Abstract
A defect in cell trafficking and chemotaxis plays an important role in the immune deficiency observed in Wiskott-Aldrich syndrome (WAS). In this report, we show that marrow cells from WAS protein (WASP)–deficient mice also have a defect in chemotaxis. Serial transplantation and competitive reconstitution experiments demonstrated that marrow cells, including hematopoietic progenitors and stem cells (HSCs), have decreased homing capacities that were associated with a defect in adhesion to collagen. During development, HSCs migrate from the liver to the marrow and the spleen, prompting us to ask if a defect in HSC homing during development may explain the skewed X-chromosome inactivation in WAS carriers. Preliminary evidence has shown that, in contrast to marrow progenitor cells, fetal liver progenitor cells from heterozygous females had a random X-chromosome inactivation. When fetal liver cells from WASP-carrier females were injected into irradiated recipients, a nonrandom inactivation of the X-chromosome was found at the level of hematopoietic progenitors and HSCs responsible for the short- and long-term hematopoietic reconstitution. Therefore, the mechanism of the skewed X-chromosomal inactivation observed in WAS carriers may be related to a migration defect of WASP-deficient HSCs.
Introduction
Wiskott Aldrich syndrome (WAS) is an X-linked hereditary disease characterized by the association of a thrombopenia with small platelet volume, eczema, and increased susceptibility to infection.1 The phenotype observed in this syndrome is caused by mutation in the Wiskott-Aldrich syndrome protein (WASP) gene mapped on Xp11;22 that leads to a loss of function.2 In contrast, gain of function mutations may be responsible for neutropenia.3 WASP expression is restricted to hematopoietic cells4 and has defined a new family of scaffold proteins involved in the transduction of signals from membrane receptors to the actin cytoskeleton.5 WASP was identified as a binding partner of the small guanosine triphosphatase (GTPase) Cdc42.2 The WASP/GTPase interaction is mediated by a region called CRIB (Cdc42 Rac-interactive binding). The interaction between the GTP-bound Cdc42 and the CRIB domain of WASP induces conformation changes that allow the carboxyl terminus verprolin cofilin acid (VCA) domain of WASP to bind the actin-related protein (Arp) complex Arp2/3.6 The VCA-Arp2/3 interaction initiates branching structures by promoting addition of actin monomers to the barbed end of actin filament.7 Moreover, WASP contains a proline-rich sequence that can bind to profilin, vasodilator-stimulated phosphoprotein (VASP), Src homology 3 (SH3) domains of signaling proteins, and cytoplasmic tyrosine kinases.8 The result of the interaction between incoming signals and specific domains of the protein is the induction of actin polarization required for directed motility and phagocytosis.9
Studies of hematopoietic cells from human patients have strongly suggested that the biological mechanisms responsible for the physiopathology of WAS is due to a deregulation of the actin cytoskeleton in response to stimuli. WASP-deficient B lymphocytes are impaired in interleukin 4 (IL-4) and CD40-dependent induction of polarization and spreading.10 In immature WASP-deficient dendritic cells (DCs), podosomes are absent, residual dysmorphic lamellipodia and filopodia are nonpolarized, and migration is severely compromised.11 In addition, T cells from WAS patients contained reduced numbers and sizes of surface micovilli12 and responded poorly to stromal cell–derived factor 1 (SDF-1) in migration assays13 and to antigen receptor–induced stimulation in vitro.14 Taken together, these results suggest that a defect in cell migration may play an important role in the WASP syndrome as has been already suggested by Thrasher et al.15 However, other defects such as alteration in phagocytosis, capping, or receptor clustering are also involved in the molecular mechanisms of the disease.16
It has been shown that a great majority of hematopoietic cells from the blood of heterozygous females with a nonsense WASP mutation (obligate carriers of the WAS gene) normally express WASP.17 Furthermore, DNA extracted from CD34+ cells of WAS carriers exhibited a pattern of nonrandom X inactivation,18 demonstrating that the selective advantage of cells expressing the normal X-chromosome occurs early during hematopoietic differentiation. At present, the mechanism responsible for this nonrandom X-chromosome inactivation is poorly understood because WASP is not directly involved in the mechanism of X inactivation. A defect in WASP may impair the mobility and migration abilities of the cells; one might thus speculate that the nonrandom X-chromosome inactivation could be due to a selective advantage for engraftment of cells expressing WASP. To test this hypothesis, we compared the hematopoiesis of wild-type and WASP-deficient mice and analyzed the capability of hematopoietic progenitors to migrate into hematopoietic tissues. We show that despite a normal marrow and spleen hematopoiesis in WASP-deficient mice, hematopoietic stem cells from WASP-deficient mice have a defect in their ability to migrate into the hematopoietic microenvironment. These results sustain the hypothesis that skewed X inactivation in WAS carriers is the consequence of a defect in the homing capacities of HSCs during fetal life.
Materials and methods
Animals
WASP-deficient mice were provided by S.B.S.19 To disrupt the murine WASP gene, the neomycin-resistance gene (neo) was inserted into exon 7 of WASP in the reverse-transcriptional orientation in TC-1 embryonic stem (ES) cells. Female WASP–/– offspring were bred with 129 wild-type male mice. Wild-type (WT) 129 Sv/Ev mice were purchased from Iffa Credo (L'arbresle, France).
Mice were housed in animal facilities at the Institut Gustave Roussy (Villejuif, France) under specific pathogen-free conditions.
Irradiated recipient mice were exposed to 9.5 Gy total body irradiation administrated in one dose 2 to 3 hours before transplantation.
Cell migration
The migration assay was performed with the use of 5-μm or 8-μm pore filters (24-well cell clusters) (Transwell; Costar, Cambridge, MA). Nucleated bone marrow cells were suspended in serum-free medium to a final concentration of 2.5 × 106/mL and 100 μL cell suspension was loaded into the upper chamber whereas 600 μL medium, with or without SDF-1α (100 ng/mL) (R&D Systems, Minneapolis, MN), was introduced into the lower chamber. SDF-1α was diluted in serum-free medium and placed in the lower chamber. Cells were allowed to migrate for 1 hour at 37°Cin5%CO2 and 95% air. Cells in the bottom chambers were collected and enumerated in triplicate. Data are presented as the chemotaxis index.
Homing ability of WASP-deficient bone marrow cells
Cells from wild-type 129Sv mice were stained with CSFE (5- and 6-carboxyfluorescent diacetate succinimidyl ester) (Molecular Probes Eugene, OR) (green dye), and cells from WASP-deficient mice were stained with PKH26 (Sigma, St Louis, MO) (red dye) as already described.20,21 In a similar experiment, wild-type cells were stained with PKH26 and WASP-deficient cells with CSFE to ensure that labeling had no specific effect on the properties of hematopoietic progenitors. Then, 10 million labeled wild-type nucleated cells were mixed with an equal number of labeled WASP-deficient cells, and the mixture was injected into lethally irradiated mice. Bone marrow and spleen cells of recipient mice were harvested 24 hours after the injection, and the origins of the cells that had migrated in the bone marrow and in the spleen were analyzed by determining the proportion of either green (CSFE)– or red (PKH26)–labeled cells by means of a flow cytometer or polymerase chain reaction (PCR) analyses on individual colonies obtained by culturing the cells in methylcellulose. The presence of the neo gene testified to the WASP-deficient mice origin. Individually harvested colonies were suspended in 20 μL lysis buffer containing 0.45% Tween 20 and 400 μg/mL fresh proteinase K for 30 minutes at 56°C, followed by 10 minutes at 95°C. DNA was monitored for PCR reaction. The sequences for the PCR primers were as follows: neo sense, 5′-ATGATTGAACAAGATGGATTGCACGC-3′; neo antisense, 5′-GCTGTGCTCGACGTTGTCACTGAA-3′; actin sense, GTACCACAGGCATTGTGATG; actin antisense, GCAACATAGCACAGCTTCTC.
Animal survival and donor recovery after serial bone marrow transplantation
Wild-type 129Sv or WASP knock-out male mice were used as marrow donors. The 129Sv female recipient mice were lethally irradiated (9.5 Gy), and 3 million nucleated cells were injected intravenously into the retro-orbital sinus of irradiated mice.
Animal survival. Each group initially included 10 mice. Recipient mice were monitored daily for survival. At 2 months after each transplantation, the animals were killed, and the cells from one leg were individually injected into a new irradiated female recipient. This process was repeated 7 times.
Donor contribution monitored by PCR. At the time of each retransplantation, an aliquot of bone marrow cells from each donor mouse was cultured in semisolid medium. Twenty colonies per mouse obtained in culture were picked; DNA was prepared as already described; and mice were monitored by a semiquantitative PCR for neo, actin, and Y chromosome–specific sequences (Y sense, TCATGAGACTGCCAACCACAGAY; antisense, GACCACCACCACCACCACCAA).
Long-term bone marrow culture
To evaluate stem cell self-renewal in bone marrow of wild-type and WASP-deficient mice, long-term bone marrow cultures were performed with MS-5 used as a stromal layer. First, 25-cm2 flasks were treated with gelatin (1%) and rinsed with medium 24 hours afterwards; then, 2.5 × 105 MS-5 cells were seeded in 10 mL long-term culture medium (Myelocult; Stem Cell Technologies, Vancouver, BC, Canada). At 24 hours after this, 3 × 106 cells of wild-type or WASP-deficient bone marrow nucleated cells were cultured at 33°C in the same long-term culture medium in an atmosphere of 5% CO2 in air. Each week, 50% of the culture medium was removed and replaced by fresh medium.
Colony-forming assay
Bone marrow or spleen cells were plated in methylcellulose as already described.22 Briefly, 105 bone marrow and 106 spleen cells were seeded in 1 mL 0.8% methylcellulose (Fluka; Sigma) in α–modified Eagle medium (αMEM) supplemented with 20% fetal calf serum (FCS), 75 μM α-thioglycerol (Sigma), 10% WEHI-conditioned medium, 50 ng/mL thrombopoietin (TPO) and 2 U/mL erythropoietin (EPO) in the presence or absence of neomycin (G418). Since the WASP gene is located on the X-chromosome, only cells from heterozygous females (WASP+/–) that have an inactivated normal X-chromosome are able to grow in the presence of G418. Cultures were incubated at 37°C in an atmosphere of 5% CO2 in air. Progenitor-derived colonies were identified by means of an inverted microscope at × 100 magnification, counted, plucked, and suspended in 10 μL phosphate-buffered saline (PBS) for PCR analysis.
Glutathione S-transferase–WASP-CRIB domain (GST-CRIB) pull-down assay
Escherichia coli strain BL21 was transformed by pGEX WASP-CRIB vector, grown to midlog phase at 37°C, and then induced with 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) for 4 hours. The GST-fusion domain was purified by glutathione (GSH)–agarose (Sigma). The GST-fusion proteins were in the beads or eluted in the solution containing 50 mM Tris (tris(hydroxymethyl)aminomethane) (pH 8.0) and 10 mM reduced glutathione (Sigma). Protein purification was visualized by Coomassie blue staining after sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) to assess the amount and purification of the GST-CRIB domain.
Lineage-negative (Lin–) bone marrow cells from 129Sv and WASP-deficient mice were obtained by depletion of Lin+ cells with a cocktail of monoclonal antibodies (TER 119, GR1, Mac1, B220, Lyt 1, GK 1.5) and immunomagnetic beads (Dynal, Compiègne, France). Purified Lin– bone marrow cells (2 × 106 cells per lane) were treated with 100 ng/mL SDF-1 for 5, 10, or 20 minutes; washed with cold PBS; and pelleted. The pellets were resuspended in PBS and lysed in 50 mM Tris (pH 7.5), 500 mM NaCl, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, 10% glycerol, 10 mM MgCl2,10 μg/mL leupeptin and aprotinin, and 1 mM phenylmethyl sulfonylfluoride (PMSF) (Sigma). Triton-X-100–insoluble material was removed by centrifugation (10 minutes, 9500g), and equal volumes of lysates were incubated with 20 μg bacterially produced GST-CRIB domain, bound to glutathione-coupled Sepharose beads, for 45 minutes at 4°C.
Beads and bound proteins were washed 4 times in washing buffer (25 mM Tris-HCl, pH 7.5; 1 mM dithiothreitol [DTT]; 30 mM MgCl2; 40 mM NaCl; 0.5% Nonidet P-40 [Sigma, St Quentin, France]) and resuspended in 20 μL Laemmli sample buffer. Then, proteins were separated by SDS-PAGE on 12% acrylamide gels and blotted by specific antibody for Cdc42 (1:400, kindly provided by J. Bertoglio, Chatenay Malabry, France). Prior to the incubation with the beads, 50 μL aliquots were removed from all samples to control for equal loading of total Cdc42 protein. The immunoblots were detected with enhanced chemiluminescence (ECL) Western blotting detection kit (Amersham, Buckinghamshire, United Kingdom) and Kodak Biomax film (Kodak, Rochester, NY).
Immunofluorescence studies
Glass coverslips were coated with fibronectin (20 μg/mL, overnight at 4°C) (Invitrogen, Carlsbad, CA), fibrillar collagen I (25 μg/mL, overnight at 4°C) (Horm Nycomed, Munich, Germany), or poly-l-lysine (20 μg/mL, 1 hour at room temperature) and washed with PBS. Lin– cells were stimulated for the indicated times with SDF-1 (100 ng/mL) and allowed to adhere on substrate-coated coverslips for 1 hour at 37°C, fixed in 2% paraformaldehyde for 10 minutes, washed with PBS, and permeabilized with Triton X-100 (0.2%) for 4 minutes. Cells were stained with rhodamine-phalloidin and washed in PBS, and DNA was labeled with DAPI (4,6-diamidino-2-phenylindole) in the antifading mounting medium Vectashield (Vector, Burlingame, CA). Slides were examined with a fluorescence microscope equipped with the appropriate filter (Nikon, Tokyo, Japan). Images were captured with a charge-coupled device (CCD) camera and CoolSnap software (Roper Scientific, Ottobrunn, Germany).
Flow cytometry
Lin– cells were labeled with a fluorescein isothiocyanate (FITC) or a phycoerythrin (PE) anti–stem cell antigen (Sca)–1 monoclonal antibody, an FITC anti–very late activation antigen 4 (anti–VLA-4), or a PE anti–VLA-5 monoclonal antibody, and an allophycocyanin (APC) anti–c-kit monoclonal antibody (all from Pharmingen, San Diego, CA). Conjugated isotype antibodies were used as controls. Cells were analyzed on a FACS Sort (Becton Dickinson, Franklin Lakes, NJ). VLA-4 and VLA-5 expression was studied in the Lin–Sca+c-kit+ cell population and compared with the isotype controls.
5-Fluorouracil exposure in vivo
The antimetabolite 5-fluorouracil (5-FU) was used to selectively deplete cycling cells in vivo. The 5-FU was administered intraperitonally at a dose of 150 mg/kg, and the bone marrow cells were harvested 4 days later.
Results
Reduced in vitro response of WASP-deficient bone marrow cells to SDF-1 compared with wild-type cells
SDF-1 is a chemokine that plays an important role in the homing of hematopoietic stem cells. T cells from WAS patients have a profound defect in their chemotactic response to SDF-1.13 These results prompted us to study the response to SDF-1 of bone marrow cells from WASP-deficient mice that were used as a model of the human disease. We compared the ability of bone marrow cells from WASP-deficient and wild-type mice to migrate in response to SDF-1 in transwell chambers. In all experiments, the proportion of marrow cells that migrated after SDF-1 stimulation into the bottom chamber after 1 hour at 37°C cells was 2-fold higher in wild-type mice (28.2% ± 4.4%) than in WASP-deficient mice (13.7% ± 3.3%) (Table 1; n = 4; results are means ± SD, P = .019). Similar results were found when bone marrow cells from 5-FU–treated mice were assayed (Table 1). However, this last result, performed on only 2 experiments, was not statistically significant. Nevertheless, all together these results show that marrow cells from WASP knock-out mice have a defect in SDF-1 response.
Reduced homing capacity of WASP-deficient bone marrow cells compared with wild-type cells
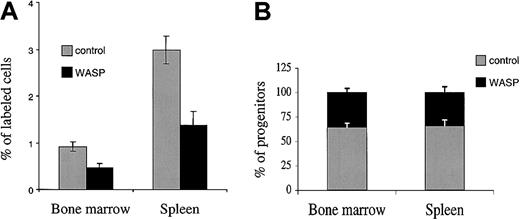
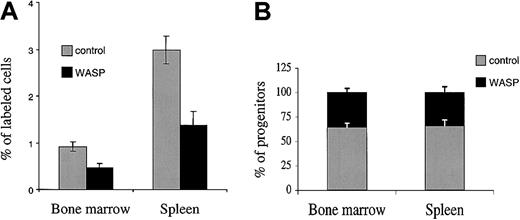
To examine if this defect observed in SDF-1 response is associated with a homing defect, we used an in vivo tracking assay.21 Bone marrow cells from wild-type or WASP-deficient mice were stained with 2 different dyes (10 × 106 cells labeled with CSFE and 10 × 106 cells labeled with PKH26) and injected simultaneously in equal proportions. At 24 hours after the graft, 7 × 106 nucleated cells were found in the bone marrow, and individual bone marrow and spleen cells were analyzed for the proportion of green (CSFE)– or red (PKH26)–labeled cells by flow cytometry. Figure 1A shows that 24 hours after the graft, WASP-deficient marrow cells homed to the different hematopoietic tissues 2-fold less efficiently than normal cells. A similar result was obtained independently of the dye used to stain WASP-deficient cells. To assess if this defect in homing also involves hematopoietic progenitors, we used the strategy previously described. At 24 hours after the graft of the mixture of labeled wild-type and WASP-deficient cells, the bone marrow and spleen cells of recipients were harvested and cultured in methylcellulose in the presence of a growth factor combination. After 7 days, colonies were individually plucked, and the presence of the neo gene was determined by PCR in the cells of each colony. The presence of the neo gene demonstrated that cells originated from WASP-deficient mice. Pooled data are shown on Figure 1B. Only 35.3% ± 4% of the 140 colonies arising from the bone marrow of the recipient mice were positive for the presence of the neo gene, demonstrating that more than 50% (64.7%) of the progenitors found 24 hours after the graft were from normal origin. This result was highly significant (P ≤ .01) and was not due to the persistence of radiation-resistant hematopoietic progenitors in the donor because no hematopoietic colonies could be grown in the absence of a transplant. A similar result was obtained in the spleen with 66.1% ± 6% clonogenic progenitors of normal donor origin (140 colonies were individually studied).
Reduced homing capacity of WASP-deficient bone marrow cells as compared with wild-type cells. Lethally irradiated female mice received transplants of a mixture of 10 × 106 wild-type and 10 × 106 WASP-deficient bone marrow cells. The bone marrow cells were stained with 2 different dyes: wild-type mice were stained with CSFE, and WASP-deficient cells with PKH26, or vice versa. (A) At 24 hours after the graft, bone marrow and spleen were harvested from each recipient, and nucleated cells were analyzed by flow cytometry to evaluate the proportion of red and green cells in the 2 hematopoietic tissues. Results shown are means ± SD of 4 experiments done with 3 mice in each group. (B) Bone marrow and spleen cells of recipient mice were used for progenitor assays, and 20 colonies per mouse were picked and monitored by Y and neo PCR to determine the origin of the cell that gives rise to the colony. Seven recipient mice were analyzed, and 40 colonies per mouse were harvested (20 from the bone marrow and 20 from the spleen; a total of 140 colonies from the bone marrow and 140 from the spleen were analyzed). Results shown are means ± SD of 7 analyzed mice.
Reduced homing capacity of WASP-deficient bone marrow cells as compared with wild-type cells. Lethally irradiated female mice received transplants of a mixture of 10 × 106 wild-type and 10 × 106 WASP-deficient bone marrow cells. The bone marrow cells were stained with 2 different dyes: wild-type mice were stained with CSFE, and WASP-deficient cells with PKH26, or vice versa. (A) At 24 hours after the graft, bone marrow and spleen were harvested from each recipient, and nucleated cells were analyzed by flow cytometry to evaluate the proportion of red and green cells in the 2 hematopoietic tissues. Results shown are means ± SD of 4 experiments done with 3 mice in each group. (B) Bone marrow and spleen cells of recipient mice were used for progenitor assays, and 20 colonies per mouse were picked and monitored by Y and neo PCR to determine the origin of the cell that gives rise to the colony. Seven recipient mice were analyzed, and 40 colonies per mouse were harvested (20 from the bone marrow and 20 from the spleen; a total of 140 colonies from the bone marrow and 140 from the spleen were analyzed). Results shown are means ± SD of 7 analyzed mice.
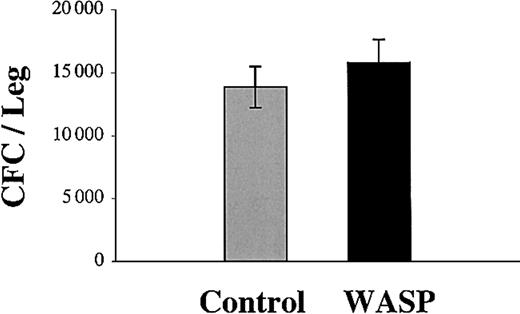
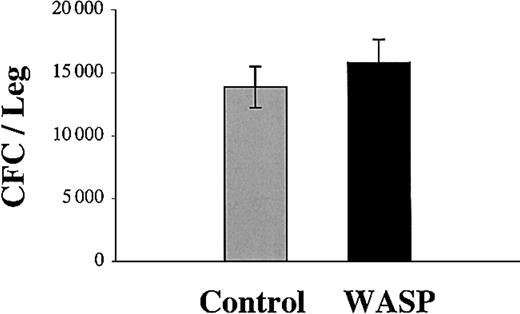
We asked if these differences in engraftment could be due to differences in the number of infused clonogenic progenitors. This hypothesis was entertained because of a prior report suggesting that WAS patients may have a quantitative defect in hematopoietic progenitors.23 Thus, we directly compared the number of clonogenic progenitors in the bone marrow of knock-out and normal mice. Bone marrow cells from normal adult 129Sv mice or from WASP knock-out mice of the same age were harvested and cultured in methylcellulose. The same numbers of total clonogenic progenitors were found in the control and in the WASP knock-out bone marrow (Figure 2). Thus, the wild-type and WASP-grafted populations contained the same number of progenitors.
Comparison of progenitor numbers in wild-type and WASP-deficient bone marrow. The number of progenitors is the same in the bone marrow of wild-type and WASP-deficient mice. Bone marrow cells from wild-type (gray column) or WASP-deficient (black column) mice were cultured in methylcellulose in the presence of IL-3, TPO, and EPO. After 8 days at 37°C in an atmosphere of 5% CO2 in air, the erythroid, megakaryocytic, and granulo-macrophagic colonies were counted under an inverted microscope. Results shown are mean ± SD of 3 independent experiments.
Comparison of progenitor numbers in wild-type and WASP-deficient bone marrow. The number of progenitors is the same in the bone marrow of wild-type and WASP-deficient mice. Bone marrow cells from wild-type (gray column) or WASP-deficient (black column) mice were cultured in methylcellulose in the presence of IL-3, TPO, and EPO. After 8 days at 37°C in an atmosphere of 5% CO2 in air, the erythroid, megakaryocytic, and granulo-macrophagic colonies were counted under an inverted microscope. Results shown are mean ± SD of 3 independent experiments.
This result demonstrates that progenitors expressing the normal WASP protein have an advantage in migration and homing over WASP-deficient progenitors.
The kinetics of Cdc42 activation is different in Lin– cells than in wild-type and WASP-deficient mice
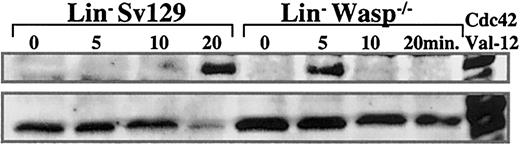
Previous studies demonstrated that activation of Rho GTPase Cdc42, which has been shown to induce cell polarization and filopodia formation in fibroblasts, also regulates shape, migration, and mobilization of hematopoietic progenitor cells.24 Hence, we examined if the observed differences between hematopoietic progenitor cells and their counterpart WASP-deficient cells could be due to a different status of Cdc42 activation following stimulation by SDF-1, the most potent chemoattractant of primitive cells. Using the GST-CRIB binding domain to pull down the active form of Cdc42 (Cdc42-GTP), we studied the kinetics of Cdc42 GTPase activation of SDF-1–primed cells (Figure 3). SDF-1 activation of Lin– cells from 129Sv mice induced strong activation of GTP-bound Cdc42 at 20 minutes, to a level comparable to that observed in cells transfected by dominant activated Cdc42 (CDc42-Val12). In WASP–/– cells, Cdc42 activation was transient, although substantially increased at 5 minutes (Figure 3, upper panel).
Time course of Cdc42 activation following SDF-1 stimulation. Cdc42 activation was studied after SDF-1 (100 ng/mL) activation of Lin– cells from 129Sv and WASP-deficient mice by a pull-down assay. The GST-WASP-CRIB–binding domain was used to evaluate GTP-bound Cdc42 (upper panel). The immunoblot of total lysates by Cdc42 antibody is shown in the lower panel. The last line represents the pull-down and total Cdc42 lysates of transfected cells by a dominant activated Cdc42 (CDc42-Val12).
Time course of Cdc42 activation following SDF-1 stimulation. Cdc42 activation was studied after SDF-1 (100 ng/mL) activation of Lin– cells from 129Sv and WASP-deficient mice by a pull-down assay. The GST-WASP-CRIB–binding domain was used to evaluate GTP-bound Cdc42 (upper panel). The immunoblot of total lysates by Cdc42 antibody is shown in the lower panel. The last line represents the pull-down and total Cdc42 lysates of transfected cells by a dominant activated Cdc42 (CDc42-Val12).
Actin cytoskeleton reorganization is altered in Lin– WASP-deficient cells following SDF-1 activation and specific adhesion
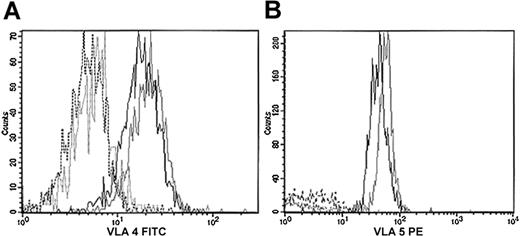
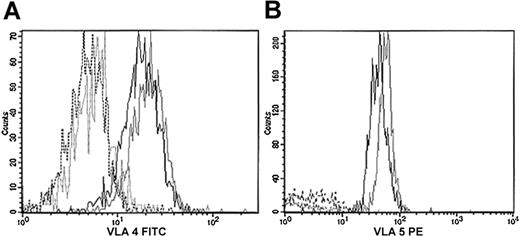
We further investigated if the difference of this timing had any effect on actin cytoskeleton reorganization. We therefore tested whether adhesion to important components of bone marrow, such as fibronectin and fibrillar collagen, could affect actin reorganization in Lin– cells and their counterpart WASP-deficient cells primed by SDF-1. First, we studied whether the fibronectin receptors were normally expressed by hematopoietic cells from WASP-deficient mice. No significant differences were observed in the expression of VLA-4 and VLA-5 from Lin– (data not shown) and Lin– Sca+c-kit+ cells (Figure 4) from the 2 types of mice.
Expression of VLA-4 and VLA-5 in Lin–Sca+c-kit+ cells. Lin– cells were first purified by depletion of Lin+ cells. Lin– cells were labeled with antibodies against Sca-1, c-kit, VLA-4, VLA-5, or their isotype controls. VLA-4 expression was studied with an FITC-conjugated antibody (panel A); VLA-5 was studied with a PE-conjugated antibody (panel B). Black indicates 129Sv; gray, WASP-deficient; plain line, specific antibody; dotted line, isotype control.
Expression of VLA-4 and VLA-5 in Lin–Sca+c-kit+ cells. Lin– cells were first purified by depletion of Lin+ cells. Lin– cells were labeled with antibodies against Sca-1, c-kit, VLA-4, VLA-5, or their isotype controls. VLA-4 expression was studied with an FITC-conjugated antibody (panel A); VLA-5 was studied with a PE-conjugated antibody (panel B). Black indicates 129Sv; gray, WASP-deficient; plain line, specific antibody; dotted line, isotype control.
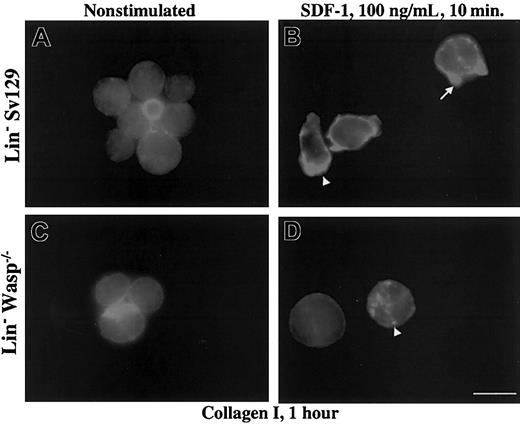
Then, we studied the actin reorganization of Lin– cells after adhesion. The distribution of F-actin in the 2 cell types was no different whether cells were allowed to adhere to collagen I, fibronectin fragment, or poly-l-lysine without SDF-1 activation. We also found qualitatively similar F-actin distribution in SDF-1–prestimulated Lin– adherent cells to fibronectin or poly-l-lysine, even though cell spreading was greater in 129Sv Lin– cells compared with WASP-deficient cells (data not shown). Interestingly, adhesion to fibrillar collagen I induced a polarized shape and the extension of cellular protrusions. F-actin was highly concentrated in small spikes at the leading edges (Figure 5B, arrow) or at the rear of polarized cells (Figure 5B, arrowhead) only after SDF-1 stimulation (Figure 5A).
Effect of collagen I on polarization and actin reorganization. Collagen I induced polarization and actin reorganization in SDF-1–pretreated Lin– 129Sv but not in WASP-deficient cells. Lin– cells from 129Sv and WASP-deficient mice (panels A-B) were either pretreated (panels B,D) or not (panels A,C) by SDF-1 for 10 minutes, allowed to adhere to collagen I–coated coverslips for 1 hour at 37°C, and processed for actin staining by rhodamine phalloidin as described in “Materials and methods.” Scale bar equals 10 μm.
Effect of collagen I on polarization and actin reorganization. Collagen I induced polarization and actin reorganization in SDF-1–pretreated Lin– 129Sv but not in WASP-deficient cells. Lin– cells from 129Sv and WASP-deficient mice (panels A-B) were either pretreated (panels B,D) or not (panels A,C) by SDF-1 for 10 minutes, allowed to adhere to collagen I–coated coverslips for 1 hour at 37°C, and processed for actin staining by rhodamine phalloidin as described in “Materials and methods.” Scale bar equals 10 μm.
Conversely, in WASP-deficient cells, most of cells maintained a round shape (Figure 5C-D). F-actin displayed diffuse distribution or was aggregated in dotlike structures (Figure 5D, arrowhead). These results indicate that activation of hematopoietic progenitor cells by SDF-1 is potentiated by specific adhesion to a major component of extracellular matrix in bone marrow microenvironment, such as collagen I. WASP is probably involved in the process of actin reorganization induced by both SDF-1 activation and adhesion to collagen I.
Reduced survival and donor recovery after transplantation of WASP-deficient bone marrow cells
To investigate whether the defect in migration and/or homing of WASP-deficient progenitors observed in the short term has consequences on long-term repopulating ability, we used a serial transplantation approach. Three million bone marrow cells from wild-type male (129Sv) or WASP-deficient male mice were engrafted into irradiated 129Sv female mice. Every 2 months, total bone marrow cells from one leg of each grafted animal were individually injected into a new irradiated female recipient mouse. As shown on Figure 6A, recipient animals began to die after the fifth serial transplantation when WASP-deficient cells were grafted and one transplantation later (sixth transplantation) when wild-type bone marrow cells were used for transplantation. The percentage of survival also decreased faster when WASP-deficient cells were used for bone marrow transplantation. Indeed, 50% survival was achieved 1 month after the seventh transplantation of WASP-null cells while 90% of recipient animals receiving serial transplants of wild-type bone marrow cells survived. This result shows a decrease in the ability of WASP-deficient hematopoietic stem cells to be serially transplanted.
Animal survival and chimerism of animals after bone marrow transplantation (BMT). Male mice were used as bone marrow donors. Female recipient mice were letally irradiated, and 3 million nucleated cells were injected intravenously. The mice were killed 2 months after the graft, and the cells from one leg were injected into a new irradiated female mouse. This process was repeated 7 times. (A) Cumulative survival after serial BMT. Each group included 10 mice. The donor marrow from one leg of the previous transplant was individually injected into a new recipient. There was no mortality during the first 3 transplantations. Results of the fourth to seventh transplantations are shown here. (B) Numbers of nucleated cells per leg. At each transplantation, one leg was harvested from each mouse and cells were enumerated; results shown are the means ± SD for the 2 groups after the fourth to the sixth transplantation. (C) Numbers of colony-forming cells (CFCs) per leg at the fourth to the sixth transplantation. (D) Percentage of donor cells observed in recipient mice after each transplantation. At each transplantation, bone marrow cells from recipient mice were cultured in methylcellulose. Twenty colonies per mouse were picked, and monitored by Y and neo PCR.
Animal survival and chimerism of animals after bone marrow transplantation (BMT). Male mice were used as bone marrow donors. Female recipient mice were letally irradiated, and 3 million nucleated cells were injected intravenously. The mice were killed 2 months after the graft, and the cells from one leg were injected into a new irradiated female mouse. This process was repeated 7 times. (A) Cumulative survival after serial BMT. Each group included 10 mice. The donor marrow from one leg of the previous transplant was individually injected into a new recipient. There was no mortality during the first 3 transplantations. Results of the fourth to seventh transplantations are shown here. (B) Numbers of nucleated cells per leg. At each transplantation, one leg was harvested from each mouse and cells were enumerated; results shown are the means ± SD for the 2 groups after the fourth to the sixth transplantation. (C) Numbers of colony-forming cells (CFCs) per leg at the fourth to the sixth transplantation. (D) Percentage of donor cells observed in recipient mice after each transplantation. At each transplantation, bone marrow cells from recipient mice were cultured in methylcellulose. Twenty colonies per mouse were picked, and monitored by Y and neo PCR.
To determine whether this reduced capacity of WASP-deficient stem cells to repopulate hematopoiesis of irradiated animals is due to a defect in their ability to migrate into hematopoietic tissues, the contribution of donor cells from normal or WASP-deficient mice was determined in the bone marrow of each recipient at each transplantation by semisolid culture assays in the presence or absence of G418. The absolute number of hematopoietic cells and of clonogenic progenitors is not statistically different (Figure 6B-C) in the 2 groups. Colonies growing in the absence of G418 were harvested; DNA was prepared; and the presence of the neo gene and the Y sequence was determined by PCR. After the fifth retransplantation of wild-type bone marrow or WASP-deficient cells, 68.2% and 37.5% of the colonies, respectively, were of donor origin. This percentage was calculated as the percentage of colonies containing the Y sequence for wild-type bone marrow cells. For WASP-deficient cells, the percentage of colonies was calculated in 2 ways: the percentage of colonies growing in the presence of G418 (39.8%) and the percentage of colonies positive for neo-resistant gene in PCR (36.8%) (Figure 6D). In this case, the result was expressed as the mean of the 2 determinations, which were nearly identical.
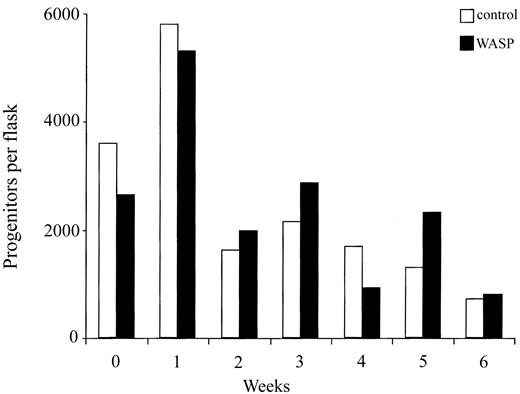
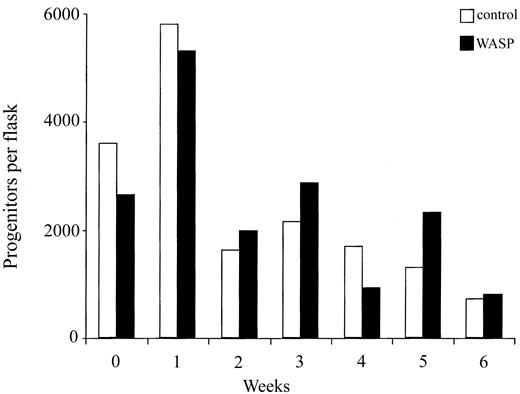
These results demonstrate that at each transplantation the number of progenitors of donor origin found in the bone marrow of recipient mice decreased more sharply when WASP-deficient cells were used for serial transplantation. This result can be the consequence either of a quantitative defect in hematopoietic stem cells or of their decreased homing capacity. Thus, we compared the stem cell activity of WASP-deficient and wild-type bone marrow cells using in vitro assays. Long-term bone marrow cultures were established with bone marrow cells from wild-type or WASP-null mice and maintained for 6 weeks. Every week, one flask of the 2 origins was killed, and nucleated cells were counted and assayed for their content in progenitors. As shown in Figure 7, cell proliferation occurred during the first week of both cultures, and then decreases in nucleated (data not shown) and progenitor cells numbers were observed. However, the numbers of nucleated or progenitor cells did not differ notably between wild-type and WASP-deficient cultures during the period of observation (6 weeks). These results indicate that the stem cell compartment of adult mutant mice is not significantly altered.
No defect in self-renewal in stem cells from WASP-deficient mice. Long-term bone marrow cultures were established on MS-5 feeder layer with bone marrow cells from wild-type and WASP-deficient mice. First, 3 × 106 bone marrow cells were seeded into 10 mL culture medium in a 25-cm2 flask. Half of each culture was removed every week and replaced with fresh medium. A flask of each culture (one from wild-type and one from WASP-deficient cells) was stopped every week. Nucleated cells were counted and a progenitor assay was performed. No differences were found between the numbers of nucleated cells or clonogenic progenitors of wild-type and WASP-deficient cultures.
No defect in self-renewal in stem cells from WASP-deficient mice. Long-term bone marrow cultures were established on MS-5 feeder layer with bone marrow cells from wild-type and WASP-deficient mice. First, 3 × 106 bone marrow cells were seeded into 10 mL culture medium in a 25-cm2 flask. Half of each culture was removed every week and replaced with fresh medium. A flask of each culture (one from wild-type and one from WASP-deficient cells) was stopped every week. Nucleated cells were counted and a progenitor assay was performed. No differences were found between the numbers of nucleated cells or clonogenic progenitors of wild-type and WASP-deficient cultures.
Collectively, these results show that the decrease in the repopulating ability of WASP-deficient progenitors can be explained by a decrease in the stem cell ability to home in the hematopoietic tissues.
Selective advantage of cells expressing the normal X-chromosome in the bone marrow of heterozygous females
It was demonstrated that in blood of WAS-obligate carriers there is a nonrandom inactivation of the X-chromosome. This may be due to a selective advantage of cells expressing the normal WASP. It has been suggested that this selective advantage occurs early during hematopoietic differentiation. Furthermore, a somatic reversion of a mutation in the WASP gene of a WAS patient with a somatic mosaicism has been recently reported.25 This reverse mutation has been explained by a selective growth advantage of WASP+ lymphocytes during thymic development. We hypothesized that stem cells expressing WASP have an advantage over WASP-deficient stem cells in migrating into hematopoietic tissues. During development, at the end of the fetal life, hematopoietic stem cells migrate from the liver to the marrow. There is evidence that this migration is regulated by the chemokine SDF-1.26-28 Thus, it was tempting to speculate that WASP-expressing stem cells may have a selective advantage precisely at the stage of migration from liver to bone marrow.
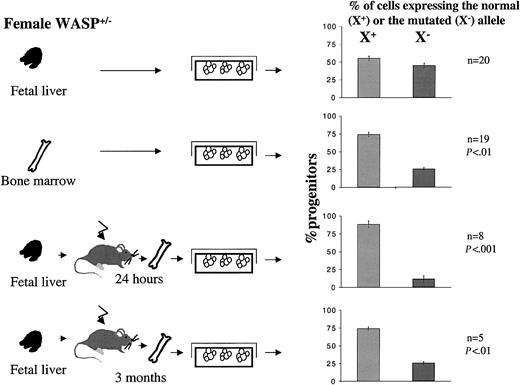
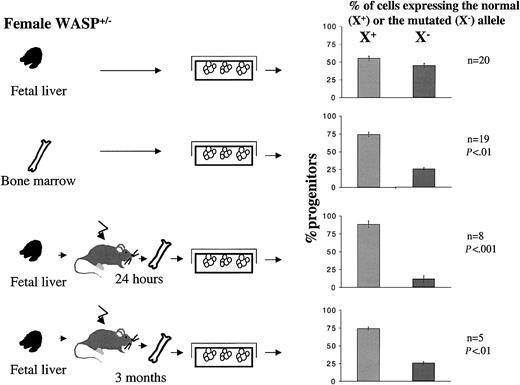
To test this hypothesis, we used heterozygous female mice as a model. First, we determined if hematopoietic progenitors isolated from the bone marrow or the fetal liver of heterozygote female mice exhibited random or nonrandom X-chromosome inactivation. Inactivation of the X-chromosome in progenitor cells was analyzed by culturing hematopoietic cells from heterozygous females in semisolid medium in the presence or absence of G418, which selects the growth of progenitors expressing the mutated X-chromosome. Our results showed clearly that the X inactivation was random in the fetal liver (Figure 8). In contrast, it was not random in the bone marrow because the proportion of progenitors that have inactivated the mutated X-chromosome was 75%, and thus only 25% have inactivated the normal X-chromosome (P ≤ .001, Wilcoxon test and χ2).
Percentage of progenitor cells from bone marrow or fetal liver of heterozygous female expressing normal (X+) or mutated (X–) allele. Bone marrow and fetal liver cells from heterozygous females were seeded into methylcellulose in the presence or absence of G418. Only the cells expressing the X-chromosome carrying the mutated WASP gene are able to grow in the presence of G418. Fetal liver cells from heterozygous females were also injected intravenously into irradiated recipient mice, and at 24 hours or 3 months after the graft, bone marrow cells were harvested and cultured in methylcellulose in the presence or absence of G418. At 7 days later, the number of colonies were scored. Results are expressed as the percentage of clonogenic progenitors expressing the normal (X+) or the mutated (X–) allele. This proportion was compared (by Wilcoxon test and χ2 test) to the proportion 50:50 that is observed when random X-chromosome inactivation occurs. As this skewed X inactivation is observed 24 hours after transplantation, this indicates that hematopoietic progenitors expressing WASP have a selective advantage over the WASP-deficient progenitors in homing ability. Error bars indicate SD.
Percentage of progenitor cells from bone marrow or fetal liver of heterozygous female expressing normal (X+) or mutated (X–) allele. Bone marrow and fetal liver cells from heterozygous females were seeded into methylcellulose in the presence or absence of G418. Only the cells expressing the X-chromosome carrying the mutated WASP gene are able to grow in the presence of G418. Fetal liver cells from heterozygous females were also injected intravenously into irradiated recipient mice, and at 24 hours or 3 months after the graft, bone marrow cells were harvested and cultured in methylcellulose in the presence or absence of G418. At 7 days later, the number of colonies were scored. Results are expressed as the percentage of clonogenic progenitors expressing the normal (X+) or the mutated (X–) allele. This proportion was compared (by Wilcoxon test and χ2 test) to the proportion 50:50 that is observed when random X-chromosome inactivation occurs. As this skewed X inactivation is observed 24 hours after transplantation, this indicates that hematopoietic progenitors expressing WASP have a selective advantage over the WASP-deficient progenitors in homing ability. Error bars indicate SD.
To determine if this difference in the X-chromosome inactivation between the fetal liver and the bone marrow was due to an advantage of cells expressing the normal WASP in migrating into the bone marrow, fetal liver cells of heterozygous females (with a random X-chromosome inactivation) were grafted into irradiated animals. Figure 8 shows that at both 24 hours and 3 months after the graft, fewer than 25% of the hematopoietic progenitors found in the bone marrow had inactivated the nonmutated X-chromosome. Thus, the same pattern of X inactivation was observed in the marrow of adult WASP-carrier females and in the marrow of normal mice engrafted with fetal liver from heterozygous embryos. These results indicate that nonrandom X inactivation in heterozygous WASP female mice is due to an advantage in migrating in the marrow present in fetal hematopoietic stem cells expressing the WASP gene.
Discussion
Hematopoietic stem cells are characterized by their multipotence, self-renewal, and migration/homing properties. WASP deficiency has been demonstrated to induce marked defect in cell trafficking.13,15 Because the migration and homing properties of stem cells play a key role in blood homeostasis, at stem cell transplantation, and during development when sites of hematopoiesis change, we used WASP knock-out mice to investigate19 whether WASP deficiency in stem cells impairs their ability to colonize hematopoietic tissues.
Using 3 independent approaches, we demonstrated that WASP-deficient hematopoietic progenitors have a defect in their homing capacities. First, WASP-deficient marrow cells display a 2-fold decrease in SDF-1 chemotactic response compared with wild-type marrow cells. Second, by means of an in vivo homing assay, a similar quantitative defect was observed in hematopoietic progenitor cells. Third, a serial transplantation assay demonstrated reproducible defects in WASP-deficient stem cell activity.
The signaling induced by the binding of SDF-1 with its receptor, CXCR4, may be involved in the defective homing properties of WASP-deficient stem cells. It has been shown that CXCR4 is absolutely required for the engraftment of human stem cells in nonobese diabetic–severe combined immunodeficient (NOD-SCID) mice,29 while in contrast, murine fetal liver CXCR4-deficient stem cells are able to reconstitute hematopoiesis in transplantation experiments.27 SDF-1, like other chemokines, plays a pivotal role not only by its chemoattractant properties and the induction of diapedesis, but also by activating the different integrins, especially VLA-4,30 which are important for transendothelial migration. The precise mechanism by which SDF-1 induces activation of integrins is not well understood, but may be mediated through activation of the Rac 2 pathway.31,32 Rac interferes with WASP-family verprolin homologous (WAVE) proteins, which belong to the same family of scaffold proteins as WASP.9,33 Because activation of integrins can be mediated through clustering, a function regulated by Cdc42, WASP-deficient stem cells may potentially also have a defect in adhesion. According to this hypothesis, we found a defect both in Cdc42 activation and in adhesion to collagen I but not to fibronectin after SDF-1 stimulation of Lin– cells from WASP-deficient mice.
In comparison with CXCR4–/– stem cells, which are unable to repopulate secondary recipients,34 we found that WASP-deficient stem cells retain this characteristic, but at 50% less than normal. As hematopoietic cells also express low levels of neural (N)–WASP,35 a ubiquitously expressed protein that is very similar to WASP, N-WASP may partially rescue WASP deficiency.
A skewed X-chromosomal inactivation pattern has been reported in a female carrier of WAS.17 This skewed X-chromosome inactivation was also found in marrow CD34+ cells of female carriers,18 showing that the hematopoietic progenitors are already skewed. However, it has been shown that a random X inactivation is observed in carriers of a missense mutation responsible for X-linked thrombocytopenia or mild WAS.36 It has been suggested that skewed X-chromosomal inactivation could be related to a negative selection of WASP-deficient cells.25 WASP deficiency may impair hematopoiesis at some level of differentiation, and thus cells expressing the normal WASP have an advantage over cells bearing the product of the defective gene. More recently, it has been shown that differences in the expression of WASP in female carriers were observed between monocytes and lymphocytes.37 This result suggests that the nonrandom X inactivation is not definitively fixed at the stem cell lineage, but may progress during differentiation in a lineage-dependent manner. In favor of this hypothesis, it has been recently demonstrated that WASP and presumably other members of its family play an important role in early T-cell development.25
We next speculated that WASP deficiency may be indirectly responsible for the nonrandom X inactivation in hematopoietic cells as a consequence of a reduction in homing capacity of the WASP-deficient HSCs during ontogeny. Such a hypothesis has been previously proposed by Thrasher et al.15 Because the WASP gene is located on the X-chromosome, WASP expression in heterozygous female cells is either on or off. Using heterozygous (X–/X+) WASP-carrier mice, we found strong evidence in favor of abnormal migration of WASP-deficient stem cells. First, fetal liver hematopoietic progenitors had randomly inactivated the X-chromosome, whereas only 25% of the adult marrow cells had inactivated the nonmutated X-chromosome. Second, when cells from fetal liver with a random X inactivation were injected into irradiated mice, 75% of the hematopoietic stem cells engrafted in the marrow had inactivated the X-mutated chromosome. These 2 results clearly demonstrate that a skewed X inactivation pattern can be attributed to a defect of homing of WASP-deficient fetal liver cells into the marrow and occurs in stem cells capable of long-term reconstitution.
It is noteworthy that a minority of bone marrow progenitor cells had not inactivated the X-mutated allele, in contrast to the total absence of cells with activated mutated alleles in the human disease. However, in the human disease, the skewed X inactivation has essentially been studied in peripheral mature cells. Thus, it is possible that WASP-deficient cells are further negatively selected during differentiation, as has been recently suggested.37
During development, hematopoietic stem cells migrate from the aorta-gonad-mesonephros (AGM) to fetal liver and then, at the end of the fetal life, to the bone marrow. This second migration appears to be regulated by SDF-1 because CXCR4-null or SDF-1–null mice have a normal fetal liver hematopoiesis contrasting with severely compromised marrow hematopoiesis.
Because complete random X inactivation was found in the fetal liver, this suggests that WASP deficiency does not affect early stages of stem cell migration. This contrasts with the results obtained with β1 integrin deficiency, which has shown that chimeric embryos contained β1-null hematopoietic cells in the yolk sac and in the fetal circulation, but not in the fetal liver, demonstrating that the β1 integrin is crucial for migration to the fetal liver.38 Several explanations could account for these differences: (1) The precise mechanism of the migration from the AGM to the fetal liver is still unknown and may not involve the same set of chemokines involved in later stages. (2) The relative expression of N-WASP and WASP in hematopoietic cells has not been studied during development, and one cannot exclude the possibility that N-WASP may rescue WASP deficiency at this stage of development. (3) The fact that WASP-null mice have a normal hematopoiesis suggests that WASP deficiency may essentially alter the capacity of stem cell homing and/or engraftment. Stem cells expressing WASP could have a marked advantage over WASP-deficient HSCs for entering stem cell niches. Thus, if the number of fetal stem cell niches is in excess during embryogenesis, WASP+ and WASP-null HSCs will not compete for engraftment, and no skewed X inactivation will be observed in the fetal liver.
Overall, these results clearly demonstrate an advantage of stem cells expressing a normal WASP gene over WASP-deficient cells in their capacity to migrate and home to the bone marrow, which could explain the nonrandom inactivation of the X-chromosome in the blood of obligate female carriers. Futhermore, for therapeutic treatment of human patients, our data strongly argue that gene therapy correction of only a fraction of hematopoietic stem cells may be sufficient to cure this disease.
Prepublished online as Blood First Edition Paper, May 1, 2003; DOI 10.1182/blood-2002-07-2099.
Supported by European Commission (contract no. QLG1CT 1999-01090, WASPNEST program) and a contract with the French Ministry of Research (ACI); S.S. is a recipient of a postdoctoral fellowship from the European Commission.
C.L. and E.H. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Drs A. Galy, I. Godin, A. Fischer, and J. Dando for helpful discussions. We would like to thank P. Ardouin and A. Rouches (Institut Gustave Roussy) for animal facilities.