Abstract
Mutations in the Nramp1 gene (Slc11a1) cause susceptibility to infection by intracellular pathogens. The Nramp1 protein is expressed at the phagosomal membrane of macrophages and neutrophils and is a paralog of the Nramp2 (Slc11a2) iron transporter. The Nramp1 transport mechanism at the phagosomal membrane has remained controversial. An Nramp1 protein modified by insertion of a hemagglutinin epitope into the predicted TM7/8 loop was expressed at the plasma membrane of Chinese hamster ovary cells as demonstrated by immunofluorescence and surface biotinylation. Experiments in Nramp1HA transfectants using the metal-sensitive fluorophors calcein and Fura2 showed that Nramp1HA can mediate Fe2+, Mn2+, and Co2+ uptake. Similar results were obtained in transport studies using radioisotopic 55Fe2+ and 54Mn2+. Nramp1HA transport was dependent on time, temperature, and acidic pH, occurring down the proton gradient. These experiments suggest that Nramp1HA may be a more efficient transporter of Mn2+ compared to Fe2+ and a more efficient Mn2+ transporter than Nramp2HA. The membrane topology and transport properties of Nramp1HA and Nramp2HA were indistinguishable, suggesting that Nramp1 divalent-metal transport at the phagosomal membrane is mechanistically similar to that of Nramp2 at the membrane of acidified endosomes. These results clarify the mechanism by which Nramp1 contributes to phagocyte defenses against infections.
Introduction
Mutations in the mouse Nramp1 gene (natural resistance associated macrophage protein 1, also known as Slc11a1; OMIM [Online Mendelian Inheritance in Man] #600266) cause susceptibility to infection by several intracellular pathogens including Mycobacterium, Leishmania, and Salmonella.1 Likewise, polymorphic variants of NRAMP1 are associated with human susceptibility to tuberculosis and leprosy in endemic areas of disease.2,3 Nramp1 mRNA is abundant in mouse macrophages and human neutrophils, where it encodes a 90- to 100-kDa integral membrane phosphoglycoprotein4 present in the Lamp1-positive late endosomes and lysosomes5 and in gelatinase-positive tertiary granules,6 respectively. Following phagocytosis, Nramp1 is rapidly recruited to and remains associated with the membrane of phagosomes containing either inert particles5 or live bacteria/parasites.7-9 Recruitment of Nramp1 to the membrane of Mycobacteria containing phagosomes is associated with bacteriostasis, bacterial damage, and appears to antagonize the ability of Mycobacterium to block phagolysosomal fusion and acidification.10,11 Similarly, Nramp1 appears to impair the ability of Salmonella to shelter in a vacuole that does not fuse to early endosomes and that remains negative for the mannose-6-phosphate receptor.9
Nramp1 has a close mammalian paralog Nramp2 (OMIM #600523; also known as Dmt1, Dct1, Slc11a2)12 that has been functionally characterized. Transport studies using Xenopus laevis oocytes13 and mammalian cell lines14,15 have demonstrated that Nramp2 is a broad specificity divalent-metal transporter that functions in a pH-dependent fashion, stimulated by acidic pH, which is suggestive of a proton/metal-symport mechanism.13 In mice, Nramp2 is expressed at the duodenum brush border, where it is responsible for transferrin-independent uptake of dietary iron from the intestinal lumen.16 Nramp2 also colocalizes with transferrin in the recycling endosomes of many cell types, including reticulocytes,17,18 where it transports iron from the acidified lumen of the endosomes into the cytoplasm.19-21 A mutation in Nramp2 impairs both of these aspects of iron homeostasis and causes microcytic anemia in mk mice and Belgrade rats.17,20,22
These studies of Nramp2 have suggested a role for Nramp1 in metal transport at the phagosomal membrane. However, controversy regarding the mechanism of Nramp1 metal transport with respect to protein topology and the direction of metal transport in relation to the proton gradient has persisted.23 Previously, we used a metal-sensitive fluorophor (Fura-FF6) chemically coupled to zymosan particles to monitor divalent-metal flux by means of real-time microfluorescence imaging across the membrane of single phagosomes formed in live Nramp1+/+ and Nramp1–/– primary macrophages.24 Nramp1-positive phagosomes exhibited reduced intraphagosomal accumulation of externally added Mn2+ ions. Likewise, Nramp1-positive phagosomes showed increased release of Mn2+ ions from preloaded Fura-FF6–zymosan particles. Mn2+ transport by Nramp1 was abrogated by bafilomycin (vacuolar H+/ATPase inhibitor), suggesting that Nramp1 functions to efflux Mn2+ ions in a pH-dependent fashion from acidified phagosomes down the proton gradient. This mechanism is similar to Fe2+ transport by Nramp2 across the membrane of acidified endosomes, implying that Nramp1/2 share a common transport mechanism and suggests that Nramp1 exerts its antimicrobial activity through depletion of divalent metals from the phagosomal space. This hypothesis is in agreement with results from other independent studies.25-28
In contrast, increased Nramp1-dependent accumulation/binding of isotopic Fe2+ into isolated phagosomes containing either Latex beads or M avium has been reported.29-31 Increased Fe2+ accumulation was blocked by anti-Nramp1 antibodies, suggesting that Nramp1 may transport cytoplasmic Fe2+into phagosomes. In an independent study, injection of Nramp1 mRNA into X laevis oocytes induced small Zn2+-dependent inward currents suggestive of metal uptake.32 Additional studies of pH-dependent transport of isotopic Zn2+ led these authors to conclude that Nramp1 may transport cytoplasmic metals into the phagosome by a proton/divalent-metal antiport mechanism. These authors proposed that increased phagosomal Fe2+ would stimulate oxygen radical production in situ via the Fenton reaction resulting in increased bactericidal activity.29-32 However, phagosomal metal influx mediated by Nramp1 requires that Nramp1 would have to be mechanistically distinct from Nramp2, with respect to direction of transport, use of the transmembrane pH gradient, and/or membrane topology of the proteins.
In order to address these differing conclusions regarding the transport function of Nramp1, we sought to directly compare Nramp1 and Nramp2 proteins. Should Nramp1/2 have the same membrane organization and work by the same mechanism, ectopic expression of Nramp1 at the plasma membrane of whole cells would, like Nramp2, be expected to result in the pH-dependent uptake of metals from the extracellular milieu. To test this proposal, independent Chinese hamster ovary (CHO) transfectants expressing HA-tagged Nramp1 protein at the plasma membrane were created. Comparative analysis of Nramp1 and Nramp2 activity was performed with respect to transport function, pH dependence, and metal ion selectivity. These studies show that the 2 proteins are mechanistically indistinguishable but may have different substrate selectivity.
Materials and methods
Cell culture
A mammalian expression plasmid (Nramp1HA-pCB6) containing a neomycin resistance gene together with the complete Nramp1 coding sequence modified by the in-frame insertion of a hemagglutinin (HA) epitope (YPYDVPDYAS) at amino-acid position 330 was constructed as was previously described for the corresponding HA-tagged Nramp2 construct (Nramp2HA-pCB6).15 CHO LR73 cells33 were cultured as previously described15 and transfected with Nramp1HA-pCB6 using a calcium-phosphate coprecipitation method.34 Stably transfected clones were selected (geneticin, 1 mg/mL; Invitrogen, Burlington, ON, Canada) for 10 days, followed by isolation and expansion of individual clones. Total protein extracts were prepared from CHO transfectants, and Nramp1HA protein expression was analyzed by immunoblotting with anti-HA monoclonal antibodies. The CHO transfectant N2-310a stably expressing Nramp2HA was previously reported,15 as were the CHO transfectants stably expressing Nramp1/2 proteins modified with a C-terminal c-Myc epitope (Nramp1Myc, Nramp2Myc).5,18 All chemicals were purchased from Sigma Chemical (Oakville, ON, Canada) unless otherwise noted.
Protein preparations and immunoblotting
Total cell protein extracts were prepared by solubilizing cell pellets in Tris [tris(hydroxymethyl)aminomethane]-buffered saline (TBS; 100 mM Tris-HCl pH 7.5, 150 mM NaCl) containing 1% Triton X-100, 1 mM PMSF (phenylmethanesulfonyl fluoride), 2 μg/mL leupeptin, 2 μg/mL aprotinin, 1 μg/mL pepstatin, 2 mM EDTA (ethylenediaminetetraacetic acid), and 20% glycerol (20 minutes, on ice), and the insoluble material was removed by centrifugation (16 000 g, 10 minutes, 4°C). Crude membrane fractions were prepared as previously described.35 Protein concentrations were determined using the Bradford assay (BioRad, Missisauga, ON, Canada). Discontinuous sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was done with a 4% polyacrylamide stacking gel and a 10% separating gel.36 Proteins were mixed with sample buffer36 and denatured (20°C, 20-30 minutes) prior to loading onto gels. Prestained molecular mass markers (BioRad) were included in all SDS-PAGE experiments.
For immunoblotting, proteins separated by SDS-PAGE were transferred electrophoretically to polyvinylidine difluoride membranes (4°C, 450 V/hr; Schleicher and Schuell, Keene, NH) in buffer containing 20% methanol and 0.01% SDS.37 Membranes were blocked (30 minutes-1 hour, 20°C) in TBS containing 0.25% Tween-20 (TBST) and 5% nonfat skim milk. Membranes were then incubated in blocking buffer for either 3 hours at 20°C or 16 hours at 4°C with one of the following primary antibodies: mouse monoclonal anti–HA 16B12 or anti–c-Myc 9E10 antibodies (both used at 1/1000; Covance, Princeton, NJ), or affinity purified polyclonal rabbit anti-Nramp2NT or anti-Nramp1NT antibodies (used at 1/1000), which are directed against the amino-terminus of Nramp2 and Nramp1, respectively.4,18 Membranes were washed in TBST (3 × 5 minutes, 20°C) prior to incubation with horseradish peroxidase–conjugated goat anti–rabbit IgG or anti–mouse IgG (1/10 000; Perkin-Elmer, Woodbridge, ON, Canada) secondary antibodies (1 hour, 20°C) in blocking buffer. Membranes were washed in TBST (4 × 5 minutes, 20°C), and specific immune complexes were revealed by enhanced chemiluminescence (ECL; Perkin-Elmer) and autoradiography (Kodak, Rochester, NY).
Immunofluorescence
Immunofluorescence on nonpermeabilized whole cells was done as previously described.15 Live cells grown on coverslips were blocked (15 minutes, 4°C) and then incubated (1 hour, 4°C) with mouse anti–HA monoclonal antibodies (diluted 1/50) or with affinity-purified rabbit polyclonal anti–Nramp1NT antiserum (diluted 1/50). Cells were then washed extensively, fixed with 4% paraformaldehyde (20 minutes, on ice), and incubated (1 hour, 20°C) with either goat anti–mouse IgG Cy3 (1/200; Jackson Laboratories, West Grove, PA) or goat anti–rabbit IgG rhodamine (1/100; Jackson Laboratories) secondary antibodies. Following extensive washing, the coverslips were mounted onto glass slides using Permafluor antifade reagent (Shandon, Pittsburgh, PA). The cells were photographed by epifluorescence microscopy using a Nikon (Tokyo, Japan) Eclipse E800 microscope mounted with a 60 × oil-immersion objective lens and a Nikon DXM1200 digital camera.
Surface biotinylation
Attached cells were rinsed twice with ice-cold phosphate-buffered saline (PBS) and once with ice-cold borate buffer (10.0 mM boric acid, 154 mM NaCl, 7.2 mM KCl, 1.8 mM CaCl2, pH 9.0), and then incubated (15 minutes, on ice) in the same buffer containing Sulfo-NHS-LC-biotin (0.5 mg/mL; Pierce, Milwaukee, WI). Unreacted biotin was removed by 3 washes with TBS containing 0.2 M glycine and one wash with TBS. Total cell protein extracts were prepared from the biotinylated cells. Biotinylated proteins were captured by incubation (16 hours, 4°C) of 100 μg of total cell extracts with 50 μL of a 50% (wt/vol) slurry of streptavidin-agarose beads (Pierce) in 500 μL (total) of TBS buffer containing 1% Triton X-100 and protease inhibitors. Beads were washed 5 times with TBS/1% Triton X-100, and bound proteins were eluted in 50 μL of sample buffer36 containing 5% β-mercaptoethanol (30 minutes, 20°C). Captured proteins were analyzed by SDS-PAGE and immunoblotted with either anti–HA or anti–cMyc monoclonal antibodies, or with anti–Nramp1NT or anti–Nramp2NT affinity-purified antisera.
Divalent-metal transport by fluorescence quenching
Cells were detached using PBS/citrate (5-10 minutes, 37°C) and resuspended in loading medium (α-MEM [minimum essential media], 0.5 mg/mL BSA [bovine serum albumin], 25 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] pH 7.4). Cells (1 × 106 per assay) were centrifuged and resuspended in prewarmed (37°C) loading medium containing either 0.25 μM calcein-AM (acetoxy-methylester) or 2 μM Fura2-AM (1 mM stock solutions in 100% DMSO [dimethyl sulfoxide]; Molecular Probes, Eugene, OR) followed by incubation at 37°C for 10 minutes or 1 hour, respectively. After this loading period, cells were washed and resuspended in loading medium. Transport assays were performed with a Perkin-Elmer LS-50B fluorometer equipped with a stirring and water-jacketed cuvette holder. To measure metal-dependent calcein/Fura-2 quenching, the fluorometer settings were excitation λ = 488/360 nm and emission λ = 517/510 nm with 5/7.5 micron bandpass slit widths. Immediately prior to each transport assay, an aliquot of cells was washed once in PBS (37°C), resuspended in 500 μL transport buffer (37°C; 150 mM NaCl, MES [2-(N-morpholino)ethanesulfonic acid] pH 5-6 or HEPES pH 7-8), transferred into a prewarmed cuvette, and thereafter cell-associated fluorescence was recorded continuously (0.5-second intervals). After a stabilization period (60 seconds), divalent-metal was added and cell-associated fluorescence was recorded for an additional 120 seconds. Divalent-metals MnCl2 and CoCl2 were prepared as 2 mM stock solutions in water. Iron was prepared fresh as a 2 mM FeNH4SO4 stock solution in transport buffer with a 25:1 molar ratio of sodium ascorbate (50 mM) to maintain the metal in its reduced form. Fluorescence quenching data were normalized for individual samples to the fluorescence value taken at 70 seconds to facilitate visual presentation of the data.
Radioisotopic divalent-metal transport assay
Cells were harvested as described for the fluorescent assay and resuspended (107 cells per assay) in 1.5 mL of transport buffer (25 mM Tris, 25 mM MES, 140 mM NaCl, 5.4 mM KCl, 5 mM glucose, 1.8 mM CaCl2, pH 5.5). Transport was initiated by addition of 1 mL of radioisotope buffer, followed by incubation at 20°C. Mn2+ radioisotope buffer was transport buffer containing 0.45 μM 54Mn (54MnCl2, 13.4 Ci/mmol [495.8 GBq/mmol]; Perkin-Elmer) and 22.05 μM MnCl2 (22.5 μM total Mn, 49:1 molar ratio of cold MnCl2:54Mn), giving a final concentration of 9 μM Mn2+ in each transport reaction. Fe2+ radioisotope buffer contained 1.125 μM 55Fe (55FeCl3, 3.012 Ci/mmol [111.4 GBq/mmol]; Perkin-Elmer) and 21.375 μM FeNH4SO4 (22.5 μM total Fe, 19:1 molar ratio of cold FeNH4SO4/55Fe) together with 1.125 mM sodium ascorbate (50:1 molar ratio ascorbate–Fe) giving a final concentration of 9 μM Fe2+. At predetermined time intervals (0, 5, 15, 30 minutes), 500 μL cell aliquots were transferred to microcentrifuge tubes containing a 200-μL oil cushion (4:1 silicon oil-mineral oil). Cells were pelleted by centrifugation (12 000 g, 10 seconds) through the oil cushion, the aqueous phase was removed, and the walls of the tube were washed with transport buffer. The oil cushion was removed, and the cell pellets were digested with 0.1N NaOH. Lysates were neutralized by addition of an equal volume of 0.1N HCl, and cell-associated radioactivity was determined by liquid scintillation counting. The protein content of each lysate was determined using the Bradford assay (BioRad). Background radioisotope binding was determined by parallel control transport experiments performed on ice. For metal ion selectivity studies, cells (3 × 106) were resuspended in 375 μL transport buffer, and transport was initiated by addition of an equal volume of radioisotope buffer. Stock Mn2+ radioisotope buffer contained 0.4 μM 54Mn and 19.6 μM MnCl2 (20 μM total Mn), and stock Fe2+ radioisotope buffer contained 1 μM 55Fe and 19 μM FeNH4SO4 (20 μM total Fe) in 1 mM sodium ascorbate. Cells were incubated for 10 minutes with serial 2-fold dilutions (0.3125-10 μM final) of these stocks, and the amount of cell-associated radioactivity was determined as described above.
Results
PM expression of Nramp1HA detected by immunofluorescence
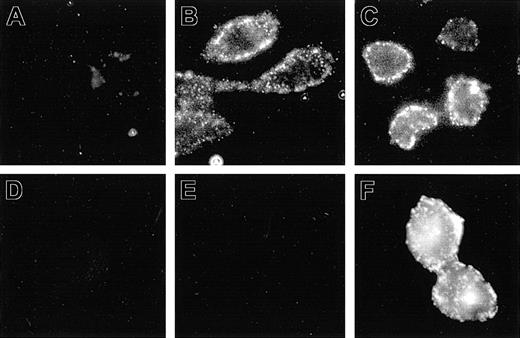
The aim of the present study was to express Nramp1 at the plasma membrane (PM) of CHO cells, where its transport properties could be studied and compared to Nramp2. Previous studies done in primary macrophage, together with RAW264.7 and/or CHO cells transfected with Nramp1 and Nramp2 constructs modified by the addition of a C-terminal c-Myc tag (Nramp1Myc, Nramp2Myc), indicated different subcellular distributions of the 2 proteins.5,18 Nramp2Myc was found to be expressed at the PM and in recycling endosomes, whereas Nramp1Myc was not found at the PM but was detected in lysosomes/late endosomes. Likewise, an Nramp2 protein modified by the insertion of an HA epitope (Nramp2HA) into the predicted loop delineated by putative transmembrane (TM) domains 7 and 8 was shown to be functional and expressed at the PM of CHO cells with the HA epitope extracellularly located and accessible to antibodies added to the external medium.15 In an attempt to identify cell lines expressing Nramp1 at the PM, Nramp1 cDNA was similarly modified by insertion of HA epitope in the TM7/8 loop, followed by transfection into CHO cells. Several transfectants stably expressing Nramp1HA protein were identified (Figures 1, 2, 3; N1-1816, N1-94, N1-116, and N1-123). The possibility of PM localized Nramp1HA protein was examined by immunofluorescent detection of the HA epitope in nonpermeabilized cells, and representative images are shown in Figure 1. In nonpermeabilized N1-1816 cells, Nramp1HA staining was detected at the cell periphery by extracellular anti–HA monoclonal antibodies (Figure 1B), revealing a staining pattern similar to that observed in control N2-310a cells expressing Nramp2HA (Figure 1C). PM staining of N1-1816 cells was Nramp1HA-specific, as it was absent in untransfected CHO cell controls (Figure 1A) and in N1-1816 cells stained with the secondary antibody alone (Figure 1D). This demonstrates that the predicted TM7/8 loop of Nramp1HA is extracellular in N1-1816 cells. Additionally, Nramp1HA was detected by an anti–Nramp1NT rabbit polyclonal antiserum directed against the amino terminus of the protein in Triton X-100 permeabilized N1-1816 cells (Figure 1F), but not in intact N1-1816 cells (Figure 1E), demonstrating that the N-terminus of Nramp1HA in N1-1816 cells was intracellular. Together, these results indicate that Nramp1HA was expressed at the PM, and that it has a membrane topology similar to that of Nramp2HA.
Detection of Nramp1HA and Nramp2HA proteins by immunofluorescence in nonpermeabilized cells. Mouse Nramp1 and Nramp2 cDNAs were modified by in-frame insertion of a hemagglutinin (HA) epitope (YPYDVPDYAS) into the predicted loop delineated by putative TM7 and TM8 followed by transfection into CHO cells. Surface expression was monitored by immunofluorescent detection of the HA epitope in nonpermeabilized cells with the mouse monoclonal anti–HA antibody 16B12. Control CHO cells (A), Nramp2HA-expressing CHO cell line N2-310a (C), and Nramp1HA-expressing CHO cell line N1-1816 (B,D,E,F) were grown on glass coverslips and incubated (1 hour, 4°C) with mouse anti–HA monoclonal primary antibodies (A-C; 1/50 dilution), affinity-purified rabbit polyclonal anti–Nramp1NT antibodies (E; diluted 1/50), or without primary antibody (D). Cells were then fixed, and incubated with goat anti–mouse-IgG-Cy3 (A-D; 1/200) or goat anti–rabbit IgG-rhodamine (E-F; 1/100) secondary antibodies. In panel F, N1-1816 cells were fixed with 4% paraformaldehyde and then permeabilized with 0.5% Triton X-100 prior to addition of primary antibodies to detect both intracellular and PM-localized Nramp1HA protein. Original magnification, × 600 for all panels.
Detection of Nramp1HA and Nramp2HA proteins by immunofluorescence in nonpermeabilized cells. Mouse Nramp1 and Nramp2 cDNAs were modified by in-frame insertion of a hemagglutinin (HA) epitope (YPYDVPDYAS) into the predicted loop delineated by putative TM7 and TM8 followed by transfection into CHO cells. Surface expression was monitored by immunofluorescent detection of the HA epitope in nonpermeabilized cells with the mouse monoclonal anti–HA antibody 16B12. Control CHO cells (A), Nramp2HA-expressing CHO cell line N2-310a (C), and Nramp1HA-expressing CHO cell line N1-1816 (B,D,E,F) were grown on glass coverslips and incubated (1 hour, 4°C) with mouse anti–HA monoclonal primary antibodies (A-C; 1/50 dilution), affinity-purified rabbit polyclonal anti–Nramp1NT antibodies (E; diluted 1/50), or without primary antibody (D). Cells were then fixed, and incubated with goat anti–mouse-IgG-Cy3 (A-D; 1/200) or goat anti–rabbit IgG-rhodamine (E-F; 1/100) secondary antibodies. In panel F, N1-1816 cells were fixed with 4% paraformaldehyde and then permeabilized with 0.5% Triton X-100 prior to addition of primary antibodies to detect both intracellular and PM-localized Nramp1HA protein. Original magnification, × 600 for all panels.
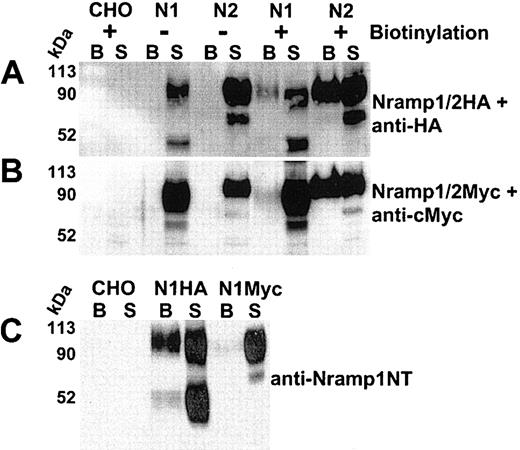
Cell-surface biotinylation of CHO cells expressing Nramp1/2HA or Nramp1/2Myc proteins. Cell-surface biotinylation was used to assess plasma membrane expression of Nramp1 (N1) and Nramp2 (N2) proteins modified either by insertion of an HA epitope into the loop delineated by predicted TM7/8 (panel A) or by a c-Myc epitope at the C-terminus (panel B). Live cells were labeled with membrane impermeant Sulfo-NHS-LC-biotin (see “Materials and methods”). Total cell protein extracts were prepared, and biotinylated proteins were isolated by affinity capture with streptavidin-agarose beads (from 100 μg of cell extract). Captured biotinylated proteins (B, the entire eluate), and postcapture supernatant (S; 10% of remaining supernatant volume) from CHO controls and from Nramp1/2HA (panel A) or Nramp1/2Myc (panel B) transfectants were analyzed by SDS-PAGE and immunoblotted with the corresponding anti–HA (panel A) or anti–cMyc monoclonal antibodies (panel B). Panel C shows a direct comparison of biotinylated proteins from CHO control, Nramp1HA, and Nramp1Myc expressing CHO transfectants immunoblotted with affinity-purified rabbit anti–Nramp1NT antibodies that recognize both Nramp1HA and Nramp1Myc proteins. The size of the molecular mass markers is shown to the left of the immunoblots.
Cell-surface biotinylation of CHO cells expressing Nramp1/2HA or Nramp1/2Myc proteins. Cell-surface biotinylation was used to assess plasma membrane expression of Nramp1 (N1) and Nramp2 (N2) proteins modified either by insertion of an HA epitope into the loop delineated by predicted TM7/8 (panel A) or by a c-Myc epitope at the C-terminus (panel B). Live cells were labeled with membrane impermeant Sulfo-NHS-LC-biotin (see “Materials and methods”). Total cell protein extracts were prepared, and biotinylated proteins were isolated by affinity capture with streptavidin-agarose beads (from 100 μg of cell extract). Captured biotinylated proteins (B, the entire eluate), and postcapture supernatant (S; 10% of remaining supernatant volume) from CHO controls and from Nramp1/2HA (panel A) or Nramp1/2Myc (panel B) transfectants were analyzed by SDS-PAGE and immunoblotted with the corresponding anti–HA (panel A) or anti–cMyc monoclonal antibodies (panel B). Panel C shows a direct comparison of biotinylated proteins from CHO control, Nramp1HA, and Nramp1Myc expressing CHO transfectants immunoblotted with affinity-purified rabbit anti–Nramp1NT antibodies that recognize both Nramp1HA and Nramp1Myc proteins. The size of the molecular mass markers is shown to the left of the immunoblots.
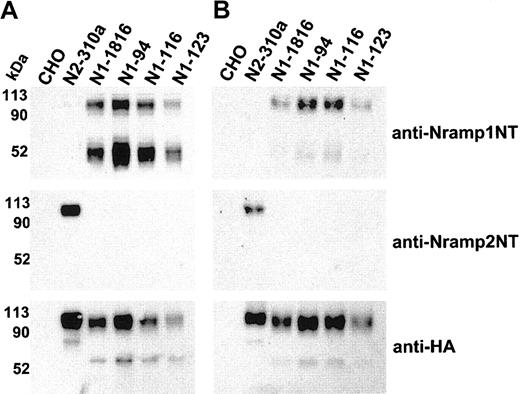
Immunoblot detection of cell-surface biotinylated Nramp1HA in independent CHO transfectants. Membrane protein fractions were prepared from independent CHO transfectants stably expressing Nramp1HA (N1-94, N1-116, N1-123, N1-1816) or Nramp2HA (N2-310a) and from CHO controls. These proteins (10 μg/lane) were analyzed by immunoblotting with affinity-purified rabbit polyclonal anti–Nramp1NT or anti–Nramp2NT, or with monoclonal anti–HA antibodies (panel A). In panel B, the cell lines were labeled by surface biotinylation (as described in “Materials and methods”), and biotinylated proteins captured with streptavidin-agarose beads were analyzed by immunoblotting with affinity-purified rabbit polyclonal anti–Nramp1NT or anti–Nramp2NT antibodies, or monoclonal anti–HA antibodies. The size of the molecular mass markers is shown to the left of the immunoblots.
Immunoblot detection of cell-surface biotinylated Nramp1HA in independent CHO transfectants. Membrane protein fractions were prepared from independent CHO transfectants stably expressing Nramp1HA (N1-94, N1-116, N1-123, N1-1816) or Nramp2HA (N2-310a) and from CHO controls. These proteins (10 μg/lane) were analyzed by immunoblotting with affinity-purified rabbit polyclonal anti–Nramp1NT or anti–Nramp2NT, or with monoclonal anti–HA antibodies (panel A). In panel B, the cell lines were labeled by surface biotinylation (as described in “Materials and methods”), and biotinylated proteins captured with streptavidin-agarose beads were analyzed by immunoblotting with affinity-purified rabbit polyclonal anti–Nramp1NT or anti–Nramp2NT antibodies, or monoclonal anti–HA antibodies. The size of the molecular mass markers is shown to the left of the immunoblots.
PM expression of Nramp1HA detected by surface biotinylation
Cell-surface biotinylation was used to verify PM expression of Nramp1/2HA proteins. Live CHO, N1-1816 (Nramp1HA), and N2-310a (Nramp2HA) cells were reacted with a membrane-impermeant biotinylating reagent (Sulfo-NHS-LC-biotin), and biotinylated proteins were captured using streptavidin-conjugated agarose beads. Proteins present in the streptavidin-captured fraction (the entire eluate was loaded; “B” in Figure 2A) were analyzed together with proteins remaining in the supernatant fraction after streptavidin-capture (10% [vol] of the postcapture supernatant was loaded; “S” in Figure 2A) by immunoblotting with anti-HA antibody. Both Nramp1HA and Nramp2HA proteins were biotinylated and subsequently detected in the streptavidin-captured fraction (“B”) of the corresponding CHO transfectants (Figure 2A). Nramp1/2HA protein detection was specific, as it was absent in both biotinylated untransfected CHO controls and in unbiotinylated Nramp1/2HA cell extracts incubated with streptavidin beads (Figure 2A). Neither the abundant cytosolic protein tubulin nor the endo/lysosomal mannose-6-phosphate receptor proteins were detected in biotinylated fractions of the cell lines examined (data not shown). These data demonstrate that biotinylated Nramp1HA and Nramp2HA proteins in the streptavidin-captured fraction were due to surface labeling of PM-localized Nramp1/2HAproteins, as opposed to labeling of intracellular protein pools by the biotinylating reagent.
In previous studies of CHO cells expressing Nramp1/2Myc proteins, Nramp2Myc was detected by immunofluorescence at the PM, while Nramp1Myc was not.5,18 Thus, the surface biotinylation of Nramp1/2Myc (Figure 2B) proteins expressed in CHO cells was compared to that of Nramp1/2HA (Figure 2A). Both Nramp1Myc and Nramp2Myc (Figure 2B) were biotinylated and detected in the streptavidin-captured fraction (“B”) by immunoblotting with an anti-Myc antibody. However, the relative proportion of Nramp1Myc in this fraction compared to that of Nramp1HA was considerably lower (Figure 2A-B, compare “B” to “S”), whereas surface-biotinylated Nramp2HA and Nramp2Myc proteins appeared to be detected in streptavidin-captured fractions in similar amounts under the same conditions (Figure 2A-B, compare “B” to “S”). Direct comparison of Nramp1HA and Nramp1Myc proteins in streptavidin-captured fractions by immunoblotting with anti–Nramp1NT polyclonal antiserum (Figure 2C, compare “B” to “S”) further illustrates that Nramp1Myc was detectable in surface-biotinylated fractions but in a significantly lower proportion compared to Nramp1HA. This suggests that insertion of the HA tag in the predicted TM7/8 loop of Nramp1 was responsible for the increased PM localization of Nramp1HA protein.
Immunoblot analysis also showed that in contrast to Nramp1Myc, which is expressed as a glycoprotein of ∼ 90 to 100 kDa in CHO cells, Nramp1HA is present as 2 prominent bands of ∼ 90 to 100 kDa and of ∼ 50 kDa. The ∼ 50 kDa was particularly apparent when the proteins were immunoblotted against anti–Nramp1NT polyclonal antibodies. Pulse-chase metabolic labeling of Nramp1HA protein with 35S-methionine followed by immunoprecipitation suggested that the lower ∼ 50 kDa band consisted of a mixture of newly synthesized polypeptide as well as degradation products and/or partially glycosylated Nramp1HA (data not shown).
Three additional stably transfected CHO clones expressing significant amounts of Nramp1HA (N1-94, N1-116, N1-123) were identified (Figure 3). Crude membrane preparations from these clones were analyzed by immunoblotting with anti-Nramp1NT, anti-Nramp2NT, or anti-HA antibodies (Figure 3A). The results confirmed the presence of a ∼ 50 kDa and ∼ 90 to 100 kDa immunoreactive species in all Nramp1HA transfectants. These clones were analyzed for presence of Nramp1HA protein at the PM by surface biotinylation, followed by analysis of the streptavidin-captured proteins by immunoblotting (Figure 3B). N2-310a (Nramp2HA) and N1-1816 (Nramp1HA) transfectants were used as positive controls and CHO cells as negative controls. These experiments confirmed results of transfectant N1-1816 (Figure 2) by showing surface labeling/PM localization of Nramp1HA in transfectants N1-94, N1-116, and N1-123. Interestingly, the ∼ 90 to 100 kDa species was predominantly biotinylated, compared to the 50 kDa species, indicating that the former comprises most of the Nramp1HA protein present at the cell surface.
Nramp1HA divalent-metal transport properties monitored by fluorescence quenching assays
The possibility of Nramp1HA metal transport activity at the PM was investigated using transfectants N1-94, N1-1816, N1-116, N1-123 that express different amounts of Nramp1HA. Nramp2HA expressing transfectant N2-310a and CHO cells were used as positive and negative controls, respectively (Figure 4). Metal transport was investigated in whole cells using a fluorescence quenching assay.15 Cells were loaded with the membrane-permeant acetoxymethylester (AM) form of the dyes calcein or Fura-2, which are subsequently de-esterified in the cytosol releasing the fluorescent, membrane-impermeant, metal-sensitive probe. The effect of extracellularly added Fe2+ (Figure 4A) or Co2+ (Figure 4C) on intracellular calcein fluorescence was measured. Similarly, the effect of extracellularly added Mn2+ on intracellular Fura-2 fluorescence (Figure 4B) was monitored for 200 seconds. Experiments were carried out at pH 6.0, a pH known to be optimal for Nramp2 activity in this assay.15,38 Typical fluorescence quenching traces are shown in Figure 4A-C, and the rate of quenching was calculated from the initial linear slope of individual traces generated in 3 to 6 independent experiments (Figure 4D-F). For each of the metal/fluorophor combinations tested, Nramp1HA-expressing cells demonstrated substantial, rapid, time-dependent, and statistically significant (Figure 4) fluorescence quenching compared to CHO controls. Background fluorescent quenching in CHO-negative control cells was identical in 5 independent isolates (data not shown). Examination of the rate of quenching measured in independent Nramp1HAtransfectants for each divalent-metal/fluorophor combination (Figure 4D-F) suggested that divalent-metal uptake was proportional to the amount of Nramp1HA protein expressed in each cell line and to the amount of Nramp1HA protein localized to the PM as determined by immunoblot analysis of the corresponding total cell membrane or surface biotinylated fraction (Figure 2). Clone N1-94, which expresses the largest amount of Nramp1HA, exhibited initial rates of Fe2+,Mn2+, and Co2+ uptake that were respectively 2.1 ± 0.1-, 2.7 ± 0.2-, and 2.1 ± 0.1-fold greater than background measured in CHO cells. These results demonstrate that Nramp1HAprotein is capable of Fe2+,Mn2+, and Co2+ uptake at the PM.
Metal transport by Nramp1HA and Nramp2HA in CHO transfectants measured by quenching of calcein or Fura2 fluorescence. Metal transport was measured in independent transfected CHO cell lines stably expressing Nramp1HA (N1-94, N1-116, N1-123, N1-1816) or Nramp2HA (N2-310a) and in untransfected CHO controls (color coded and identified in the inset). For Fe2+ (A) and Co2+ (C) transport assays, cells were loaded with calcein-AM (0.25 μM), and the effect of extracellular Fe2+ (added as sodium ascorbate: Fe2+) or Co2+ on intracellular calcein fluorescence was continuously monitored at 37°C, pH 6.0, for 200 seconds (0.5-second intervals) using a Perkin-Elmer LS-50B fluorometer (excitation λ = 488 nm; emission λ = 517 nm; 5 μM bandpass slit width). For Mn2+ transport assays (B), cells were loaded with Fura2-AM (2 μM) and the effect of extracellular Mn2+ on intracellular Fura2 fluorescence quenching was monitored (excitation λ = 360 nm, emission λ = 510 nm, 7.5 μM bandpass slit width). Representative quenching curves are shown in panels A-C as relative fluorescence (Rel. Fluor.) normalized to the 70-second time point (immediately following addition of metal) for all groups. The average rate (with standard errors) of fluorophor quenching was calculated for Fe2+ (D), Mn2+ (E), and Co2+ (F) from the initial slope of 3-6 individual quenching curves. The mean quenching rates of all Nramp1/2HAtransfectants were statistically different (P < .005) than those of CHO controls with the following (still significant) exceptions: N1-116 (Fe2+; P = .010), N1-123 (Co2+; P = .05) as determined using the Student t test.
Metal transport by Nramp1HA and Nramp2HA in CHO transfectants measured by quenching of calcein or Fura2 fluorescence. Metal transport was measured in independent transfected CHO cell lines stably expressing Nramp1HA (N1-94, N1-116, N1-123, N1-1816) or Nramp2HA (N2-310a) and in untransfected CHO controls (color coded and identified in the inset). For Fe2+ (A) and Co2+ (C) transport assays, cells were loaded with calcein-AM (0.25 μM), and the effect of extracellular Fe2+ (added as sodium ascorbate: Fe2+) or Co2+ on intracellular calcein fluorescence was continuously monitored at 37°C, pH 6.0, for 200 seconds (0.5-second intervals) using a Perkin-Elmer LS-50B fluorometer (excitation λ = 488 nm; emission λ = 517 nm; 5 μM bandpass slit width). For Mn2+ transport assays (B), cells were loaded with Fura2-AM (2 μM) and the effect of extracellular Mn2+ on intracellular Fura2 fluorescence quenching was monitored (excitation λ = 360 nm, emission λ = 510 nm, 7.5 μM bandpass slit width). Representative quenching curves are shown in panels A-C as relative fluorescence (Rel. Fluor.) normalized to the 70-second time point (immediately following addition of metal) for all groups. The average rate (with standard errors) of fluorophor quenching was calculated for Fe2+ (D), Mn2+ (E), and Co2+ (F) from the initial slope of 3-6 individual quenching curves. The mean quenching rates of all Nramp1/2HAtransfectants were statistically different (P < .005) than those of CHO controls with the following (still significant) exceptions: N1-116 (Fe2+; P = .010), N1-123 (Co2+; P = .05) as determined using the Student t test.
A hallmark of metal transport by eukaryotic and prokaryotic members of the Nramp protein superfamily is that transport is pH dependent, suggesting a proton-symport mechanism.23 Therefore, the pH dependence of divalent-metal transport by Nramp1HA at the PM was investigated. For this, CHO and N2-310a (Nramp2HA) controls together with N1-94 cells (Nramp1HA) were loaded with Fura-2 and Nramp1/2HA-dependent Mn2+ uptake was monitored by fluorescence quenching at different extracellular pH (Figure 5). Typical fluorescence quenching traces for each cell line are shown for pH 5.0 (Figure 5A), 6.0 (Figure 5B), and 7.0 (Figure 5C). The rate of quenching at each pH was calculated from the initial linear slope of individual traces generated in 3 to 5 independent experiments (Figure 5D). These rate data are also expressed relative to the CHO cell quench rate at each pH (Figure 5E). Nramp1HA-dependent quenching of Fura-2 fluorescence by Mn2+ was strongly pH dependent: Transport compared to CHO controls was substantial at acidic pH 5.0 and 6.0, but was minimal at neutral pH 7.0 (Figure 5E). This behavior was identical to that seen for Nramp2HA tested under the same experimental conditions (Figure 5). Similar results were obtained when Co2+ and Fe2+ were used as transport substrates (data not shown). Therefore, results from fluorescence quenching assays demonstrate that Nramp1HA is transport competent at the PM and indicate that metal transport by Nramp1HA is pH dependent and mechanistically similar to Nramp2HA.
pH-dependence of metal transport by Nramp1HA and Nramp2HA expressed in CHO transfectants. CHO control cells along with Nramp1HA (N1-94) and Nramp2HA (N2-310a) expressing CHO transfectants were loaded with Fura2-AM, and the quenching of intracellular Fura2 fluorescence by extracellular Mn2+ ions was determined as described in “Materials and methods” and in the legend to Figure 4. Transport was conducted in buffer systems adjusted to pH 5, 6, or 7. Data were collected (0.5-second intervals) for 200 seconds. Typical fluorescence quenching traces for each cell line are shown for pH 5.0 (A), 6.0 (B), and 7.0 (C). The average rate (with standard errors) of Nramp1/2-dependent Fura2 quenching by Mn2+ was calculated at each pH from the initial slope of 3-5 independent quenching curves (D). These rate data are also expressed relative to background metal uptake in CHO-negative controls (E). The means of normalized quenching rates (E) of Nramp1/2HA transfectants were statistically different than those of CHO controls at pH 5, pH 6 (P < .0005), and pH 7.0 (P = .0015 and P = .015, respectively) as determined using the Student t test. The normalized mean of Nramp1HA transport (E) at pH 5 was not statistically different than that of its transport at pH 6 (P = .1). However, the normalized means of Nramp1HA transport (E) at both pH 5 (P = .0006) and pH 6 (P = .015) were significantly different than that of its transport at pH 7.0. The mean of normalized Nramp2HA transport (E) at pH 5 was statistically different than those of its transport at pH 6.0 and 7.0 (P < .0001). Likewise, the mean of Nramp2HA transport (E) at pH 6.0 was statistically different than that of its transport at pH 7.0 (P = .012).
pH-dependence of metal transport by Nramp1HA and Nramp2HA expressed in CHO transfectants. CHO control cells along with Nramp1HA (N1-94) and Nramp2HA (N2-310a) expressing CHO transfectants were loaded with Fura2-AM, and the quenching of intracellular Fura2 fluorescence by extracellular Mn2+ ions was determined as described in “Materials and methods” and in the legend to Figure 4. Transport was conducted in buffer systems adjusted to pH 5, 6, or 7. Data were collected (0.5-second intervals) for 200 seconds. Typical fluorescence quenching traces for each cell line are shown for pH 5.0 (A), 6.0 (B), and 7.0 (C). The average rate (with standard errors) of Nramp1/2-dependent Fura2 quenching by Mn2+ was calculated at each pH from the initial slope of 3-5 independent quenching curves (D). These rate data are also expressed relative to background metal uptake in CHO-negative controls (E). The means of normalized quenching rates (E) of Nramp1/2HA transfectants were statistically different than those of CHO controls at pH 5, pH 6 (P < .0005), and pH 7.0 (P = .0015 and P = .015, respectively) as determined using the Student t test. The normalized mean of Nramp1HA transport (E) at pH 5 was not statistically different than that of its transport at pH 6 (P = .1). However, the normalized means of Nramp1HA transport (E) at both pH 5 (P = .0006) and pH 6 (P = .015) were significantly different than that of its transport at pH 7.0. The mean of normalized Nramp2HA transport (E) at pH 5 was statistically different than those of its transport at pH 6.0 and 7.0 (P < .0001). Likewise, the mean of Nramp2HA transport (E) at pH 6.0 was statistically different than that of its transport at pH 7.0 (P = .012).
Nramp1HA transport properties monitored by radioisotopic metal transport
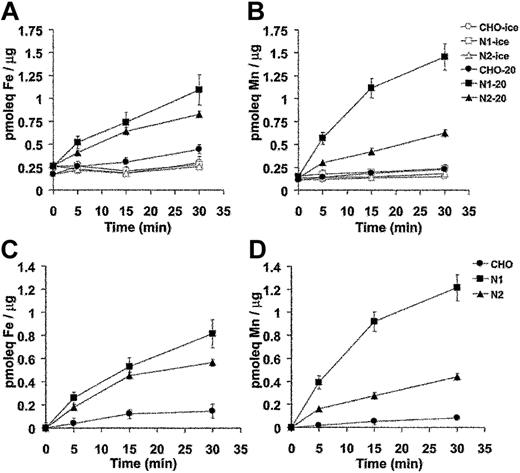
Divalent-metal transport activity for Nramp1HA also was investigated using radioisotopic 54Mn or 55Fe. For these studies, control CHO cells, N1-94 (Nramp1HA) and N2-310a (Nramp2HA) transfectants were used. In the first set of experiments, cells were incubated with tracer amounts of radioisotopic 55Fe or 54Mn in a total metal concentration of 9 μM at pH 5.5. At predetermined time intervals over a 30-minute period, cells were pelleted by centrifugation through an oil cushion to remove unincorporated metal, and the cell-associated radioactivity was determined. Results from 3 to 5 independent experiments are shown in Figure 6A (55Fe2+) and 6B (54Mn2+). Expression of Nramp1HA or Nramp2HA strongly stimulated accumulation of 55Fe2+ and 54Mn2+ into transfected CHO cells. For both proteins, metal uptake was time dependent and increased steadily over the 30-minute incubation period. Metal transport was temperature dependent, being abrogated at 4°C (Figure 6A-B). Subtraction of the nonspecific metal binding to cells measured at 4°C gives the net amount of metal uptake, which is shown in Figure 6C (55Fe2+) and Figure 6D (54Mn2+). Over 30 minutes, Nramp1HA caused a 4- and 10-fold stimulation of 55Fe2+ and 54Mn2+ uptake, respectively, compared to 3- and 4-fold stimulation for Nramp2HA-expressing cells. Thus, Nramp1HA appeared to transport Mn2+ to a greater degree than Nramp2HA, while both proteins showed similar uptake of Fe2+.
Time and temperature dependence of Mn2+ and Fe2+ transport by Nramp1HA and Nramp2HA measured by a radioisotopic uptake assay. Incorporation of 55Fe2+ (A) and 54Mn2+ (B) into CHO control cells, and Nramp1HA expressing (clone N1-94) or Nramp2 HA expressing (clone N2-310a) CHO cell transfectants was measured as described in “Materials and methods.” Briefly, cells were resuspended in transport buffer (107 cells/1.5 mL), and transport was initiated by addition of 1 mL of radioisotope buffer containing tracer amounts of either 54Mn2+ (total Mn2+ concentration of 9 μM) or 55Fe2+ (total Fe2+ concentration of 9 μM), followed by a 30-minute incubation at 20°C. At predetermined time points (0, 5, 15, 30 minutes), metal accumulation was calculated and expressed as picomolar equivalents (pmoleq) divalent-metal/μg total cellular protein as shown in panels A (Fe2+) and B (Mn2+), which represent the average (with standard error) from 3 or 4 independent experiments. Parallel experiments were conducted at 4°C to establish the temperature-independent component of cell-associated radioactivity (binding). These values were subtracted from the 20°C accumulation data to deduce net uptake values, which are shown in panels C (Fe2+) and D (Mn2+).
Time and temperature dependence of Mn2+ and Fe2+ transport by Nramp1HA and Nramp2HA measured by a radioisotopic uptake assay. Incorporation of 55Fe2+ (A) and 54Mn2+ (B) into CHO control cells, and Nramp1HA expressing (clone N1-94) or Nramp2 HA expressing (clone N2-310a) CHO cell transfectants was measured as described in “Materials and methods.” Briefly, cells were resuspended in transport buffer (107 cells/1.5 mL), and transport was initiated by addition of 1 mL of radioisotope buffer containing tracer amounts of either 54Mn2+ (total Mn2+ concentration of 9 μM) or 55Fe2+ (total Fe2+ concentration of 9 μM), followed by a 30-minute incubation at 20°C. At predetermined time points (0, 5, 15, 30 minutes), metal accumulation was calculated and expressed as picomolar equivalents (pmoleq) divalent-metal/μg total cellular protein as shown in panels A (Fe2+) and B (Mn2+), which represent the average (with standard error) from 3 or 4 independent experiments. Parallel experiments were conducted at 4°C to establish the temperature-independent component of cell-associated radioactivity (binding). These values were subtracted from the 20°C accumulation data to deduce net uptake values, which are shown in panels C (Fe2+) and D (Mn2+).
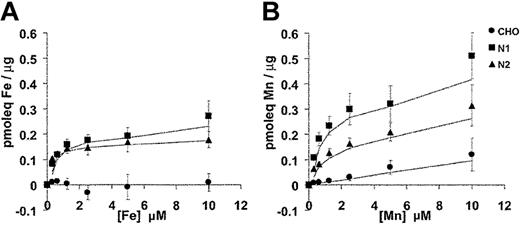
The substrate selectivity of the Nramp1/2HA transporters for Fe2+ and Mn2+ was characterized in dose-response experiments (Figure 7). In these experiments, the total concentration of Fe2+ and Mn2+ in the transport buffer was varied between 0.31 μM and 10 μM. Cell-associated radioactivity was determined (after 10 minutes) at each divalent-metal concentration. Parallel transport assays were carried out at 20°C and at 4°C. The 4°C binding data were subtracted from each corresponding 20°C data point. In the case of Fe2+ (Figure 7A), results were very similar for Nramp1HA and Nramp2HA with transport reaching a plateau at approximately 1 μM, closely approximating that previously determined for Nramp2 in fluorescence quenching transport studies.15 In the case of Mn2+ (Figure 7B), transport appeared to plateau at approximately 2.5 μM for both Nramp1HA and Nramp2HA. However, Nramp1HA appeared to be a more efficient transporter for Mn2+ than Nramp2HA, with a minimum of 2-fold increases in total cellular accumulation of the metal over all concentrations tested, in agreement with results shown in Figure 6B. Together, these results confirm and extend those obtained in fluorescence quenching studies (Figures 4, 5) and establish that Nramp1HA (1) is active at the plasma membrane, (2) can transport Fe2+, Mn2+, and Co2+, (3) is acid-pH dependent, (4) is mechanistically indistinguishable from Nramp2HA, and (5) appears to be a more efficient transporter of Mn2+ than is Nramp2HA.
Divalent-metal selectivity of Nramp1HA and Nramp2HA. The divalent-metal selectivity of Nramp1HA and Nramp2HA was compared in dose-response experiments. Transfected CHO cell lines N1-94 (Nramp1HA) and N2-310a (Nramp2HA), along with untransfected CHO cells, were resuspended in transport buffer (3 × 106 cells/0.375 mL), and transport was initiated by addition of an equal volume of radioisotope buffer containing tracer amounts of 55Fe2+ (A) or 54Mn2+ (B), followed by incubation at 20°C. The final concentration of Fe2+ and Mn2+ in the transport reaction was varied between 0.31 μM and 10 μM total divalent metal (by 2-fold serial dilution), while the specific radioactivity of each was held constant. For Fe2+ transport buffer, a 50:1 molar excess of ascorbate to Fe2+ was used and kept constant at each Fe2+ concentration. Transport was allowed to proceed for 10 minutes at 20°C, and cell-associated radioactivity was determined as described in “Materials and methods” and in the legend to Figure 6. Parallel transport assays were conducted at 4°C, and these values were subtracted from those obtained at 20°C to determine the net amount of metal uptake, which is expressed as pmoleq of divalent-metal/μg of total cellular protein.
Divalent-metal selectivity of Nramp1HA and Nramp2HA. The divalent-metal selectivity of Nramp1HA and Nramp2HA was compared in dose-response experiments. Transfected CHO cell lines N1-94 (Nramp1HA) and N2-310a (Nramp2HA), along with untransfected CHO cells, were resuspended in transport buffer (3 × 106 cells/0.375 mL), and transport was initiated by addition of an equal volume of radioisotope buffer containing tracer amounts of 55Fe2+ (A) or 54Mn2+ (B), followed by incubation at 20°C. The final concentration of Fe2+ and Mn2+ in the transport reaction was varied between 0.31 μM and 10 μM total divalent metal (by 2-fold serial dilution), while the specific radioactivity of each was held constant. For Fe2+ transport buffer, a 50:1 molar excess of ascorbate to Fe2+ was used and kept constant at each Fe2+ concentration. Transport was allowed to proceed for 10 minutes at 20°C, and cell-associated radioactivity was determined as described in “Materials and methods” and in the legend to Figure 6. Parallel transport assays were conducted at 4°C, and these values were subtracted from those obtained at 20°C to determine the net amount of metal uptake, which is expressed as pmoleq of divalent-metal/μg of total cellular protein.
Discussion
Both the mechanism of transport and the substrate specificity of Nramp1 at the phagosomal membrane have proven difficult to study. In order to overcome this difficulty, we have inserted an HA epitope into the TM7/8 loop of Nramp1 (Nramp1HA) and expressed the recombinant protein at the plasma membrane of CHO cells. PM expression in CHO transfectants was demonstrated by direct extracellular accessibility of the HA tag to antibodies in whole cells using immunofluorescence (Figure 1), as well as by cell-surface biotinylation experiments (Figures 2, 3). These studies provide topologic information for Nramp1 and establish that the TM7/8 loop, which bears a number of predicted N-linked glycosylation sites,39 is extracellular when Nramp1HA is expressed at the PM. By inference, the TM7/8 loop of Nramp1 would be found in the lumen of phagosomal vesicles. We show also that the N-terminus of Nramp1HA, which is only accessible to anti–Nramp1NT antibodies in permeabilized but not in whole cells, is cytoplasmic. Thus, the membrane organization of Nramp1HA at the PM is identical to that established previously for Nramp2HA,15 in agreement with the high degree of shared sequence similarity (78%) and identity (64%).12
The PM localization of Nramp1HA in transfected CHO cells is unique and different from that of the endogenous protein found in either primary macrophages or neutrophils, where Nramp1 was not at the PM but was found in lysosomes or tertiary granules, respectively.5-7 Likewise, recombinant Nramp1Myc expression in CHO cells or in RAW264.7 macrophages was restricted to the late endosomes and lysosomes and was not detected at the PM.5 Unsurprisingly, expression of Nramp1Myc in CHO cells did not stimulate uptake of extracellular divalent-metal.15 This suggests that insertion of the HA epitope into the TM7/8 loop may directly alter targeting, maturation, or processing of Nramp1HA, resulting in its accumulation at the PM. Several endosomal/lysosomal proteins transiently pass through the PM, prior to final localization via endocytotic retrieval.40,41 The relatively high level of Nramp1HA PM accumulation compared to Nramp1Myc detected in transfected CHO cells may reflect partial or complete uncoupling of this retrieval process due to the presence of HA tag. Also, the TM7/8 loop is predicted to have several N-glycosylation sites.39 The structure or processing of these sites may be altered by the inserted HA tag, thereby contributing to increased PM Nramp1HA accumulation. Indeed, apical PM targeting of Nramp2 in polarized MDCK (Madin Darby canine kidney) cells has been shown to depend on N-glycosylation.42 Likewise, the effect of N-glycosylation on lysosomal protein targeting recently has been demonstrated for the protein endolyn.43 Disruption of N-glycosylation in MDCK cells was shown to cause redistribution of endolyn from an apical PM lysosomal sorting pathway to the basolateral PM.
Our results from fluorescence quenching transport assays (Figures 4, 5) and transport studies with isotopic 55Fe2+ and 54Mn2+ (Figures 6, 7) show that Nramp1HA expressed at the PM is transport competent and functions as a multispecific divalent-metal transporter. Divalent-metal transport by Nramp1HA was time and temperature dependent and required an acidic pH. These Nramp1HA transport characteristics are identical to those demonstrated for Nramp2HA in our study, and they closely parallel results from independent transport studies of Nramp2 expressed either in Xenopus laevis oocytes13 or transfected mammalian cells,14,15 as well as those reported for other eukaryotic and bacterial Nramp homologs.23 PM expression of Nramp1HA has permitted the analysis of its substrate selectivity compared to that of Nramp2HA. Our experiments have shown that like other Nramp family transporters,13,44-46 Nramp1HA can transport both Fe2+ and Mn2+ with apparent affinity in the low micromolar range. These values are in the physiologically relevant concentration range and are consistent with the known concentration of chelatable Fe2+ in mammalian cells (0.2-1 μM),47 as well as cellular and tissue concentrations of Mn2+ (< 4 μM).48 In addition, Nramp1HA appears to have a preference for Mn2+ ions when compared to Fe2+, suggesting that the former may be a preferred substrate at the phagosomal membrane. More detailed transport studies in membrane vesicles will be required to fully characterize the ion selectivity of Nramp1 and Nramp2.
Despite this potential difference in substrate preference, our results indicate that Nramp1 and Nramp2 are functionally equivalent multispecific divalent-metal transporters. The major physiologic difference between the 2 proteins appears to be at the level of the cell type–specific expression and subcellular site of transport. Nramp2 is expressed in recycling endosomes, where it transports transferrin-delivered iron from the acidified endosomal lumen into the cytoplasm down a proton gradient.17-21 This endosomal Fe2+ efflux is impaired in reticulocytes from microcytic anemia mk mice, which bear a loss-of-function mutation at Nramp2.17,22 It is very likely that Nramp1 functions in an analogous fashion to remove divalent metals from the phagosome. Indeed, microfluorescence imaging studies of Nramp1-positive phagosomes containing zymosan particles labeled with the metal-sensitive fluorophor Fura-FF6 have demonstrated pH-dependent metal efflux by Nramp1 at the phagosomal membrane within live primary macrophage.24 For both Nramp1 and Nramp2, luminal acidification is achieved by recruitment of the vacuolar H+/ATPase, which provides the proton gradient necessary for Nramp protein function.11,24,49 Altogether, these results strongly support a model in which Nramp1 transports Fe2+, Mn2+, and likely other divalent metals from the lumen of acidified phagosomes and into the cytoplasm.
The mechanism by which Nramp1-mediated depletion of divalent metals from the phagosomal space affects the survival and replication of unrelated intracellular parasites is not fully understood. Several lines of evidence indicate that an adequate supply of divalent metals such as Fe2+, Mn2+, and Mg2+ is critical for successful intracellular parasitism.50,51 First, bacteria such as Salmonella express, under different conditions, a surprising number of diverse high- or low-affinity adenosine triphosphate (ATP)–dependent or proton-coupled iron (Fe2+, Fe3+) and/or manganese transporters such as fepBCDG, sitAD, FeoABC, CorAD,52-56 and the Nramp homolog MntH.45,57,58 Second, mutation of several of these transporter genes abrogate virulence and impair intracellular replication in vivo.53,59-61 Third, intracellular replication of Salmonella within permissive Nramp1-negative RAW264.7 macrophage was partly abrogated by incubation with the metal chelator dipiridyl.59 Interestingly, recent studies using Salmonella-bearing gene-specific reporter constructs have established that phagosomal divalent-metal depletion by Nramp1 creates a stressful environment, which is sensed by bacteria within macrophage. The intracellular bacteria responded to the presence of Nramp1 by the transcriptional induction of a number of “virulence” genes that map within Salmonella pathogenicity island 2 (SPI2) including ssrA and sseJ.62 Similarly, infection of human macrophage by M tuberculosis results in induction of several mycobacterial genes required for siderophore-mediated iron uptake.63 More specifically, preliminary comparative transcriptional profiling studies of M bovis–infected RAW264.7 macrophages (Nramp1-negative) and RAW264.7-Nramp1 transfectants suggest that Nramp1 has a direct effect on the level of transcriptional induction of the mycobacterial MbtB gene involved in iron acquisition (J.R.F. and P.G., unpublished data, 2002). Together, these results suggest that Nramp1 can act as an important antagonist of bacterial metal acquisition systems in the microenvironment of the phagosome.
Prepublished online as Blood First Edition Paper, May 15, 2003; DOI 10.1182/blood-2003-02-0425.
Supported by research grant RO1 AI35237-08 from the National Institute of Allergy and Infectious Diseases (P.G.). J.R.F. is supported by a fellowship from the Canadian Institutes of Health Research, and P.G. is supported by a Distinguished Scientist salary award from the Canadian Institutes of Health Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We acknowledge Dr Francois Canonne-Hergaux and Dr Samantha Gruenheid for the preparation of anti-Nramp1NT and Nramp2NT polyclonal antibodies, and Dr Virginie Picard for the kind gift of cell lines and cDNA. We thank Steven Lam-Yuk-Tseung for advice with the fluorescent quenching assays.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal