Abstract
High plasma levels of total homocysteine (tHcy) are a risk factor for deep vein thrombosis. Because no information on the relationship between cerebral vein thrombosis and hyperhomocysteinemia is available, a case–control study of 121 patients with a first episode of cerebral vein thrombosis and 242 healthy control subjects was carried out. Fasting plasma levels of tHcy and their postmethionine load (PML) increments, together with other laboratory markers of thrombophilia, were measured in plasma or DNA. Hyperhomocysteinemia (high fasting tHcy and/or PML increments) was diagnosed in 33 patients (27%) and 20 control subjects (8%) (odds ratio, 4.2; 95% confidence interval [CI], 2.3-7.6). Low levels of serum folate and the 677TT methylene tetrahydrofolate reductase were associated with hyperhomocysteinemia, but in a multivariate model hyperhomocysteinemia only was associated with an increased risk of cerebral vein thrombosis. Oral contraceptive intake was associated with the disease with an odds ratio of 6.1 (95% CI, 3.3-11.0). The combined presence of the latter and hyperhomocysteinemia increased the risk of the disease with an odds ratio of 19.5 (95% CI, 5.7-67.3). In conclusion, hyperhomocysteinemia is associated with a 4-fold increased risk of cerebral vein thrombosis; whether or not its correction with vitamins reduces the risk of the disease remains to be demonstrated.
Introduction
Cerebral vein thrombosis is a relatively rare but severe thrombotic manifestation with a high mortality rate,1 the potential to cause disability, and the tendency to recur.2 Coagulation abnormalities, particularly gain-of-function mutations in the genes encoding factor V (factor V Leiden) and prothrombin,3 are associated with an increased risk of cerebral vein thrombosis,4-6 whereas no data are available on the role of hyperhomocysteinemia, a risk factor for venous thrombosis of the lower limbs.7 High plasma levels of total homocysteine (tHcy) result from the interaction between genetic and acquired determinants.7 The latter are deficiencies of vitamins such as folic acid, pyridoxine, and cobalamin, which are involved in the metabolic pathways of homocysteine. Among genetic determinants, a homozygous substitution of cytosine by thymine at position 677 of the gene encoding for methylenetetrahydrofolate reductase (MTHFR) causes a 50% reduction in the activity of this enzyme and is associated with mild to moderate hyperhomocysteinemia in individuals with inadequate dietary intake of folic acid.8 Vitamin supplementation with folic acid, pyridoxine, and cobalamin lowers the plasma levels of tHcy in most cases.9 Therefore, if hyperhomocysteinemia is associated with cerebral vein thrombosis, vitamin therapy has the potential to decrease the risk of recurrence. With this as background, we carried out a case–control study on the role of hyperhomocysteinemia in a relatively large series of patients. The interaction of this metabolic abnormality with other genetic and acquired risk factors for venous thrombosis was also evaluated.
Patients and methods
Patients
One hundred thirty unrelated patients with cerebral vein thrombosis were referred to the Thrombosis Center between April 1991 and November 2002 for thrombophilia screening. The median time elapsed since the occurrence of cerebral vein thrombosis and blood sampling was 5 months (range, 3-96 months). The clinical records and the objective documentation of cerebral vein thrombosis were reviewed by a neurologist to confirm the diagnosis. Five patients were excluded from the analysis because of incomplete thrombophilia screening, 2 because the diagnosis was uncertain, and 2 because of previous venous thrombotic episodes. Therefore, 121 patients with a first, objectively confirmed episode of cerebral vein thrombosis were included in the study; 39 of them had been enrolled in a previous study of thrombosis risk factors in cerebral vein thrombosis.4 Diagnosis was made in 23 cases by intra-arterial angiography, in 4 cases by intravenous digital angiography, in 79 cases by magnetic resonance or magnetic resonance angiography, and in 15 cases by computed tomography scans showing the typical delta and cord signs.10 Cerebral vein thrombosis involved the superior sagittal sinus in 30 patients, the lateral sinus in 27, the straight sinus in 6, the cavernous sinus in 2, the cortical veins in 2, the deep cerebral veins in 1, and the jugular vein in 7. In 46 patients thrombosis involved more than one vein. Forty-one patients developed symptoms of isolated intracranial hypertension (headache, papilledema, and/or sixth nerve palsy), whereas the remaining 80 had various combinations of focal neurologic deficits, seizures, and impaired consciousness. Symptoms were headache in 75 cases (62%), papilledema in 26 (21%), sixth nerve palsy in 12 (10%), motor or sensory deficit in 32 (26%), seizures in 33 (27%), and impaired consciousness in 13 (4 had coma) (11%).
Control subjects
Two hundred twenty-four healthy individuals were matched for sex and age (± 5 years) with the patients with cerebral vein thrombosis. They were chosen from a large population of control subjects made up of 90% partners and 10% friends who agreed to accompany patients and to be investigated at the Thrombosis Center. Control subjects chosen for this study attended the Thrombosis Center in the same period as patients. Previous thrombosis was excluded in them with a structured questionnaire validated for the retrospective diagnosis of thrombosis.11
The presence of circumstantial thrombosis risk factors, such as oral contraceptive intake, pregnancy, postpartum period, cancer, surgery, trauma, and prolonged immobilization, were recorded for both patients and control subjects. Postpartum period was defined as the 3-month period after childbirth. The type of oral contraceptive and the period of time of its intake were recorded; women were considered to be on oral contraceptives if they had taken them until 2 weeks or less before thrombosis for patients or at the time of blood sampling for control subjects. Because smoking may increase the thrombotic risk in carriers of genetic coagulation defects,12,13 information on this habit was also recorded. Individuals who smoked at least 5 cigarettes daily were considered smokers. No subject had abnormal liver or renal function or overt autoimmune or neoplastic diseases. All subjects were of European descent. The study was approved by the institutional review board of the University of Milan. All subjects gave their informed consent to the study.
Laboratory tests
Plasma tHcy was measured in blood samples anticoagulated with EDTA (ethylenediaminetetraacetic acid). Blood was withdrawn after overnight fasting of at least 8 hours and 4 hours after an oral methionine load (3.8 g/m2 body surface area). Blood samples were immediately placed on ice to prevent the artifactual in vitro increase in plasma tHcy levels and centrifuged at 1600g at 4°C for 15 minutes within 1 hour. The supernatant platelet-poor plasma was stored at –80°C. Plasma tHcy was measured by high-performance liquid chromatography (HPLC) and fluorescence detection.14 Hyperhomocysteinemia was diagnosed when plasma levels of fasting tHcy or their postmethionine load (PML) increments above fasting levels exceeded the 95th percentile of the values obtained in the control group (fasting, 19.0 μM in men and 15.2 μM in women; PML increments, 29.6 μM in men and 24.7 μM in women). Blood samples for serum folates and cobalamin levels were collected in tubes without anticoagulant and protected from light, and measurements were performed by radioimmuno-assay (Diagnostic Product Corporation, Los Angeles, CA).
DNA analysis for the 1691G>A substitution in coagulation factor V gene (factor V Leiden) and for the 20210G>A substitution in the 3′-untranslated region of the prothrombin gene was carried out as previously described.15,16 The 677C>T substitution in the MTHFR gene was also tested.17 Functional or antigenic assays for antithrombin, protein C, and protein S were carried out on plasma samples obtained from blood anticoagulated with sodium citrate (3.8% [wt/vol]), as previously described.4 Antiphospholipid antibodies (lupus anticoagulant and anticardio-lipin antibodies) were diagnosed according to previously described methods.18
Statistical analysis
Odds ratios (ORs) and 95% confidence intervals (CIs) were used as a measure of the association between cerebral vein thrombosis and various types of thrombophilia. The Student t test (with the Satterthwaite method applicable to variables that are not normally distributed) was performed to compare plasma tHcy levels and serum vitamin levels in patients and control subjects. With the use of an unconditional logistic regression analysis, ORs were adjusted for possible confounders, such as age, body mass index, and smoking status, when appropriate. Interaction between various types of thrombophilia and oral contraceptive intake on cerebral vein thrombosis was evaluated using a logistic regression model, which included the main effects and the interaction term. Ninety-five percent CIs were calculated using the covariates matrix of this logistic model. The analyses were done using the Statistical Analysis Software package for Windows, version 8.1 (SAS Institute, Cary, NC).
Results
Table 1 shows the general characteristics of patients with cerebral vein thrombosis and of control subjects. Most patients were women. As shown in Table 2, 57 women of reproductive age were taking oral contraceptives (71%), and 4 postmenopausal women (36%) were on hormone replacement therapy at the time of thrombosis. Other risk factors were much less frequent. Table 2 also shows the prevalence of thrombophilic abnormalities other than hyperhomocysteinemia in patients and control subjects and the ORs for the disease. The most frequent thrombophilia abnormality was hyperhomocysteinemia, found in 33 (27%) patients and 20 (8%) control subjects for an OR of 4.2 (95% CI, 2.3-7.6) (Table 3). Fasting tHcy was elevated in 14 (11.6%) patients and 12 (5%) control subjects for an OR of 2.5 (95% CI, 1.1-5.6), whereas PML increments of tHcy were elevated in 26 patients and 12 control subjects for an OR of 5.2 (95% CI, 2.5-10.8). The association of fasting or PML increments of tHcy and cerebral vein thrombosis was observed both in men and women (Table 3). Hyperhomocysteinemia was present in combination with other types of thrombophilia in 11 patients (4 had also factor V Leiden, 4 prothrombin mutation, 1 both, and 2 antiphospholipid antibodies) and in no control subjects. The OR for cerebral vein thrombosis associated with isolated hyperhomocysteinemia was 5.2 (95% CI, 2.7-10.2).
General characteristics of the study population
Characteristics . | Patients . | Control subjects . |
|---|---|---|
| N | 121 | 242 |
| Male/female | 30/91 | 60/182 |
| Median age, y (range) | 33 (12-64) | 36 (13-62) |
| Body mass index, kg/m2, mean ± SD | 24.0 ± 4.0 | 22.6 ± 3.1 |
| Smoking, no. (%) | 22 (18) | 60 (25) |
Characteristics . | Patients . | Control subjects . |
|---|---|---|
| N | 121 | 242 |
| Male/female | 30/91 | 60/182 |
| Median age, y (range) | 33 (12-64) | 36 (13-62) |
| Body mass index, kg/m2, mean ± SD | 24.0 ± 4.0 | 22.6 ± 3.1 |
| Smoking, no. (%) | 22 (18) | 60 (25) |
Type of thrombophilia and transient risk factors for cerebral vein thrombosis in the study population
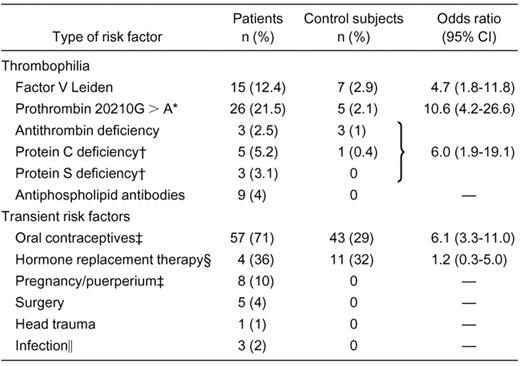
—indicates not applicable.
Four patients and no control subjects had homozygous prothrombin mutation.
Percentage calculated on the number of patients not on oral anticoagulants at the time of blood sampling.
Percentage calculated on the number of women of reproductive age.
Percentage calculated on the number of postmenopause women.
Infections were otitis in 2 patients and meningitis in 1 patient.
Prevalence of hyperhomocysteinemia in the study population and odds ratio for cerebral vein thrombosis
Hyperhomocysteinemia* . | Patients n (%) . | Controls n (%) . | Odds ratio (95% CI) . |
|---|---|---|---|
| Total | 33 (27.3) | 20 (8.3) | 4.2 (2.3-7.6) |
| Men | 10 (33.3) | 4 (6.6) | 7.0 (2.0-24.9) |
| Women | 23 (25.3) | 16 (8.8) | 3.5 (1.7-7.1) |
| High fasting tHcy levels | |||
| Total | 14 (11.6) | 12 (5.0) | 2.5 (1.1-5.6) |
| Men | 5 (16.6) | 3 (5.0) | 3.8 (0.8-17.2) |
| Women | 9 (9.9) | 9 (4.9) | 2.1 (0.8-5.6) |
| High PML increments | |||
| Total | 26 (21.5) | 12 (5.0) | 5.2 (2.5-10.8) |
| Men | 6 (20.0) | 3 (5.0) | 4.8 (1.1-26.0) |
| Women | 20 (22.0) | 9 (4.9) | 5.4 (2.4-12.5) |
Hyperhomocysteinemia* . | Patients n (%) . | Controls n (%) . | Odds ratio (95% CI) . |
|---|---|---|---|
| Total | 33 (27.3) | 20 (8.3) | 4.2 (2.3-7.6) |
| Men | 10 (33.3) | 4 (6.6) | 7.0 (2.0-24.9) |
| Women | 23 (25.3) | 16 (8.8) | 3.5 (1.7-7.1) |
| High fasting tHcy levels | |||
| Total | 14 (11.6) | 12 (5.0) | 2.5 (1.1-5.6) |
| Men | 5 (16.6) | 3 (5.0) | 3.8 (0.8-17.2) |
| Women | 9 (9.9) | 9 (4.9) | 2.1 (0.8-5.6) |
| High PML increments | |||
| Total | 26 (21.5) | 12 (5.0) | 5.2 (2.5-10.8) |
| Men | 6 (20.0) | 3 (5.0) | 4.8 (1.1-26.0) |
| Women | 20 (22.0) | 9 (4.9) | 5.4 (2.4-12.5) |
Hyperhomocysteinemia is high fasting tHcy level or high PML increment, or both.
Table 4 shows that the mean values (± SD) of plasma fasting tHcy levels and their PML increments were higher in patients than in control subjects. The mean serum levels of folate were lower in patients, whereas those of cobalamin did not differ between patients and control subjects (Table 3). The 677C>T mutation in the MTHFR gene was found in its homozygous form in 20 patients (17%) and 59 control subjects (25%), for an OR of 0.7 (95% CI, 0.3-1.4). The homozygous mutation was more frequent among patients and control subjects with hyperhomocysteinemia (24% and 60%, respectively) than in those without (14% and 21%, respectively). The mean serum levels of folates and cobalamin were lower among patients and control subjects with hyperhomocysteinemia than in those without (Table 5). Multivariate analysis including hyperhomocysteinemia, serum folate, cobalamin, and the 677C>T MTHFR mutation showed that the only variable associated with a heightened risk of cerebral vein thrombosis was hyperhomocysteinemia (OR, 5.4 [(95% CI, 2.6-11.4]).
Mean levels (± SD) of plasma tHcy, serum folates, and cobalamin
Blood test . | Patients . | Control subjects . | P . |
|---|---|---|---|
| Fasting tHcy, μM | 14.9 ± 27.5 | 10.0 ± 4.9 | .06 |
| Postmethionine load tHcy increments, μM* | 21.1 ± 10.9 | 14.2 ± 6.6 | < .0001 |
| Folate, nM | 5.6 ± 2.8 | 6.6 ± 3.3 | .01 |
| Cobalamin, pM | 412.9 ± 238.9 | 408.5 ± 172.1 | .88 |
Blood test . | Patients . | Control subjects . | P . |
|---|---|---|---|
| Fasting tHcy, μM | 14.9 ± 27.5 | 10.0 ± 4.9 | .06 |
| Postmethionine load tHcy increments, μM* | 21.1 ± 10.9 | 14.2 ± 6.6 | < .0001 |
| Folate, nM | 5.6 ± 2.8 | 6.6 ± 3.3 | .01 |
| Cobalamin, pM | 412.9 ± 238.9 | 408.5 ± 172.1 | .88 |
Increments are defined as the difference between PML and fasting plasma tHcy levels.
Mean levels (± SD) of serum folates and cobalamin in patients and control subjects with and without hyperhomocysteinemia
. | Patients . | . | . | Controls . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | With hyperhomocysteinemia (n = 33) . | Without hyperhomocysteinemia (n = 88) . | P . | With hyperhomocysteinemia (n = 20) . | Without hyperhomocysteinemia (n = 222) . | P . | ||||
| Folate, nM | 4.1 ± 2.0 | 6.3 ± 2.9 | .0003 | 4.2 ± 1.9 | 6.8 ± 3.4 | < .0001 | ||||
| Cobalamin, pM | 335.6 ± 141.4 | 450.1 ± 267.1 | .02 | 320.9 ± 191.8 | 416.4 ± 168.4 | .04 | ||||
. | Patients . | . | . | Controls . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | With hyperhomocysteinemia (n = 33) . | Without hyperhomocysteinemia (n = 88) . | P . | With hyperhomocysteinemia (n = 20) . | Without hyperhomocysteinemia (n = 222) . | P . | ||||
| Folate, nM | 4.1 ± 2.0 | 6.3 ± 2.9 | .0003 | 4.2 ± 1.9 | 6.8 ± 3.4 | < .0001 | ||||
| Cobalamin, pM | 335.6 ± 141.4 | 450.1 ± 267.1 | .02 | 320.9 ± 191.8 | 416.4 ± 168.4 | .04 | ||||
When women of reproductive age were stratified according to the presence of hyperhomocysteinemia and oral contraceptive intake (Table 6), the OR for the disease in those with hyperhomocysteinemia and taking oral contraceptives was 19.5 (95% CI, 5.7-67.3). Data did not substantially change after adjustment for confounding factors (age, smoking, and body mass index). Mean levels of tHcy were similar in women taking and not taking oral contraceptives (data not shown). Stratification for the presence of factor V Leiden or prothrombin mutation and the intake of oral contraceptives gives similar point estimates for the coagulation abnormalities alone and the use of oral contraceptives alone, whereas the presence of both risk factors gave an OR of 30.0 (95% CI, 3.4-263.0) for factor V Leiden and 79.3 (95% CI, 10.0-629.4) for the prothrombin mutation.
Interaction between hyperhomocysteinemia and the use of oral contraceptives in determining cerebral vein thrombosis in 80 patients and 148 control subjects of reproductive age
Hyperhomocysteinemia . | Oral contraceptive intake . | Patients, n . | Control subjects, n . | Odds ratio (95% CI) . | Odds ratio (95% CI)* . |
|---|---|---|---|---|---|
| No | No | 16 | 96 | 1 (reference) | 1 (reference) |
| No | Yes | 44 | 39 | 6.8 (3.4-13.4) | 8.6 (3.9-18.8) |
| Yes | No | 7 | 9 | 4.7 (1.5-14.3) | 7.1 (2.1-23.5) |
| Yes | Yes | 13 | 4 | 19.5 (5.7-67.3) | 18.3 (4.9-68.1) |
Hyperhomocysteinemia . | Oral contraceptive intake . | Patients, n . | Control subjects, n . | Odds ratio (95% CI) . | Odds ratio (95% CI)* . |
|---|---|---|---|---|---|
| No | No | 16 | 96 | 1 (reference) | 1 (reference) |
| No | Yes | 44 | 39 | 6.8 (3.4-13.4) | 8.6 (3.9-18.8) |
| Yes | No | 7 | 9 | 4.7 (1.5-14.3) | 7.1 (2.1-23.5) |
| Yes | Yes | 13 | 4 | 19.5 (5.7-67.3) | 18.3 (4.9-68.1) |
Adjusted for age, body mass index, and smoking status.
Discussion
This study shows that hyperhomocysteinemia increases the risk of cerebral vein thrombosis by approximately 4-fold. At variance with deep vein thrombosis of the lower limbs, in which the magnitude of the risk of thrombosis associated with fasting and PML tHcy was similar,7,19,20 the association with cerebral vein thrombosis of high PML tHcy increments was stronger than that of fasting tHcy levels. The data of this study add to the evidence in the literature that the 2 parameters are not interchangeable and should be measured in conjunction for a more accurate evaluation of the risk of thrombosis, even though a causal role of high PML values remains to be demonstrated.7 Determinants of hyperhomocysteinemia were low levels of serum folate and the homozygous 677C>T mutation in the MTHFR gene, but in a multivariate model hyperhomocysteinemia only was associated with an increased risk of the disease. Because approximately two thirds of patients were women, we looked at the interaction between hyperhomocysteinemia and another established and frequent risk factor for venous thrombosis, ie, the intake of oral contraceptives, finding an odds ratio of approximately 20 that indicates an additive interaction between the 2 risk factors. Hence, hyperhomocysteinemia is another risk factor, in addition to factor V Leiden and the prothrombin mutation,4,21 that interacts with oral contraceptives in increasing the risk of cerebral vein thrombosis.
Among the limitations of this study is that patients referred for thrombophilia screening to our specialized center are selected. However, the exclusion of the most severe patients, such as those with previous venous thrombosis, should have limited the possibility of risk overestimation. A diagnostic bias is thought to be minimized by the revision of the medical records and imaging of patients with cerebral vein thrombosis by an expert radiologist. It could be noted that the 95th percentile of PML increments among control subjects was higher in men than in women, probably because of the small number of men included in the study. Despite this, the association between hyperhomocysteinemia and cerebral vein thrombosis was observed in both sexes. Whether hyperhomocysteinemia is a cause or merely a consequence of cerebral vein thrombosis cannot be established by studies with a retrospective design like this. However, the rarity of cerebral vein thrombosis renders the organization of prospective and/or intervention studies very difficult.
To date, thrombophilia screening, including coagulation factor abnormalities such as factor V Leiden, prothrombin mutation, deficiencies of antithrombin, protein C, and protein S, and the presence of antiphospholipid antibodies, is recommended in the diagnostic work up in patients with cerebral vein thrombosis.6 The results of this study suggest that measurements of plasma tHcy should be included in thrombophilia screening. At variance with other types of thrombophilia, hyperhomocysteinemia can be easily and safely treated with vitamin supplementation (folic acid alone or in combination with cobalamin and pyridoxine). Whether or not the correction of hyperhomocysteinemia with vitamin therapy will help to reduce the high risk of recurrent cerebral vein thrombosis (up to 20%)2 is not demonstrated by this retrospective study, but it should be tested in proper studies.
Prepublished online as Blood First Edition Paper, April 24, 2003; DOI 10.1182/blood-2003-02-0443.
Supported by Ministero dell'Università e della Ricerca Scientifica e Tecnologica (grant no. 2001065344).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr. G. Landi for reviewing the medical records and helping in selecting patients for this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal