Abstract
Collagen stimulates platelet activation through a tyrosine kinase–based pathway downstream of the glycoprotein VI (GPVI)–Fc receptor (FcR) γ-chain complex. Genetic ablation of FcR γ-chain results in a complete inhibition of aggregation to collagen. In contrast, a steady increase in light transmission is induced by collagen in phospholipase Cγ2–deficient (PLCγ2–/–) platelets in a Born aggregometer, indicating a weak level of activation. This increase is inhibited partially in the presence of an α2β1-blocking antibody or an αIIbβ3 antagonist and completely by a combination of the 2 inhibitors. It is also abolished by the Src kinase inhibitor PP1 and reduced in the presence of the phosphatidylinositol (PI) 3-kinase inhibitor wortmannin. The GPVI-specific agonists convulxin and collagen-related peptide (CRP) also stimulate weak aggregation in PLCγ2–/– platelets, which is inhibited by wortmannin and PP1. Collagen and CRP stimulate tyrosine phosphorylation of PLCγ1 at its regulatory site, Tyr 783, in murine but not in human platelets through a Src kinase–dependent pathway. Adhesion of PLCγ2–/– platelets to a collagen monolayer is severely reduced at a shear rate of 800 s–1 , relative to controls, whereas it is abolished in FcR γ-chain–/– platelets. These results provide strong evidence that engagement of GPVI stimulates limited integrin activation in PLCγ2–/– platelets via PLCγ1 and PI3-kinase.
Introduction
Adhesion and activation of platelets by subendothelial collagen fibers initiates aggregate formation at sites of vessel damage. Because of its central role in hemostasis and pathological thrombosis, the mechanism of collagen-induced platelet activation has been extensively studied. Among several candidates, the integrin α2β1 and the glycoprotein VI (GPVI)–Fc receptor (FcR) γ-chain complex are recognized as the major platelet collagen receptors.
GPVI-FcR γ-chain plays a critical role in platelet activation by collagen, as shown by the lack of response to collagen in human and murine platelets deficient in the receptor complex.1,2 Additionally, a collagen-related peptide, known as CRP, and a snake venom toxin, convulxin, interact specifically with GPVI and mimic many of the responses to collagen.3-5 On the other hand, collagen induces full aggregation of human platelets in the presence of α2β1-blocking antibodies6 and in murine platelets that lack α2 or β1, demonstrating that the integrin is not essential for activation.7,8 However, aggregation by collagen is delayed in the absence of functional α2β1, demonstrating an important role for the integrin in the initial events that underlie activation.7,8
The mechanism of the GPVI-mediated signaling system has been extensively investigated.9-11 GPVI is present as a complex with the FcR γ-chain in the platelet membrane.9,12,13 The Src family kinases Fyn and Lyn are associated with the proline-rich domain in the GPVI cytoplasmic tail via their SH3 domains14 and upon receptor crosslinking initiate activation through phosphorylation of the immunoreceptor tyrosine–based activation motif (ITAM) in the FcR γ-chain, leading to binding and activation of the tyrosine kinase Syk. A series of adapter molecules including linker for T-cell activation (LAT) and Src homology 2 domain-containing leukocyte phosphoprotein of 76 kDa (SLP-76) orchestrate a carefully regulated signaling network leading to activation of phospholipase C γ2 (PLCγ2), phosphatidylinositol (PI) 3 (PI3)–kinase, and platelets.10,11,15,16
Studies using PLCγ2–/– mice have shown that the phospholipase plays a critical role in activation by collagen.17 In the present report, however, we show that collagen and GPVI-specific agonists are able to induce weak activation of PLCγ2–/– platelets via PLCγ1 and PI3-kinase, and we provide evidence that this is functionally relevant under shear conditions.
Materials and methods
Materials
Anti-PLCγ2 polyclonal antibody (pAb) was a gift from Dr Mike Tomlinson (DNAX, Palo Alto, CA). C57Bl/6 mice deficient in FcR γ-chain were kindly donated by Dr Takashi Saito (Chiba University, Japan). Lotrafiban was a gift from GlaxoSmithKline (Philadelphia, PA). PLCγ2–/– mice17 were bred from heterozygotes on a B6 background. Mice were genotyped by polymerase chain reaction17 and wild-type littermates were used as controls. Collagen reagent Horm, as native type I fibrils from equine tendons, was from Nycomed Pharma (Munich, Germany). CRP (GKO[GPO]10GKOG; single-letter code, where O is hydroxyproline) was synthesized by Tana Laboratories (Houston, TX); it was cross-linked with 0.25% glutaraldehyde for 3 hours on ice and then dialysed into phosphate-buffered saline (PBS). PP1 and PP2 were from Biomol Research Laboratories (Plymouth Meeting, PA). U73143 and U73122 were obtained from Calbiochem (San Diego, CA). Antifibrinogen fluorescein isothiocyanate (FITC)–conjugated antibody was from DAKO (High Wycombe, United Kingdom). Antiphosphotyrosine monoclonal antibody (mAb; 4G10) and anti-PLCγ1 monoclonal antibody were obtained from Upstate Laboratories (Syracuse, NY). Fura PE3-am was from TEF Labs (Austin, TX). Purified hamster antimouse α2 integrin mAb (HMα2) and FITC-conjugated antimouse P-selectin were from BD Pharmingen (Oxford, United Kingdom). Thrombin, adenosine diphosphate (ADP), wortmannin, apyrase, fibrinogen, fatty acid–free bovine serum albumin (BSA), D-Phe-Pro-Arg chloromethyl ketone (PPACK), and tetramethyl rhodamine isothiocyanate (TRITC)–conjugated phalloidin were from Sigma (St Louis, MO). Anti-phospho-PLCγ1 (Tyr783) pAb was from New England Biolabs (Pickering, ON, Canada). All other reagents were from previously named sources.18
Preparation of murine and human platelets
Murine blood was drawn by cardiac puncture from mice terminally anesthetized with CO2 and taken into 100 μL acid citrate dextrose (ACD). Washed platelets were prepared as previously described.19 In some experiments, murine blood was taken into 300 μL heparin solution (10 U/mL) and platelet-rich plasma (PRP) was obtained by centrifugation at 300g for 10 minutes. For aggregation or flow cytometry studies in the presence of fibrinogen and their control studies, platelets were washed with 10 mM EGTA (ethylene glycol tetraacetic acid), 10% ACD, and 1 μg/mL prostaglandin I2 (PGI2); 30 minutes after washing, 200 μM CaCl2 was added before experiments. Cell density was adjusted by adding platelet-poor plasma and modified Tyrode buffer (137 mM NaCl, 11.9 mM NaHCO3, 0.4 mM Na2HPO4, 2.7 mM KCl, 1.1 mM MgCl2, and 5.6 mM glucose, pH 7.3) at a cell density of 2 × 108/mL. Venous blood from healthy drug-free volunteers was taken into 10% sodium citrate. Washed platelets were obtained as described elsewhere.18 Human and murine washed platelets were resuspended in modified Tyrode buffer (137 mM NaCl, 11.9 mM NaHCO3, 0.4 mM Na2HPO4, 2.7 mM KCl, 1.1 mM MgCl2, and 5.6 mM glucose, pH 7.3) at a cell density of 1 to 5 × 108/mL.
Platelet aggregation and shape change
A quantity of 300 μL PRP or washed platelets (2 × 108 /mL) was used for aggregation studies. Aggregation was monitored by light transmission using a Born aggregometer (Alpha Laboratories, Eastleigh, Hants, United Kingdom) with high-speed stirring (1200 rpm) at 37°C. For measurement of platelet shape change in the absence of aggregation in response to CRP, 300 μL washed platelets (1 × 108 cells/mL) were incubated with 10 μM lotrafiban and 1 mM EGTA. Shape change was monitored by light transmission using a Born aggregometer (Chronolog, Havertown, PA) with high-speed stirring (1200 rpm) at 37°C.
Flow cytometry studies
Washed platelets (5 × 106 cells) from wild-type or PLCγ2-deficient mice were stimulated with 40 μg/mL CRP or convulxin for 2 minutes and then incubated for 20 minutes with FITC-conjugated antifibrinogen (2 μL). For P-selectin expression, washed platelets (5 × 106 cells) were stimulated with 40 μg/mL convulxin or 20 μg/mL CRP for 10 minutes and then incubated with FITC-conjugated anti–P-selectin antibody (6 μL) for 10 minutes. After addition of 500 μL Tyrode buffer, samples were analyzed with a Becton Dickinson (San Jose, CA) FACScan flow cytometer. Ten thousand particles were acquired from each sample. The light scatter and the fluorescence signals were set in logarithmic gain. The results were analyzed as a histogram of fluorescence intensity channel 1 (FL1) plotted against cell count. Data were recorded and analyzed using CellQuest software (Becton Dickinson).
Immunoprecipitation and immunoblotting
Washed platelets (5 × 108/mL) were left for 30 minutes and then incubated with 10 μM lotrafiban, 10 μM indomethacin, and 3 U/mL apyrase. Where indicated, washed platelets were incubated with dimethyl sulfoxide (DMSO), 20 μM PP2, or 100 nM wortmannin for 5 minutes before stimulation. Platelet stimulations were terminated by the addition of an equal volume of ice-cold lysis buffer (2% [volume/volume] Nonidet P-40, 20 mM Tris [tris(hydroxymethyl)aminomethane], 300 mM NaCl, 2 mM EDTA [ethylenediamine tetraacetic acid], 2 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 2 mM Na3VO4, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 1 μg/mL pepstatin A, pH 7.3). Detergent-insoluble debris was removed by centrifugation at 15 000g for 10 minutes, and the supernatant was precleared with protein A-sepharose (50% (weight/volume) in Tris-buffered saline plus Tween 20 (TBS-T: 20 mM Tris, 137 mM NaCl, 0.1% [volume/volume] Tween 20, pH 7.6) for 1 hour at 4°C. Anti-PLCγ1 antibody (4 μg) and protein A-sepharose were added and each sample was rotated at 4°C overnight. The sepharose pellet was washed sequentially in lysis buffer and TBS-T before the addition of Laemmli sample buffer. Anti-PLCγ2 antibody (2 μL) and protein A-sepharose were added to the resultant supernatant and the same procedure as for PLCγ1 immunoprecipitation was repeated. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 8% gels, electrotransferred, and blotted as described previously.18
Calcium fluorometry
Intracellular calcium increase was measured in Jurkat cells (1 × 108/mL) suspended in Tyrode buffer containing 1 mM CaCl2 upon 40 μg/mL convulxin or 20 μg/mL CRP stimulation as described previously,20 except that platelets were incubated with 10 μM Fura-PE3-am, a calcium-sensitive fluorescent dye, and 3 U/mL apyrase.
Adhesion under flow conditions
Mouse blood was collected into 0.5 volume of modified Tyrode buffer containing 120 μM PPACK and 15 U/mL heparin (volume of blood to buffer ratio of 2:1). Capillary tubes (0.3 × 1.2 mm, 50 mm long, Camlab, Cambridge, United Kingdom) were coated with 0.1 mg/mL collagen overnight at 4°C. The capillaries were washed and blocked with PBS containing 2% BSA for 2 hours at room temperature. Then they were rinsed with modified Tyrode buffer supplemented with 2 mM CaCl2 and 1 U/mL heparin, and connected to a syringe filled with the anticoagulated blood. The blood was perfused through the collagen-coated microcapillary at a shear rate of 800 s–1 for 2 minutes. Nonadherent cells were washed for 8 minutes at the same shear rate, using the modified Tyrode buffer. After washing, microscope images were recorded using a video record from at least 6 different microscope fields (63 × objectives). Image analysis was performed off-line using ImagePro plus software (DataCell Limited, Berkshire, United Kingdom). The platelet adhesion results are expressed as the mean surface area covered by platelets or percentage of controls.
Platelet adhesion under static conditions
Collagen (50 μg/mL) was incubated on a cover slip overnight at 4°C. After being washed twice with PBS, the cover slip was blocked with 1% fatty acid–free purified BSA in PBS for 2 hours at room temperature and washed twice with modified Tyrode buffer. Washed murine platelets (3 × 107/mL) were incubated on the cover slips at 30°C. After removal of unbound platelets by washing with modified Tyrode buffer, adhered platelets were fixed with 3% paraformaldehyde, permeabilized with 0.2% Triton X-100, and stained by TRITC-conjugated phalloidin as described previously.21 Platelets were viewed on an inverted fluorescent microscope (Ziess, Jena, Germany) and digital imaging (× 400) was carried out using Openlab software for Macintosh (Improvision, Coventry, United Kingdom). The number and diameter of platelets was counted on the printed images (0.036 mm2 per image).
Statistical evaluation
Statistical analysis was performed using the unpaired Student t test.
Results
Collagen stimulates integrin activation in PLCγ2–/– platelets
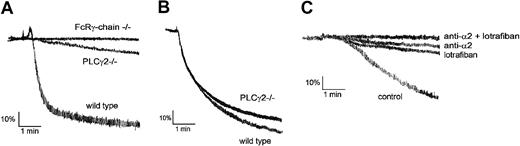
Collagen (10 μg/mL) stimulates shape change and robust aggregation in murine platelets with a characteristic delay of approximately 20 seconds (Figure 1A). A similar delay is seen in PLCγ2–/– platelets, but in this case collagen (10 μg/mL) stimulates a slow, continuous increase in light transmission that reaches 20% of maximal response after 5 minutes and is not preceded by shape change (Figure 1A). Collagen (10 μg/mL) has no effect on light transmission in FcR γ-chain–/– mice over the same period (Figure 1A). The final extent of aggregation to thrombin (0.1-1 U/mL) is slightly (< 10%) reduced in PLCγ2–/– platelets, although there is no effect on the initial phase of the response (Figure 1B and not shown). A small increase in light transmission in response to collagen was seen in the original description of the PLCγ2–/– mice but was not discussed.17
PLCγ2–/– platelets exhibit a small increase in light transmission upon stimulation by collagen. (A) Heparinized PRP from wild-type, PLCγ2–/–, and FcRγ-chain–/– mice was stimulated with 10 μg/mL collagen for 5 minutes. (B) Washed platelets from wild-type and PLCγ2–/– mice were stimulated with 1 U/mL thrombin for 5 minutes. (C) Washed platelets from wild-type, PLCγ2–/–, and FcRγ-chain–/– mice were incubated without inhibitors (control) or with 20 μg/mL anti-α2 (HMα2), 10 μM lotrafiban, or a combination of 20 μg/mL HMα2 and 10 μM lotrafiban for 5 minutes. Platelets were stimulated with 10 μg/mL collagen for 5 minutes. The data are representative of 3 experiments.
PLCγ2–/– platelets exhibit a small increase in light transmission upon stimulation by collagen. (A) Heparinized PRP from wild-type, PLCγ2–/–, and FcRγ-chain–/– mice was stimulated with 10 μg/mL collagen for 5 minutes. (B) Washed platelets from wild-type and PLCγ2–/– mice were stimulated with 1 U/mL thrombin for 5 minutes. (C) Washed platelets from wild-type, PLCγ2–/–, and FcRγ-chain–/– mice were incubated without inhibitors (control) or with 20 μg/mL anti-α2 (HMα2), 10 μM lotrafiban, or a combination of 20 μg/mL HMα2 and 10 μM lotrafiban for 5 minutes. Platelets were stimulated with 10 μg/mL collagen for 5 minutes. The data are representative of 3 experiments.
The increase in light transmission induced by collagen in the PLCγ2–/– platelets could reflect a limited degree of αIIbβ3-dependent aggregation and/or adhesion to α2β1. To investigate this, we examined the effect of an inhibitory antibody against murine α2, HMα2, and the αIIbβ3 antagonist lotrafiban on activation by collagen. Lotrafiban has established its role as an αIIbβ3 blocker in murine and human platelets.19,22,23 It also blocks αIIbβ3-dependent adhesion to von Willebrand factor (VWF) under flow (not shown). We used a slightly higher concentration of collagen (20 μg/mL) in these experiments to accentuate the response. Lotrafiban or the antibody HMα2 inhibited the increase in light transmission in PLCγ2–/– platelets induced by collagen by 60% to 70% but did not cause a complete abolition of the increase in light transmission. When given in combination, the 2 inhibitors completely blocked the response (Figure 1C). This combination of the 2 inhibitors also blocks aggregation to collagen in wild-type platelets (not shown). These findings demonstrate that the increase in light transmission induced by collagen in the PLCγ2–/– platelets is made up of a combination of α2β1-dependent adhesion and αIIbβ3-dependent aggregation.
GPVI agonists stimulate weak activation of PLCγ2–/– platelets
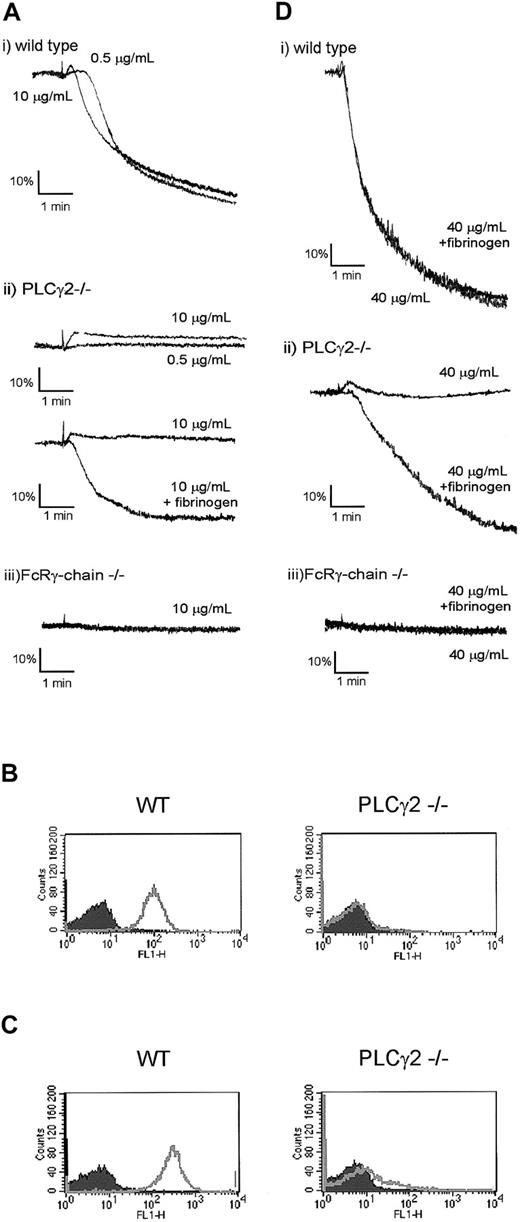
We used the selective agonists CRP and convulxin to establish whether the partial response to collagen is mediated through activation of GPVI. Platelet shape change and aggregation induced by a low concentration of CRP (0.5 μg/mL) were abolished in PLCγ2–/– platelets (Figure 2A). In contrast, a 20-fold higher concentration of CRP (10 μg/mL) induced shape change (Figure 2A), which was followed by weak aggregation in some experiments (not shown). We reasoned that the variability in the aggregation response was due to inhibition of secretion of fibrinogen from α-granules. In support of this explanation, we observed that CRP was unable to induce expression of the α-granule membrane marker P-selectin in PLCγ2–/– platelets (Figure 2B) and that shape change was unaltered in the presence of the ADP scavenger apyrase or cyclo-oxygenase inhibitor indomethacin, demonstrating that significant dense granule secretion and activation of phospholipase A2 had not occurred (not shown). Signifi-cantly, aggregation to CRP (10 μg/mL) was restored in the presence of added fibrinogen (Figure 2A), whereas the GPVI-specific peptide did not induce activation of FcR γ-chain–/– platelets under the same conditions (Figure 2A). Direct evidence for activation of αIIbβ3 in the PLCγ2–/–platelets by CRP was demonstrated by flow cytometry, using fibrinogen and an FITC-conjugated secondary antibody (Figure 2C).
GPVI agonists induce fibrinogen binding to integrin αIIbβ3 but not secretion in FcRγ-chain–/– platelets. (A,D) Washed platelets from wild-type (i), PLCγ2–/– (ii), and FcRγ-chain–/– (iii) mice were stimulated with indicated concentrations of CRP (A) or 40 μg/mL convulxin (D) for 5 minutes in the presence or absence of fibrinogen (200 μg/mL). (B) Washed platelets from wild-type (WT) and PLCγ2–/– mice were stimulated without (shaded area) or with (unshaded area) 20 μg/mL CRP and incubated with FITC-labeled anti–P-selectin antibody for 10 minutes. Fluorescence was analyzed by flow cytometry. (C) Washed platelets from wild-type (WT) and PLCγ2–/– mice were stimulated without (shaded area) or with (unshaded area) 20 μg/mL CRP in the presence of fibrinogen (200 μg/mL) and incubated with FITC-labeled anti-fibrinogen antibody for 20 minutes. Fluorescence was analyzed by flow cytometry.
GPVI agonists induce fibrinogen binding to integrin αIIbβ3 but not secretion in FcRγ-chain–/– platelets. (A,D) Washed platelets from wild-type (i), PLCγ2–/– (ii), and FcRγ-chain–/– (iii) mice were stimulated with indicated concentrations of CRP (A) or 40 μg/mL convulxin (D) for 5 minutes in the presence or absence of fibrinogen (200 μg/mL). (B) Washed platelets from wild-type (WT) and PLCγ2–/– mice were stimulated without (shaded area) or with (unshaded area) 20 μg/mL CRP and incubated with FITC-labeled anti–P-selectin antibody for 10 minutes. Fluorescence was analyzed by flow cytometry. (C) Washed platelets from wild-type (WT) and PLCγ2–/– mice were stimulated without (shaded area) or with (unshaded area) 20 μg/mL CRP in the presence of fibrinogen (200 μg/mL) and incubated with FITC-labeled anti-fibrinogen antibody for 20 minutes. Fluorescence was analyzed by flow cytometry.
A similar set of results was observed with a more powerful GPVI-specific agonist, the snake toxin convulxin. Convulxin stimulated robust aggregation of wild-type platelets but induced only weak aggregation in PLCγ2–/– platelets in the presence of fibrinogen, whereas it had no effect in the absence of the FcR γ-chain (Figure 2D). Convulxin also stimulated activation of αIIbβ3, but it was unable to induce a significant level of increase in expression of P-selectin (not shown).
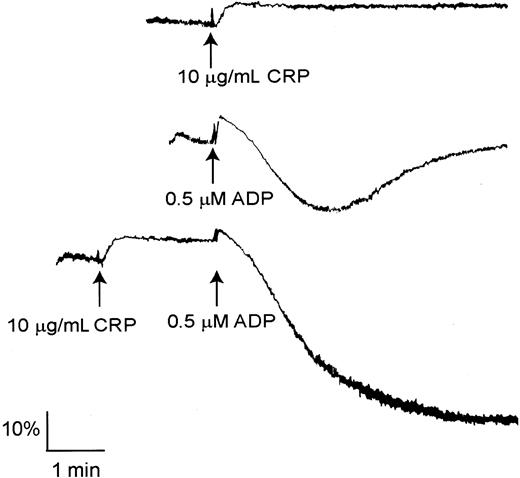
It is well established that the P2Y12 ADP receptor can undergo synergy with a number of platelet agonists, including those that act through GPVI, to induce aggregation.24,25 Significantly, a submaximal concentration of ADP (0.5 μM), which stimulates reversible aggregation, induces irreversible aggregation in PLCγ2–/– platelets in the presence of a concentration of CRP (10 μg/mL) that induces shape change (Figure 3).
CRP stimulates shape change and undergoes synergy with ADP in PLCγ2–/– platelets. Washed platelets from PLCγ2–/– mice were stimulated with 10 μg/mL CRP (upper trace) or 0.5 μM ADP (middle trace) for 5 minutes. Washed platelets from PLCγ2–/– mice were stimulated with 0.5 μM ADP for 2 minutes and then stimulated with 10 μg/mL CRP for 5 minutes (lower trace). The data are representative of 3 to 5 experiments.
CRP stimulates shape change and undergoes synergy with ADP in PLCγ2–/– platelets. Washed platelets from PLCγ2–/– mice were stimulated with 10 μg/mL CRP (upper trace) or 0.5 μM ADP (middle trace) for 5 minutes. Washed platelets from PLCγ2–/– mice were stimulated with 0.5 μM ADP for 2 minutes and then stimulated with 10 μg/mL CRP for 5 minutes (lower trace). The data are representative of 3 to 5 experiments.
These observations demonstrate that GPVI is able to mediate PLCγ2-independent activation signals that lead to shape change and potentiation of ADP-induced aggregation.
GPVI induces integrin activation in PLCγ2–/– platelets via Src kinases and PI3-kinase
We sought to investigate the signals that mediate the changes in optical density in PLCγ2–/– platelets in response to collagen and the 2 GPVI agonists CRP and convulxin. It is well established that Src family kinases associate with GPVI and mediate phosphorylation of the FcR γ-chain.14 Importantly, the Src family kinase inhibitor PP1 completely inhibited the change in optical density induced by collagen (Figure 4A), CRP (Figure 4B), and convulxin (Figure 4C) in PLCγ2–/– platelets. PI3-kinase also plays a role in collagen-induced platelet activation.26 The irreversible PI3-kinase inhibitor wortmannin inhibited aggregation of PLCγ2–/– platelets (measured in the presence of fibrinogen) induced by CRP, convulxin, and collagen (Figure 4).
Src kinases and PI3-kinase support limited activation of PLCγ2-deficient platelets via GPVI. (A) Washed platelets from PLCγ2–/– mice were incubated with DMSO, 30 μM PP1, or 100 nM wortmannin for 5 minutes and then stimulated with 20 μg/mL collagen for 5 minutes. (B) Washed platelets from PLCγ2–/– mice were stimulated with 10 μg/mL CRP for 5 minutes in the presence of fibrinogen (200 μg/mL) and the inhibitors described for panel A. (C) Washed platelets from PLCγ2–/– were stimulated with 40 μg/mL convulxin for 5 minutes under the same conditions as described for panel B. The data are representative of at least 2 experiments.
Src kinases and PI3-kinase support limited activation of PLCγ2-deficient platelets via GPVI. (A) Washed platelets from PLCγ2–/– mice were incubated with DMSO, 30 μM PP1, or 100 nM wortmannin for 5 minutes and then stimulated with 20 μg/mL collagen for 5 minutes. (B) Washed platelets from PLCγ2–/– mice were stimulated with 10 μg/mL CRP for 5 minutes in the presence of fibrinogen (200 μg/mL) and the inhibitors described for panel A. (C) Washed platelets from PLCγ2–/– were stimulated with 40 μg/mL convulxin for 5 minutes under the same conditions as described for panel B. The data are representative of at least 2 experiments.
These findings demonstrate that Src kinases and PI3-kinase mediate activation of PLCγ2–/– platelets by GPVI.
PLCγ1 is activated in murine platelets
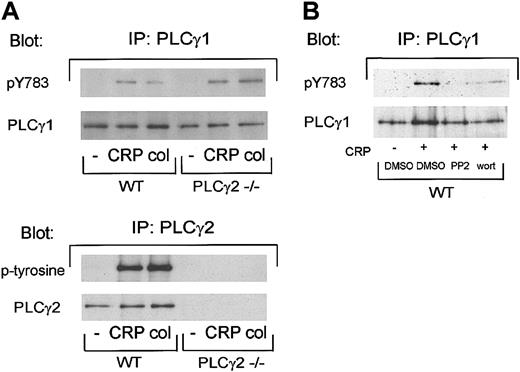
The similar inhibitory actions of PP1 and wortmannin on platelet responses to collagen and GPVI-specific agonists in wild-type and PLCγ2–/– platelets led us to consider whether murine platelets express PLCγ1 and whether this could account for the weak activation of PLCγ2–/– platelets. PLCγ1 is ubiquitously expressed, although it is only a minor component of human platelets and is not phosphorylated by collagen in these cells.27,28 We used a phosphospecific antibody to the regulatory Tyr783 to monitor activation of PLCγ1. Collagen and CRP stimulated phosphorylation of Tyr783 of PLCγ1 in control and PLCγ2–/– platelets, with a slightly greater response observed in the phospholipasedeficient platelets (Figure 5A, upper panel). As entirely expected, tyrosine phosphorylation of PLCγ2 was observed only in wild-type mice (Figure 5A, lower panel). Tyr783 phosphorylation of PLCγ1 by CRP was completely inhibited by Src kinase inhibition and was reduced in the presence of wortmannin (Figure 5B).
Collagen and CRP stimulate phosphorylation of PLCγ1 on Tyr 783 in murine platelets. (A) Washed platelets were stimulated with 10 μg/mL CRP or collagen for 30 seconds and phosphorylation was measured as described in “Materials and methods.” In brief, proteins were precipitated with anti-PLCγ1 or PLCγ2 antibody, resolved by 8% SDS-PAGE, transferred to PVDF membranes, and immunoblotted with antibodies against phosphotyrosine 783 in PLCγ1, phosphotyrosine, PLCγ1 or PLCγ2. (B) Before stimulation, washed platelets were incubated with DMSO, 20 μM PP2, or 100 nM wortmannin. Immunoprecipitation with anti-PLCγ1 antibody and immunoblotted with antiphosphotyrosine 783 in PLCγ1 or anti-PLCγ1 antibody were performed as described for panel A. The data are representative of at least 2 experiments.
Collagen and CRP stimulate phosphorylation of PLCγ1 on Tyr 783 in murine platelets. (A) Washed platelets were stimulated with 10 μg/mL CRP or collagen for 30 seconds and phosphorylation was measured as described in “Materials and methods.” In brief, proteins were precipitated with anti-PLCγ1 or PLCγ2 antibody, resolved by 8% SDS-PAGE, transferred to PVDF membranes, and immunoblotted with antibodies against phosphotyrosine 783 in PLCγ1, phosphotyrosine, PLCγ1 or PLCγ2. (B) Before stimulation, washed platelets were incubated with DMSO, 20 μM PP2, or 100 nM wortmannin. Immunoprecipitation with anti-PLCγ1 antibody and immunoblotted with antiphosphotyrosine 783 in PLCγ1 or anti-PLCγ1 antibody were performed as described for panel A. The data are representative of at least 2 experiments.
We have previously reported that PLCγ1 does not undergo tyrosine phosphorylation in collagen-stimulated human platelets.27 In view of the present results, we repeated these studies using the phosphospecific antibody to Tyr783. Neither collagen, CRP, nor the more powerful GPVI-agonist convulxin induced significant tyrosine phosphorylation of PLCγ1 in human platelets (not shown), demonstrating an important difference between human and mouse cells.
Convulxin induces Ca2+ elevation in PLCγ2–/– platelets, which is blocked by a PLC inhibitor
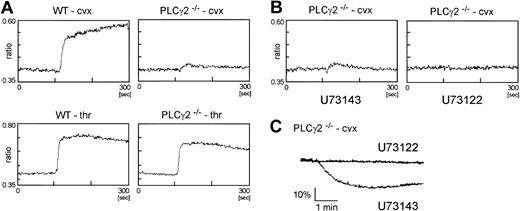
The possibility that tyrosine phosphorylation of PLCγ1 may account for the limited degree of activation of PLCγ2–/– platelets through GPVI was addressed by measurement of elevation of intracellular Ca2+ and use of a specific inhibitor of PLC, U73122. For these studies, we used the GPVI-specific agonist convulxin, as this induces powerful activation of the glycoprotein and therefore facilitates measurement of Ca2+ in the PLCγ2–/– platelets. Convulxin stimulated a rapid and sustained increase in Ca2+ in wild-type platelets, which was reduced to a small, transient response in PLCγ2–/– platelets (Figure 6A). Significantly, this increase in Ca2+ was blocked by the phospholipase inhibitor U73122 but not by its inactive analog U73243 (Figure 6A). U73122 but not U73243 also blocked convulxin-stimulated aggregation (Figure 6B). Importantly, thrombin induced a rapid and marked increase in Ca2+ in both wild-type and PLCγ2–/– platelets (Figure 6A).
PLCγ2–/– platelets show a limited Ca2+ increase, which is inhibited by U73122. (A) Washed platelets from wild-type (WT) and PLCγ2–/– mice were stimulated with 40 μg/mL convulxin (cvx) or 1 U/mL thrombin (thr). Intracellular Ca2+ was measured by the ratio of Fura-PE3 emissions. (B) Platelets were preincubated with 1 μM U73142 or 1 μM U73122 for 5 minutes. Then they were stimulated and Ca2+ was measured as described for panel A. (C) Washed platelets from PLCγ2–/– were preincubated with 4 μM U73143 or 4 μM U73122 for 5 minutes and stimulated with 40 μg/mL convulxin for 5 minutes in the presence of fibrinogen (200 μg/mL).
PLCγ2–/– platelets show a limited Ca2+ increase, which is inhibited by U73122. (A) Washed platelets from wild-type (WT) and PLCγ2–/– mice were stimulated with 40 μg/mL convulxin (cvx) or 1 U/mL thrombin (thr). Intracellular Ca2+ was measured by the ratio of Fura-PE3 emissions. (B) Platelets were preincubated with 1 μM U73142 or 1 μM U73122 for 5 minutes. Then they were stimulated and Ca2+ was measured as described for panel A. (C) Washed platelets from PLCγ2–/– were preincubated with 4 μM U73143 or 4 μM U73122 for 5 minutes and stimulated with 40 μg/mL convulxin for 5 minutes in the presence of fibrinogen (200 μg/mL).
These findings are consistent with a role for PLCγ1 in the limited activation of αIIbβ3 by GPVI agonists that is observed in the absence of PLCγ2.
Thrombus formation on collagen-coated surfaces is impaired in PLCγ2–/– platelets under flow
We sought to further investigate the significance of PLCγ2 in the interaction of platelets with collagen, using a flow-based assay. We perfused whole blood from wild-type and PLCγ2–/– mice over a collagen-coated surface at a medium shear rate of 800 s–1 for 2 minutes. In agreement with previous reports, platelets formed densely packed thrombi on collagen at this shear rate (Figure 7A-B). In marked contrast, PLCγ2–/– platelets attached along the length of collagen fibers but did not nucleate thrombus formation (Figure 7A-B). This level of attachment is greater than the residual level of adhesion observed in FcR γ-chain–/– platelets (Figure 7A-B). An antibody to murine α2β1, HMα2, reduced the limited degree of adhesion that is observed with PLCγ2–/– platelets to the same level as that seen for FcR γ-chain–/– platelets. The αIIbβ3 inhibitor lotrafiban, which blocks the murine αIIbβ3, also had a small inhibitory effect (Figure 7C). These results demonstrate that the low level of adhesion observed in the PLCγ1–/– platelets is dependent on α2β1 and αIIbβ3.
PLCγ2-deficient platelets showed limited integrin-dependent platelet adhesion on collagen at a medium rate of shear. Mouse blood anticoagulated with PPACK and heparin was perfused through a collagen-coated microcapillary at a shear rate of 800 s–1 for 2 minutes and nonadherent cells were washed for 8 minutes at the same shear rate with modified Tyrode buffer containing 2 mM CaCl2 and 1 U/mL heparin. After washing, microscope images were recorded from at least 6 different microscope fields. (A) Representative images of platelet adhesion from wild-type, PLCγ2–/–, and FcRγ-chain–/– mice. (B) Image analysis was performed off-line using ImagePro plus software. Platelet adhesion results are expressed as mean ± SEM surface area covered by platelets. (C) Blood from PLCγ2–/– mice was incubated without (control) or with 10 μM lotrafiban (lotra), 20 μg/mL anti-α2 antibody (HMα2; anti-α2) for 5 minutes before perfusion. Platelet adhesion is expressed as a percentage of control. *P < .05; **P < .01. The data were representative of 2 experiments.
PLCγ2-deficient platelets showed limited integrin-dependent platelet adhesion on collagen at a medium rate of shear. Mouse blood anticoagulated with PPACK and heparin was perfused through a collagen-coated microcapillary at a shear rate of 800 s–1 for 2 minutes and nonadherent cells were washed for 8 minutes at the same shear rate with modified Tyrode buffer containing 2 mM CaCl2 and 1 U/mL heparin. After washing, microscope images were recorded from at least 6 different microscope fields. (A) Representative images of platelet adhesion from wild-type, PLCγ2–/–, and FcRγ-chain–/– mice. (B) Image analysis was performed off-line using ImagePro plus software. Platelet adhesion results are expressed as mean ± SEM surface area covered by platelets. (C) Blood from PLCγ2–/– mice was incubated without (control) or with 10 μM lotrafiban (lotra), 20 μg/mL anti-α2 antibody (HMα2; anti-α2) for 5 minutes before perfusion. Platelet adhesion is expressed as a percentage of control. *P < .05; **P < .01. The data were representative of 2 experiments.
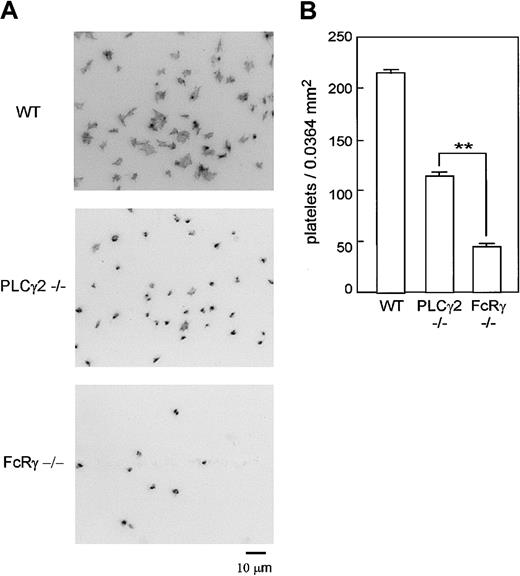
Experiments were also performed under static conditions. As shown in Figure 8, a much greater level of adhesion was observed for wild-type platelets on a collagen-coated surface than for PLCγ2–/– and FcR γ-chain–/– platelets (P < .005 vs wild type in both cases). Significantly, PLCγ2–/– platelets showed a significantly greater level of adhesion than FcR γ-chain–/– platelets (P < .005).
PLCγ2–/– platelets showed limited adhesion on collagen under static condition. (A) Washed platelets from wild-type (WT), PLCγ2–/–, and FcR γ-chain–/– were seeded on collagen-coated surfaces for 30 minutes. After unbound platelets were removed, the platelets were fixed, permeabilized, and stained by TRITC-labeled phalloidin for actin fibers and analyzed by fluorescent microscopy. (B) Mean number of adhered platelets ± SEM per 0.0364 mm2 from 14 different images from 2 experiments. **P < .01. Photo images are representative of 14 to 21 different images from 2 experiments.
PLCγ2–/– platelets showed limited adhesion on collagen under static condition. (A) Washed platelets from wild-type (WT), PLCγ2–/–, and FcR γ-chain–/– were seeded on collagen-coated surfaces for 30 minutes. After unbound platelets were removed, the platelets were fixed, permeabilized, and stained by TRITC-labeled phalloidin for actin fibers and analyzed by fluorescent microscopy. (B) Mean number of adhered platelets ± SEM per 0.0364 mm2 from 14 different images from 2 experiments. **P < .01. Photo images are representative of 14 to 21 different images from 2 experiments.
These findings demonstrate that collagen is able to induce a limited degree of adhesion in PLCγ2–/– platelets, in contrast to results observed with FcR γ-chain–/– cells.
Discussion
The present study provides strong evidence that GPVI induces weak activation of PLCγ2–/– platelets via PLCγ1. GPVI induces tyrosine phosphorylation of PLCγ1 on its regulatory site Tyr 783 in PLCγ2–/– platelets in association with slow aggregation and transient elevation of intracellular Ca2+. Significantly, functional responses to GPVI in PLCγ2–/– platelets, including phosphorylation of PLCγ1, are inhibited by PP1 and wortmannin. PLCγ1isthe only known member of the PLC superfamily, other than PLCγ2, that is regulated downstream of Src kinases and PI3-kinase. These results therefore demonstrate that GPVI mediates activation of PLCγ2–/– platelets via PLCγ1. Significantly, although this pathway induces only weak activation of platelets, it undergoes synergy with ADP to stimulate full aggregation and is able to support limited platelet adhesion to collagen under medium shear, making it likely to be of physiological relevance in vivo.
The present results are in contrast to those in human platelets, where PLCγ1 is expressed but is not phosphorylated upon stimulation by GPVI. The explanation for this difference is not known. The level of PLCγ2 in human platelets is almost 100 times greater than that of PLCγ1 and it is possible that this difference masks a role for PLCγ1.28 Alternatively, isoform-specific regulation of PLCγ1 and PLCγ2 may exist in human platelets, as demonstrated downstream of the FcϵRI (the high-affinity receptor for IgE) in mast cells.29
The small but continuous increase in light transmission observed in the collagen-stimulated platelets is made up of a combination of α2β1-mediated activation and αIIbβ3-mediated aggregation. Confirmation that these changes require intracellular signals from GPVI/FcR γ-chain is shown by the absence of these responses in FcR γ-chain–/– platelets (which also lack functional GPVI) or through the effect of inhibitors of Src kinases and PI3-kinase. These results are consistent with the modified 2-site 2-step model, which proposes that the interaction of collagen with α2β1 requires inside-out signals from GPVI.30 The results indicate that activation of PLCγ1 by GPVI leads to activation of α2β1 and αIIbβ3 and thereby promotes platelet adhesion to collagen via α2β1 and aggregation via αIIbβ3. The modified 2-site 2-step model was based on the pivotal observations of Jung and Moroi, who first showed that intracellular signals are required to promote binding of α2β1 to collagen.31-33
The observation that collagen is unable to stimulate shape change in the PLCγ2–/– platelets is explained by the dependency of this response on the release of the secondary mediators, ADP and thromboxanes,22 both of which are blocked in the absence of the phospholipase. In contrast, the more powerful agonist CRP is able to induce shape change in the absence of these mediators,22 a result that is consistent with the present observations.
Although collagen induces only weak intracellular signals in the absence of PLCγ2 (as judged by the weak nature of the aggregation), this signal is sufficient to stimulate limited α2β1-dependent adhesion under a medium rate of shear. Nevertheless, adhesion is dramatically reduced, relative to control cells, and the large thrombi seen in wild-type platelets are absent. These results indicate that the weak activation of α2β1 by GPVI in the PLCγ2–/– platelets is sufficient to promote the initial phase of adhesion but not αIIbβ3-mediated thrombus formation. We speculate that in vivo this low level of response may be sufficient to facilitate thrombus formation in association with other platelet stimuli in light of the observation that GPVI and ADP interact in a synergistic manner to induce aggregation in the PLCγ2–/– platelets.
During the course of this work, Cho et al also described a steady increase in light transmission induced by collagen in the PLCγ2–/– platelets.34 These authors speculated that the PLCγ2-independent route of activation was mediated through a novel, uncharacterized signaling pathway. The results of the present study demonstrate that this pathway involves activation of PLCγ1 downstream of GPVI.
In conclusion, we present evidence that PLCγ1 is able to support limited platelet activation by collagen and CRP in the absence of PLCγ2. These results contrast with absence of tyrosine phosphorylation of PLCγ1 in human platelets, even in response to powerful agonists at GPVI. Importantly, the low level of activation of platelets induced by collagen in the PLCγ2–/– mice is sufficient to mediate limited platelet adhesion under flow conditions, suggesting that this pathway may have functional relevance in vivo.
Prepublished online as Blood First Edition Paper, May 1, 2003; DOI 10.1182/blood-2003-01-0029.
Supported in part by grants from the Wellcome Trust, the British Heart Foundation, the Japan Clinical Pathology Foundation for International Exchange, and the Mochida Memorial Foundation for Medical and Pharmaceutical Research, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr M. Tomlinson for donating anti-PLCγ2 antibody (DN84) and to Peter Busby, Yotis Senis, Susan Adams, Ben Atkinson, Gavin Jarvis, and Andrew Pearce for their kind help with these studies.
S.P.W. holds a British Heart Foundation chair.