7Ec-3UKn-0145I02WlEqwMf-IfRXDvrGmqAS9qiSeXsz3eIsFpAPwqJY4DhQwGwkiJ3EJaUvdRLGlSy5b03d3adeG2LMmc8pcgZA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
