Abstract
We assessed the hematopoietic recovery and transplantation-related mortality (TRM) of patients who had failed peripheral blood stem cell mobilization and subsequently received high-dose chemotherapy supported by granulocyte colony-stimulating factor (G-CSF)–primed bone marrow (BM). Studied were 86 heavily pretreated consecutive patients with acute leukemia (n = 21), refractory/relapsed non-Hodgkin lymphoma (n = 41) and Hodgkin disease (n = 17), and multiple myeloma (n = 7). There were 78 patients who showed insufficient mobilization of CD34+ cells (< 10 cells/μL), whereas 8 patients collected less than 1 × 106 CD34+ cells/kg. BM was primed in vivo for 3 days with 15 to 16 μg/kg of subcutaneous G-CSF. Median numbers of nucleated cells, colony-forming unit cells (CFU-Cs), and CD34+ cells per kilogram harvested were 3.5 × 108, 3.72 × 104, and 0.82 × 106, respectively. Following myeloablative chemotherapy, median times to achieve a granulocyte count higher than 0.5 × 109/L and an unsupported platelet count higher than 20 and 50 × 109/L were 13 (range, 8-24), 15 (range, 12-75), and 22 (range, 12-180) days, respectively, for lymphoma/myeloma patients and 23 (range, 13-53), 52 (range, 40-120), and 90 (range, 46-207) days, respectively, for leukemia patients. Median times to hospital discharge after transplantation were 17 (range, 12-40) and 27 (range, 14-39) days for lymphoma/myeloma and acute leukemia patients, respectively. TRM was 4.6%, whereas 15 patients died of disease. G-CSF–primed BM induces effective multilineage hematopoietic recovery after high-dose chemotherapy and can be safely used in patients with poor stem cell mobilization.
Introduction
Transplantation of autologous mobilized peripheral blood stem cells (PBSCs) has largely replaced conventional, unprimed bone marrow (BM) transplantation because of a faster hematopoietic reconstitution, earlier hospital discharge, and more financial resource savings.1,2 Prospective studies have also demonstrated accelerated engraftment when PBSCs are transplanted in allogeneic recipients.3-5 Administration of granulocyte colony-stimulating factor (G-CSF), alone or in combination with cytotoxic drugs, is widely considered the most effective treatment to increase the number of circulating CD34+ stem cells. However, despite the superiority of mobilized blood, PBSC transplantation has some limitations. In particular, there is a general consensus that the number of CD34+ cells infused correlates with the rate of hematopoietic reconstitution.1 Therefore, below the minimum threshold level of 1 × 106 CD34+ cells/kg reinfused, the risk of delayed hematopoietic recovery and increased transplantation-related mortality (TRM) is high.6 Conversely, patients receiving at least 2 × 106 CD34+ cells/kg show a rapid granulocyte recovery, and patients infused with 5 × 106 CD34+ cells/kg or more have fast multilineage engraftment.7 Thus, the number of CD34+ cells collected and transplanted is crucial to ensure a safe procedure. Moreover, 10% to 30% of patients do not mobilize sufficient PBSCs to proceed to transplantation, depending on the amount of previous chemoradiotherapy and the interval between chemotherapy and mobilization, marrow fibrosis, or marrow involvement of the disease; addition of hematopoietic cytokines; and the number of premobilization circulating progenitor cells.8-13 However, not all reasons for poor mobilization are known, since some heavily pretreated patients or patients with marrow metastases or fibrosis mobilize well, whereas some healthy donors mobilize poorly.14
Patients with absent or poor PBSC mobilization undergoing high-dose therapy supported by BM cells do show delayed hematopoietic recovery and increased procedure-related morbidity and mortality.6 Thus, there are major concerns regarding the safety of autologous transplantation in such patients. Recently, several reports,15-23 including randomized studies,15,16 have shown that transplantation of G-CSF–primed BM induces a comparable engraftment with that of PBSCs in both autologous and allogeneic settings. Moreover, allogeneic recipients experienced reduced severity of acute graft-versus-host disease (GVHD) and less chronic GVHD.16
Based on these findings, we conducted a multicenter prospective study to assess the hematopoietic recovery and TRM of patients with hematologic malignancies who had previously failed PBSC mobilization and received high-dose chemotherapy supported by G-CSF–primed BM. Patients were defined as poor mobilizers if the peak value of CD34+ cells during mobilization was lower than 10 cells/μL or in the case of stem cell collection, if less than 1 × 106 CD34+ cells/kg was harvested.6 The minimum threshold of circulating CD34+ cells was chosen upon our and others'24 experience, indicating the minimum peak number of CD34+ cells predictive of a successful collection (ie, ≥ 2 × 106 CD34+ cells/kg body weight) as 10 cells/μL. In patients with less than that value, it is unlikely that the collection of the target CD34+ cell dose can be accomplished with few aphereses.25 Poor mobilizers were studied because the lack of autologous stem cells poses important questions for the clinical management of patients for whom autologous transplantation has proved to be clinically beneficial. Our results indicate that activated BM induces effective multilineage hematopoietic recovery and low TRM after high-dose chemotherapy and can be safely used in patients submitted to autologous transplantation programs.
Patients and methods
Patients
Studied were 86 heavily pretreated consecutive patients undergoing autologous G-CSF–primed BM transplantation between February 1995 and December 2002 at 3 transplantation centers: Bologna (40 patients), Udine (33 patients), and Reggio Emilia (13 patients). The patients' diagnoses, sex, median age, median number of lines of prior chemotherapy, and disease status at transplantation, and the percentage who received prior radiotherapy are listed in Tables 1, 2. Patients were enrolled in the study if they were eligible for autologous stem cell transplantation according to our institutional guidelines and if PBSCs were not available (see “Mobilization regimens”). Exclusion criteria were as follows: contraindication to BM harvest by general anesthesia, major organ dysfunction, myelosuppressive chemotherapy within 4 weeks of BM harvest, administration of any hematopoietic cytokine within 2 weeks before BM collection, and pelvic radiation before stem cell collection. Patients with a positive test for human immunodeficiency virus or any form of active hepatitis were also excluded. Notably, no exclusion criteria were set on the basis of the CD34+ stem cell content of BM harvest. All individuals gave written informed consent and the protocol was approved by the hospital ethics committees of all participating centers.
Mobilization regimens
Of the patients, 66 (lymphoma/myeloma [n = 63]; acute leukemia [n = 3]) attempted PBSC mobilization by administration of 7.5 μg/kg body weight twice a day of G-CSF (filgrastim, Granulokine [Roche, Milan, Italy] or Neupogen [Dompè-Biotec, Milan, Italy]) for 5 to 6 days, whereas 20 (acute leukemia [n = 18]; lymphoma/myeloma [n = 2]) received priming chemotherapy followed by daily injection of 5 μg/kg G-CSF (filgrastim, Roche/Dompè-Biotec). Patients with lymphoproliferative disorders were treated with 7 g/m2 cyclophosphamide, while acute myeloblastic leukemia (AML) patients were mobilized during the second consolidation course, which generally included fludarabine, mitoxantrone, and cytarabine. Study patients were defined as poor mobilizers (n = 78) if the peak value of circulating CD34+ cells was lower than 10 cells/μL. There were 8 patients who showed a peak value of CD34+ cells slightly higher than 10 cells/μL; however, the minimum CD34+ cell dose of 1 × 106/kg was not reached with 2 or 3 aphereses, and the clinical investigator thought it would be unlikely to reach that cell dose with few additional collections (Table 2). Regardless of the mobilization strategy, PBSC collection was attempted after a minimum of 4 weeks from the last chemotherapy cycle (median, 48 days; range, 35-56 days).
BM priming, collection, and cell processing
BM priming was performed with G-CSF (filgrastim; Roche/Dompè Biotec) at the dose of 7.5 to 8 μg/kg twice a day for 3 days. BM cells were harvested on day +4 using standard procedures (15-20 mL BM/kg body weight) and frozen in 10% dimethyl sulfoxide using controlled-rate liquid nitrogen freezing.26 The minimum number of BM cells required to proceed to autotransplantation was 1 × 108 nucleated cells/kg actual patient body weight. All patients submitted to BM harvest did achieve this threshold. At the time of reinfusion, BM cells were rapidly thawed at 37°C at bedside and reinfused via a central line.26
Progenitor cell assays
Total nucleated cells, day-14 colony-forming unit cells (CFU-Cs), and CD34+ cells were assayed on BM collections as previously reported.26-28 In brief, CFU-Cs were scored by plating in duplicate 1 × 105 light density cells in a total volume of 1 mL Iscoves modified Dulbecco medium (Gibco, Grand Island, NY) added with 20% fetal calf serum (Stem Cell Technologies, Vancouver, BC, Canada) and 1.1% (final concentration) methylcellulose. Colony-stimulating activity was provided by 10% (vol/vol) of a selected lot of phytohemagglutinin-lymphocyte–conditioned medium. Colonies were counted after 14 days of incubation at 37°C in a humidified 5% CO2 atmosphere. CD34+ cell count was determined by flow cytometry.26,27 BM cells were incubated at 4°C for 30 minutes with the anti-CD34 phycoerythrin-conjugated monoclonal antibody HPCA2 or an irrelevant isotype-matched control antibody (Becton Dickinson, Milan, Italy). Immunofluorescence analysis was performed using FACScan equipment (Becton Dickinson). A minimum of 10 000 events was collected in list mode on FACScan software.
Conditioning regimens, supportive care, and clinical monitoring
Used in this study were 4 different disease-specific preparative regimens. Carmustine, cytarabine, etoposide, and cyclophosphamide (BAVC)26 were administered to non-Hodgkin lymphoma (NHL) patients. Hodgkin disease (HD) patients received myeloablative therapy that included carmustine, cytarabine, etoposide, and melphalan (BEAM).26 Acute leukemia and multiple myeloma (MM) patients were prepared with busulphan and cyclophosphamide29,30 and high-dose melphalan,28 respectively. After completion of the conditioning regimen, patients were reinfused with autologous cryopreserved marrow cells (day 0).
All lymphoma/myeloma patients received 5 μg/kg per day of G-CSF starting day +6 after autograft. G-CSF was also used at 5 μg/kg per day in 18 of 21 acute leukemia patients upon clinical indication. Uniform supportive care measures were used for all patients on the study. Patients were nursed in single or double rooms in reverse isolation and received antimicrobial prophylaxis that consisted of oral nystatin and ciprofloxacin. Packed red blood cells and platelet transfusions were administed to maintain a hemoglobin level higher than 80 g/L (8 g/dL) and a platelet count higher than 10 × 109/L. Patients were treated with broad-spectrum antibiotics when fever developed and the absolute neutrophil count (ANC) was less than 0.5 × 109/L. Amphotericin B (1 mg/kg per day) was added if patients had persistent fever after 4 to 7 days of intravenous antimicrobial therapy.
Patients underwent daily assessment of symptoms and physical examination during hospitalization and weekly after discharge. As previously reported, laboratory studies were obtained before transplantation, daily while in the hospital, and weekly after discharge.
Hematopoietic recovery and clinical end points
The primary end point of the study was time to hematopoietic reconstitution, which was defined as the number of days to achieve an absolute neutrophil count (ANC) higher than 0.5 × 109/L (first of 3 consecutive days) and an unsupported platelet count higher than 20 and 50 × 109/L. Secondary end points were incidence of documented infections, use of intravenous antibiotics, days to hospital discharge after transplantation, and TRM. Assessment of tumor response to autologous transplantation was generally performed 30 days after reinfusion and was planned every 2 to 4 months thereafter.
Statistical analysis
The results are presented as median values and ranges where applicable. The probabilities of neutrophil and platelet recovery and overall survival of the different series of patients were compared by means of the Kaplan-Meier method. Differences with P values less than .05 were considered statistically significant. The association between the number of CD34+ cells infused and the time to hematopoietic recovery was assessed by the Pearson correlation test.
Results
Study patients
Between February 1995 and December 2002, 86 heavily pretreated consecutive patients were enrolled in this study. All together, patients for whom PBSCs were not available represented 12.5% of all individuals submitted to PBSC mobilization in the 3 participating institutions during the study period. In particular, mobilization failure, according to our stringent criteria, was observed in 58 (10.1%) of 573 lymphoma, 7 (7.3%) of 80 multiple myeloma, and 21 (60%) of 35 acute leukemia patients.
Tables 1 and 2 report patient demographics and some of the most important clinical parameters widely considered to affect BM function, such as diagnosis (ie, acute leukemia vs lymphomas and MM), burden of previous chemotherapy, and radiotherapy.
Of the patients, 66 (lymphoma/myeloma [n = 63]; acute leukemia [n = 3]) had failed PBSC mobilization with high-dose G-CSF alone, whereas 20 (acute leukemia [n = 18]; lymphoma/myeloma [n = 2]) received priming chemotherapy followed by G-CSF. Defined as poor/nonmobilizers because the peak value of circulating CD34+ cells was less than 10 cells/μL were 78 patients. An additional 8 patients did not collect the minimum CD34+ cell dose of 1 × 106/kg (Table 2).
BM harvest
Stem cell collection was performed after a minimum of 4 weeks from PBSC mobilization failure (median, 43 days; range, 32-95 days). G-CSF treatment was well tolerated and no patient required the reduction or suspension of the cytokine administration. Table 3 shows the number of nucleated cells, CFU-Cs, and CD34+ cells collected after 3 days of priming of G-CSF and those recovered and reinfused after cryopreservation. No differences were noted between lymphoma/myeloma patients and leukemia patients for nucleated cells and CFU-Cs collected. Conversely, lymphoma/myeloma patients showed a trend toward a higher number of CD34+ cells in BM grafts (P = .07) (data not shown). Of note, the median number of CD34+ cells reinfused was 0.70 × 106/kg with one patient receiving as little as 0.12 × 106 CD34+/kg.
Because G-CSF is a hematopoietic cytokine that has been shown to induce proliferation of tumor cells, especially myeloid leukemic cells, the effect of G-CSF on BM tumor cell contamination was also evaluated. Of 7 myeloma patients, 6 had a plasma cell infiltration lower than 30% before BM harvest, which was not modified by the cytokine treatment, whereas no lymphoma patients showed BM infiltration at the time of stem cell collection (data not shown). There were 3 leukemic patients who had both molecular and cytogenetic markers at diagnosis that could be traced: inv(16), t(8;21) in acute myeloblastic leukemia (AML) patients and bcr-abl rearrangement in one case of Philadelphia chromosome–positive acute lymphoblastic leukemia (ALL). AML patients were in molecular complete remission (CR) after induction/consolidation therapy and had tumor-free autografts, whereas the ALL patient who remained bcr-abl (p190) positive after chemotherapy showed molecularly detectable disease in BM harvest, although quantitative analysis did not demonstrate a higher number of copies of the transcript (data not shown).
Engraftment results
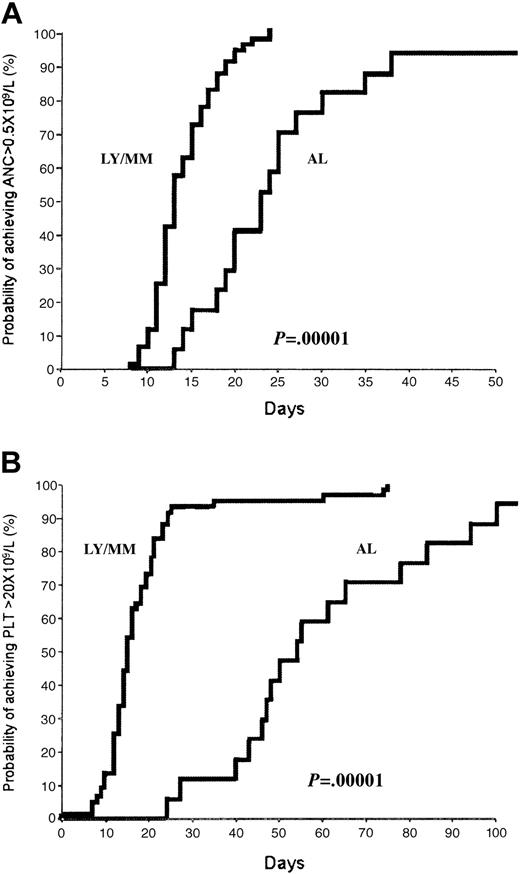
All patients submitted to BM harvest proceeded to autologous transplantation at a median of 2 months (range, 1-6 months) from stem cell collection. None of the 8 patients with inadequate PBSC collection (ie, < 1 × 106 CD34+ cells/kg) was reinfused with peripheral blood (PB) cells along with G-CSF–primed BM. Engraftment data after high-dose chemotherapy are shown in Table 4 and Figure 1. Patients are categorized according to diagnosis since the hematopoietic recovery of leukemic patients was significantly slower than that of patients with lymphoproliferative disorders. Excluded from analysis were 6 patients who died in the peritransplantation period. The median times to neutrophil engraftment were 23 and 13 days (P < .000 01) for leukemia and lymphoma/myeloma patients, respectively (Figure 1A). According to the faster neutrophil recovery, the percentage of lymphoma/myeloma patients with documented infections and submitted to intravenous antibiotic treatment was significantly lower (both P values < .05) than that of leukemia patients.
Hematopoietic recovery after myeloablative chemotherapy and reinfusion of G-CSF–primed BM. Kaplan-Meier plot of probability of recovery of neutrophils to 0.5 × 109/L (A) and recovery to an unsupported platelet count of 20 × 109/L (B) in lymphoma/myeloma (LY/MM) and acute leukemia (AL) patients. As expected, BM reconstitution was significantly slower in leukemia patients.
Hematopoietic recovery after myeloablative chemotherapy and reinfusion of G-CSF–primed BM. Kaplan-Meier plot of probability of recovery of neutrophils to 0.5 × 109/L (A) and recovery to an unsupported platelet count of 20 × 109/L (B) in lymphoma/myeloma (LY/MM) and acute leukemia (AL) patients. As expected, BM reconstitution was significantly slower in leukemia patients.
The median times to an unsupported platelet count of 20 and 50 × 109/L were 52 and 90 days and 15 and 22 days for leukemia and lymphoma/myeloma patients, respectively (both P values < .000 01) (Figure 1B). At the time of the last follow-up, 2 AML patients did not achieve platelet-transfusion independence (day +90 from transplantation). No correlation was found between the number of CD34+ cells reinfused and time to neutrophil and platelet engraftment. This finding was also confirmed when we analyzed separately the 2 groups of patients (data not shown). Furthermore, no difference of engraftment was observed between patients receiving more or less than 0.7 × 106 CD34+ cells/kg (ie, the median number of progenitors infused in our patient population). Median times of hospitalization after autograft were 27 and 17 days for leukemia and lymphoma/myeloma individuals, respectively (P < .01). No patients were readmitted into the hospital after discharge because of late infections.
TRM and overall survival after autologous transplantation
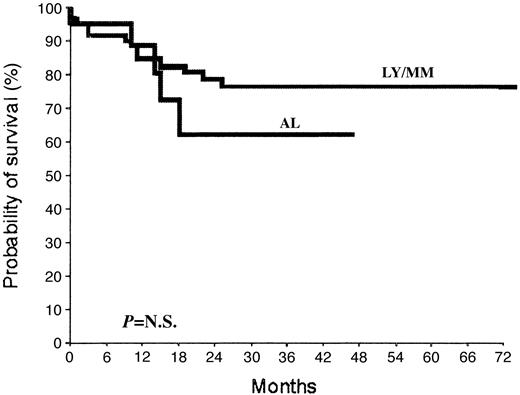
So far, 19 patients (22.1%) have died. Within 40 days from autologous transplantation, 6 patients (acute leukemia [n = 3]; lymphoma/myeloma [n = 3]; 7%) died: 4 experienced procedure-related deaths (pulmonary edema [n = 1], the day of BM reinfusion; sepsis [n = 3], at days +12, +14, and +31 from autograft) (4.65%), whereas 2 leukemic patients died following early relapse (n = 1, at day +35) or progression (n = 1, day +25) of the disease. During follow-up, 13 additional deaths occurred due to recurrence/progression of the disease. With a median follow-up for the study population of 24.5 months, the overall survival at 36 months of lymphoma/myeloma and leukemic patients is 77% and 62%, respectively (Figure 2).
Overall survival of patients submitted to autologous BM transplantation. Probability of survival for lymphoma/myeloma (LY/MM) and acute leukemia (AL) patients undergoing reinfusion of autologous primed BM to support high-dose chemotherapy. N.S. indicates not significant.
Overall survival of patients submitted to autologous BM transplantation. Probability of survival for lymphoma/myeloma (LY/MM) and acute leukemia (AL) patients undergoing reinfusion of autologous primed BM to support high-dose chemotherapy. N.S. indicates not significant.
Discussion
Randomized studies have demonstrated that G-CSF–primed BM supports hematopoietic recovery after high-dose chemotherapy recovery as rapidly as G-CSF–mobilized PBSCs15,16 and is more effective than unprimed BM.15 Fast BM reconstitution after transplantation occurs despite the slight increase, if any, of the stem/progenitor cell content of cell harvests compared with steady-state collections.15 To explain this apparent discrepancy, we previously demonstrated that G-CSF exerts a “priming effect” on BM cells by enhancing the cycling of hematopoietic progenitors and by increasing the response of BM cells to subsequent treatments (in vitro and in vivo) with cytokines.31 Despite similar engraftment data, PB is, by far, the most used source of autologous stem cells to support BM function after myeloablative chemoradiotherapy. However, there is a significative proportion of cancer patients, ranging from 10% to 30%,1 who are either poor mobilizers or nonmobilizers. For these patients, there is as yet no standard procedure for stem cell collection. Unprimed back-up marrow frequently results in delayed platelet recovery24,32 and is associated with a high TRM,6 whereas a second mobilization course has been successful in only 48% of patients who did not collect more than 2.5 × 106 CD34+ cells/kg with the first attempt.33 A more promising approach relies on the use of high doses of G-CSF, or the combination of G-CSF and granulocyte macrophage (GM)–CSF, which successfully mobilized, in small series, the majority of patients who had an insufficient cell yield during the first mobilization strategy.34-36 On the other hand, the mobilization potential of newer agents such as stem cell factor, FLT3-ligand, thrombopoietin, interleukin-8 (IL-8), and their combinations remains to be fully clarified in the clinical setting.
The number of circulating CD34+ cells during PBSC mobilization is the conventional criteria for starting leukaphereses. However, no generally agreed upon threshold exists to commence PBSC collection. The minimum peak concentration of CD34+ cells that many transplantation centers have established to start leukaphereses is 20 cells/μL.1 In addition, 1 to 2 × 106 CD34+ cells/kg is the minimum number of PBSCs to reinfuse to obtain a rapid neutrophil recovery.1,6,7 In this study we considered poor mobilizers as patients with less than 10 CD34+ cells/μL24,25 and they were submitted to G-CSF–primed BM harvest. Similarly, BM harvest was offered to patients whose PBSC collection contained less than 1 × 106 CD34+ cells/kg. By considering that autologous stem cell transplantation has proved to be the best therapeutic option for relapsed/refractory lymphoma37,38 and multiple myeloma patients,39 it appears to be crucial to develop strategies to bring transplantation to as many individuals as possible.
In our institutions, from February 1995 to December 2002, 86 consecutive patients with hematologic malignancies met these criteria and were enrolled in a prospective nonrandomized study. The study was designed to assess the hematopoietic recovery, the TRM, and all the clinical parameters correlated with effective BM reconstitution after reinfusion of G-CSF–primed BM to support high-dose chemotherapy. It should be noted that all patients who entered in our study and submitted to BM harvest subsequently underwent transplantation.
Our results demonstrated the feasibility of BM harvest in cancer patients nonmobilizing PBSCs who had been primed for 3 days with G-CSF. The content of CFU-Cs and CD34+ cells was superimposable to that of previously reported lymphoma patients who were not tested for their capacity to mobilize PBSCs.15 When reinfused, these cells engrafted rather rapidly and the results compare favorably with those observed with unprimed BM. In fact, our previous experience of autologous transplantation for AML patients in first CR conditioned with busulphan and cyclophosphamide showed a median of 25 and 36 days to neutrophil recovery and 87.4 and 150 days to platelet-transfusion independence for patients reinfused with both BM and PBSC30 or BM alone,29 respectively. However, it should be noted that in earlier studies,29,30 posttransplantation hematopoietic growth factors were administered, upon clinical indication, in only 45% of the patients. Similarly, relapsed or refractory lymphoma patients who received transplants of BM cells after BAVC or BEAM preparative regimens recovered an ANC higher than 0.5 × 109/L in a median of 14 and 11 days and a PLT higher than 20 × 109/L in 20 and 15 days according to whether they received G-CSF or G-CSF/IL-3 combination, respectively, after stem cell reinfusion.26 Notably, reinfusion of primed BM did not result in delayed PLT recovery compared with our historical controls. This is an important finding since transplantation of low numbers of PB-mobilized CD34+ cells (ie, < 2 × 106/kg) has been associated with slow PLT reconstitution. Moreover, after transplantation almost all patients enrolled in this study (including leukemia patients) were given G-CSF, which may negatively impact on PLT recovery. However, the PLT recovery of study patients appears to be slower than that of leukemia29 and lymphoma/myeloma (Lemoli et al28 ; and data not shown) patients reinfused with PBSCs in the same period of time.
Interestingly, we did not find a correlation between the number of CD34+ cells reinfused and hematopoietic engraftment, and we could not set a threshold value predictive of a rapid engraftment. This was also true when the 2 groups of study patients were analyzed separately. Thus, the speed of recovery of BM function may be due mainly to the underlying disease and the conditioning regimen. However, previous studies have demonstrated that specific CD34+ cell subsets, which were not evaluated in the present study, are more reliable predictors of fast engraftment than total CD34+ cells.40-42 The TRM rate in our series (which includes 24.4% of leukemic patients) was 4.65%, which is in the range of what is commonly observed after autologous stem cell transplantation for heavily pretreated patients. No late deaths (beyond day +40) due to infection were observed in the follow-up. As a result, the overall survival of study patients (Figure 2) was comparable with that of our historical controls26,29,30 and appears to be better than that of poor mobilizers who received transplants of unprimed BM.6
The successful engraftment in our patients rescued with primed BM indicates that failure of PBSC mobilization is not always due to poor marrow quality. Conversely, a specific impairment of mobilization, in the presence of adequate marrow, may occur. In this regard, the better understanding of the mechanisms of mobilization may contribute to the development of more effective protocols for true “nonmobilizers.” An interesting approach for such patients may include the combination of G-CSF and the growth hormone.43
In conclusion, we demonstrate that G-CSF–primed BM induces sustained and multilineage hematopoietic recovery after myeloablative chemotherapy and represents an effective source of stem cells for cancer patients eligible for autologous transplantation.
Prepublished online as Blood First Edition Paper, April 24, 2003; DOI 10.1182/blood-2003-02-0440.
Supported by the University of Bologna (funds for selected topics).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.