Abstract
During human fulminant hepatic failure (FHF) circulating levels of most hemostatic proteins fall dramatically. Concurrently, factor VIII (fVIII) procoagulant activity rises despite destruction of the hepatocytes considered responsible for fVIII synthesis. This observation suggests a role for cells other than hepatocytes in fVIII biosynthesis during FHF. We have attempted to identify nonhepatocytic sites of fVIII biosynthesis by inducing FHF in mice using acetaminophen overdose, a common cause of human FHF. Acetaminophen-treated mice consistently displayed signs characteristic of FHF, including elevated plasma aminotransferase activity. However, acetaminophen-treated mice demonstrated markedly reduced fVIII activity, contrary to the observation in human FHF. von Willebrand factor antigen levels were only mildly reduced, suggesting that the decrease in fVIII is not secondary to loss of von Willebrand factor. These results imply that there are fundamental differences in the regulation of plasma fVIII levels between humans and mice.
Introduction
Much of the knowledge concerning factor VIII (fVIII) biosynthesis has been gained through the use of heterologous expression of fVIII transgenes in cultured cells (reviewed in Kaufman et al1 ). Unfortunately, in vitro experiments cannot identify all of the factors involved in fVIII biosynthesis in vivo. Thus, a substantial gap in the understanding of fVIII gene regulation in vivo remains, including the identification of endogenous sites of fVIII production. Beyond hepatocytes, several cell types in mice have been shown to contain significant levels of fVIII mRNA,2 but only murine liver sinusoidal endothelial cells have been demonstrated to secrete fVIII in vitro.3,4 Additionally, liver is the only tissue that has been shown conclusively to produce fVIII when transplanted into fVIII-deficient (hemophilia A) canines or humans, as evident by alleviation of the bleeding phenotype.5-7 However, during the course of fulminant hepatic failure (FHF) induced by acetaminophen or viral hepatitis, plasma fVIII activity can increase to more than 10 times normal levels.8,9 The cellular source of this super-physiologic fVIII activity is currently unknown. We previously reported that fVIII levels were decreased in a murine model of FHF induced by the hepatotoxin, azoxymethane.10 Because azoxymethane has not been proven to cause FHF in humans, we have continued to identify other models that may be more representative of human FHF pathophysiology. In the current study we sought to determine whether fVIII levels are elevated in a recently described murine model of acetaminophen-induced FHF11 and possibly identify alternative sites of fVIII biosynthesis in vivo.
Study design
Twelve-week-old C57BL/6J male mice (21-26 g) were purchased from Charles River Laboratory (Wilmington, MA). Mice were fed standard rodent chow and were kept on a 12-hour light and 12-hour dark cycle. Murine fulminant hepatic failure was induced as described previously.11 Briefly, mice were given an intraperitoneal injection of 100 mg/kg phenobarbital (Sigma, St Louis, MO) suspended in corn oil, for 3 consecutive days followed by an intraperitoneal injection of 250 mg/kg acetaminophen (Sigma), dissolved in basic phosphate-buffered saline (10 mM Na2HPO4, 150 mM NaCl, pH 11), on day 4. Twenty-four hours after acetaminophen administration, the mice were killed, and blood was collected by cardiac puncture into one-tenth volume 3.8% sterile trisodium citrate. Plasma was isolated from whole blood by centrifugation at 1800g for 15 minutes at 4°C and stored at –70°C. The control plasma used in this study was pooled plasma from 6 untreated C57BL/6J male mice.
fVIII activity was determined using a chromogenic substrate assay in which the rate of activation of factor X is linearly related to the concentration of fVIII as follows. Plasma samples were diluted 1/81 in 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 0.15 M NaCl, 1% bovine serum albumen (BSA), pH 7.4. Thirty microliters of a solution containing 3.6 nM factor IXa, 150 μM phosphatidylcholine/phosphatidylserine vesicles (75%/25%), and 15 mM CaCl2 was added to 30 μL diluted sample plasma. Next, 30 μL of a solution containing 48 nM factor IIa and 900 nM factor X was added. After the reaction mixture was incubated for 5 minutes at room temperature, 30 μL of 1 mM Spectrozyme factor Xa (American Diagnostica, Stamford, CT), 400 nM desulfatohirudin, 100 mM EDTA (ethylenediaminetetraacetic acid), 20 mM HEPES, 0.15 M NaCl, 0.1% polyethylene glycol, pH 7.4 was added, and the absorbance at 405 nm was measured over a 10-minute period. Murine plasma fVIII levels were obtained by comparison to a standard curve using pooled normal human plasma (FACT; George King Biomedical, Overland Park, KS). Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities were measured using the Sigma Diagnostic Transaminases Reagent kit (Sigma) following the manufacturer's instructions. von Willebrand factor (VWF) levels were measured by enzyme-linked immunosorbent assay (ELISA) using a previously published method.12 The ELISA titer was defined as the absorbance at 490 nm obtained for a 1/20 dilution of plasma. Data acquired at this dilution were within a linear range. All numerical data are presented as means ± 1 standard deviation with the data range presented in parentheses.
Results and discussion
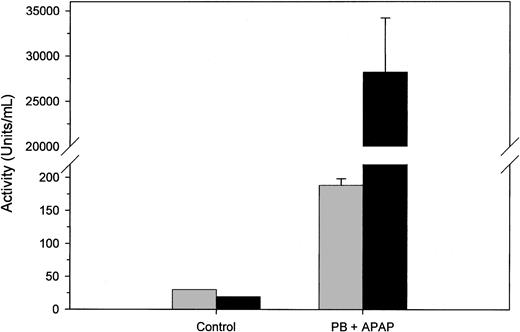
Twenty-four hours after acetaminophen treatment, all mice displayed signs of stage II hepatic encephalopathy, including reduced locomotive activity. This treatment regimen previously has been shown to induce extensive hepatic centrilobular necrosis within 24 hours.11 One indicator of liver necrosis is the elevation of serum ALT activity and a change in the AST/ALT ratio from greater than 1 to less than 1. Plasma from control and acetaminophen-treated mice was assayed for AST and ALT activity (Figure 1). Pooled plasma from untreated mice had 30 U/mL AST activity and 19 U/mL ALT activity for an AST/ALT ratio of 1.58, whereas the plasmas from all acetaminophen-treated mice demonstrated elevated AST and ALT levels and decreased AST/ALT ratios. The mean values for AST and ALT activity were 188 ± 10 (175-209) U/mL and 28 200 ± 6000 (20 300-37 300) U/mL, respectively, corresponding to an AST/ALT ratio of 0.007. These findings indicate that FHF was successfully induced in all of the mice.
Aminotransferase levels in acetaminophen-treated mice. Pooled control plasma from 6 normal mice and individual phenobarbital/acetaminophen (PB+APAP)–treated murine plasmas (n = 12) were assayed for AST (▦) and ALT (▪) activity as described in “Study design.” Error bars represent 1 SD.
Aminotransferase levels in acetaminophen-treated mice. Pooled control plasma from 6 normal mice and individual phenobarbital/acetaminophen (PB+APAP)–treated murine plasmas (n = 12) were assayed for AST (▦) and ALT (▪) activity as described in “Study design.” Error bars represent 1 SD.
Plasma fVIII activity was measured using a chromogenic substrate assay. The concentration of fVIII activity measured in control mouse plasma was 2.9 U/mL, which is consistent with previously published results.13,14 Average fVIII activity was decreased by 91% to 0.27 ± 0.07 (0.16-0.37) U/mL in the acetaminophen-treated murine plasmas (Figure 2). Therefore, we were unable to replicate the increase in plasma fVIII activity observed in human acetaminophen-induced FHF in a murine model. The decrease in fVIII activity observed in the present study corresponds well with the results previously reported in azoxymethane-treated mice in which, at the highest dose of azoxymethane tested (50 μg/g body weight), fVIII activity dropped to 10% of control levels at 24 hours after administration.10
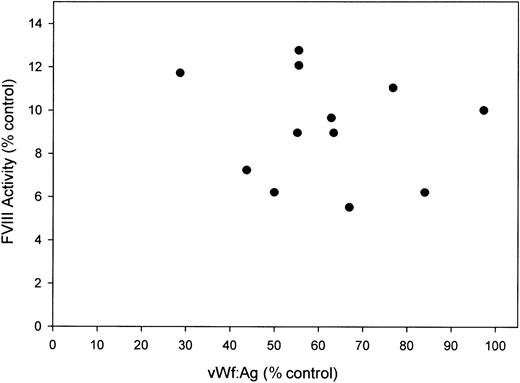
FVIII activity versus VWF antigen levels in acetaminophen-treated mice. Plasma from acetaminophen-treated mice (n = 12) was assayed for fVIII activity by chromogenic assay and VWF antigen level by ELISA. Results expressed are relative to pooled normal murine plasma.
FVIII activity versus VWF antigen levels in acetaminophen-treated mice. Plasma from acetaminophen-treated mice (n = 12) was assayed for fVIII activity by chromogenic assay and VWF antigen level by ELISA. Results expressed are relative to pooled normal murine plasma.
Several factors have been shown to influence circulating fVIII levels, including VWF whose levels display a strong positive correlation with fVIII levels (reviewed in Kamphuisen et al15 ). Accordingly, in the complete absence of VWF, fVIII levels are reduced by approximately 80% in mice.12 We measured VWF antigen levels in the acetaminophen-treated and control murine plasmas and found that the levels in acetaminophen-treated mice were mildly reduced to 62% ± 18% (29%-97%) of the control murine plasma level (Figure 2). However, the extent of decline in VWF was not equivalent to the decrease in fVIII, and there was not a significant correlation between VWF antigen and fVIII activity within the acetaminophen-treated mice. Therefore, the decrease in fVIII was not secondary to a reduction in VWF. The current data support our previous findings of reduced fVIII activity following azoxymethane-induced murine FHF. Combined, these studies argue that there are fundamental differences in the regulation of circulating fVIII levels between mice and humans and that these differences should not be ignored when using the mouse as an experimental model system to study fVIII biology.
Prepublished online as Blood First Edition Paper, May 1, 2003; DOI 10.1182/blood-2003-03-0826.
Supported by National Institutes of Health grant R01-HL40921.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.