Abstract
The recovery of circulating antigen-specific T-cell immunity to Epstein-Barr virus (EBV) was determined in ELIspot assays following allogeneic myeloablative or nonmyeloablative stem cell transplantation (MST/NST). In 8 of 12 MST patients receiving an alemtuzumab-treated graft, the frequency of the EBV-specific reactivities was similar to or greater than that seen in the healthy controls. A response was detectable in 3 of 6 and 6 of 9 patients by 3 and 6 months, respectively, and in all patients by one year following MST. In contrast, only 1 of 9 (95% confidence interval [CI], 0-2.8) patients made a detectable EBV-specific response by 6 months following NST conditioned with fludarabine, melphalan, and alemtuzumab. Responses were detected in 7 of 10 patients by 1 year after NST. Parallel surveillance demonstrated that other virus infections occurred more frequently and earlier after transplantation in NST patients. The use of alemtuzumab in vivo in the nonmyeloablative conditioning might have resulted in the delay in EBV-specific T-cell recovery and increased virus infections.
Introduction
Experimental transplantation protocols that differ in intensity of conditioning and in the strategies used to prevent graft-versus-host disease (GVHD) might impact differently on the reconstitution of antigen-specific T-lymphocyte responses. Epstein-Barr virus (EBV), a ubiquitous virus, can serve as a model source of antigen for assessing the reconstitution of functional virus-specific T-lymphocyte responses. We measured circulating functional T-cell responses against a wide range of major histocompatibility complex (MHC) class I–restricted epitopes in EBV antigens in patients following either myeloablative stem cell transplantation (MST) or nonmyeloablative stem cell transplantation (NST). Alemtuzumab (Campath-1H; Therapeutic Antibody Centre, Oxford, United Kingdom) was used to prevent GVHD as a low-dose in vitro graft treatment for MST patients or given in a higher dose in vivo for NST recipients. The patterns of (1) reconstitution of EBV-specific responses and (2) infections with other viruses were compared between MST and NST patients.
Study design
Twenty-eight consecutive patients undergoing allogeneic stem cell transplantation for hematologic malignancy at Birmingham Heartlands Hospital during 1999 were recruited to a virus surveillance program. Patients underwent MST for chronic myeloid, acute myeloid, and acute lymphoblastic leukemia. Patients underwent NST for a wider range of diseases that also included Hodgkin and non-Hodgkin lymphoma and myeloma. Serial cryopreserved peripheral blood lymphocyte (PBL) samples taken from 1 to 18 months after transplantation were available from 23 patients for analysis of EBV-specific T-cell response. Approval was obtained from the Institutional Review Board at Birmingham Heartlands Hospital for these studies. Informed consent was provided according to the Declaration of Helsinki.
Fourteen NST patients had received an unmanipulated graft following conditioning with alemtuzumab 20 mg/d for 5 days plus fludarabine and melphalan, as previously described.1 Fourteen MST patients had received a graft treated in vitro with alemtuzumab 20 mg following total body irradiation plus cyclophosphamide or etoposide.2 All patients received cyclosporin A until day 100.
Results and discussion
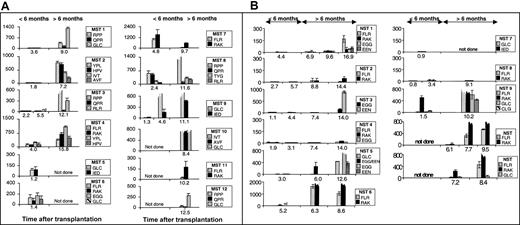
ELIspot assays3 were used to determine the proportion of functional epitope-specific T cells that released interferon γ (IFN-γ) in response to stimulation with synthetic peptide epitopes (Table 1). An unequivocal response was detected in samples taken during the first transplantation year from all 12 MST patients (Figure 1A) and from 9 of 11 NST patients (Figure 1B) on at least one occasion up to 18 months after transplantation. The data on 1 of the 2 nonresponders is limited due to his early death (NST 7).
Measurement of EBV-specific CTL response by ELIspot following myeloablative and nonmyeloablative transplantation. Panel A shows patients' responses following myeloablative transplantation and panel B shows patients' responses following nonmyeloablative transplantation. The proportion of PBLs (with 95% CI) responding to specific peptide stimulation as determined in ELIspot assays at different time points after transplantation. Individual cells producing IFN-γ in response to overnight stimulation with synthetic peptide, dimethyl sulfoxide (DMSO) solvent or phytohemagglutinin (PHA) were detected in ELIspot assays (Millipore, Bedford, MA; and Mabtech, Stockholm, Sweden) as dark spots after the cells had been discarded. The spots were counted under a dissection microscope. Frequencies exceeding 10 responders/million PBLs were considered positive. The background IFN-γ release from lymphocytes not stimulated with peptide is subtracted from the peptide-specific responses. The peptide targets are listed in Table 1. Broken bars represent estimates of the magnitude of responses too numerous to count. Y-axes show responders/million PBLs.
Measurement of EBV-specific CTL response by ELIspot following myeloablative and nonmyeloablative transplantation. Panel A shows patients' responses following myeloablative transplantation and panel B shows patients' responses following nonmyeloablative transplantation. The proportion of PBLs (with 95% CI) responding to specific peptide stimulation as determined in ELIspot assays at different time points after transplantation. Individual cells producing IFN-γ in response to overnight stimulation with synthetic peptide, dimethyl sulfoxide (DMSO) solvent or phytohemagglutinin (PHA) were detected in ELIspot assays (Millipore, Bedford, MA; and Mabtech, Stockholm, Sweden) as dark spots after the cells had been discarded. The spots were counted under a dissection microscope. Frequencies exceeding 10 responders/million PBLs were considered positive. The background IFN-γ release from lymphocytes not stimulated with peptide is subtracted from the peptide-specific responses. The peptide targets are listed in Table 1. Broken bars represent estimates of the magnitude of responses too numerous to count. Y-axes show responders/million PBLs.
Many epitope-specific reactivities in the first 15 months after transplantation were very abundant (Figure 1), with frequencies similar to or exceeding the range detected in the healthy immunocompetent controls (Table 1).3,4 This is apparent for epitopes restricted through human leukocyte antigen A2 (HLA A2) (MST 1, 9, 10 and NST 5 and 9), HLA A11 (MST 10), HLA B7 (MST 1, 3 and 8), HLA B8 (NST 6, 9, 10 and 11 and MST 4 and 7), HLA B35 (MST 2, MST 4), and HLA B44 (NST 3 and 5). Parallel ELIspot assays and flow cytometry using HLA A2 or HLA B8 tetramers using PBL samples from stem cell recipients both detected low-abundance reactivities and revealed the same hierarchy of immunodominance between different reactivities (data not shown).
Very abundant responses were detected in 9 of 9 MST patients with sufficient serial samples from the first year after transplantation compared with only 5 of 10 of the NST cohort. Furthermore, there was an apparent delay in reconstitution of the EBV-specific T-cell response in the NST compared with MST patients. An epitope-specific cytotoxic T-lymphocyte (CTL) response to EBV was detected in PBL samples taken from 3 of 6 MST patients within the first 3 months after transplantation and in 6 of 9 (95% confidence interval [CI], 3.2-8.8) MST patients by 6 months after transplantation. By contrast, 1 of 9 (95% CI, 0-2.8) NST patients made a detectable response during the first 6 months after transplantation (Figure 1B). Three of 3 MST patients had a detectable response to the immunodominant HLA B8–restricted epitopes, FLRGRAYGL and RAKFKQLL, in the first 6 months after transplantation, compared with only 1 of 6 NST recipients. Responses to the immunodominant HLA A2–restricted epitope, GLCTLVAML, were detected in the first 6 months after transplantation in 2 of 3 MST recipients but in 0 of 3 NST recipients.
We did not find the timing of first detection of EBV-specific responses to be simply dependent on the rate of recovery of either CD8+ or CD4+ T-cell numbers (data not shown). Recently a number of MHC class II–restricted epitopes within EBV antigens have been described.18 Future study of EBV-specific CD4+ and CD8+ T-cell responses may reveal interdependence of CTL and helper function following stem cell transplantation, as has been suggested for other viruses.19,20
An increase in EBV loads coincided with the first detection of an EBV-specific T-cell response 6 and 9 months following NST, although this relationship was not apparent following MST (data not shown). The abundance of specific T cells might have been driven by antigenic load as in the reconstitution of a cytomegalovirus (CMV)–specific T-cell response,21 or alternatively, might have reflected the independent recovery of B-cell numbers and T-cell function following alemtuzumab conditioning. This could not be determined because B-cell numbers were not measured. No patient in this study developed EBV–lymphoproliferative disease (EBV-LPD). The incidence of EBV-LPD is low with alemtuzumab use: 1% in 100 NST patients receiving alemtuzumab in vivo (S.C., unpublished observations, December 2001) and zero in stem cell recipients receiving alemtuzumab-treated grafts.2
The apparent delay in detection of EBV-specific immunity appeared to be indicative of a more generalized suppression of virus-specific immunity in the NST cohort. Virus infections were detected in a greater proportion of NST compared with MST patients (13/14 vs 7/14, P = .03) and earlier after transplantation (median 24 vs 60 days, P = .02). NST patients experienced more total viral episodes (mean 2.9 vs 0.9 episodes per patient, P = .01) and non-CMV viral episodes compared with the MST group (mean 2.4 vs 0.6, P = .01).
Several different factors may have contributed to the slower immune reconstitution noted in our cohort of NST patients. First, our cohorts differed in that MST was used to treat less advanced leukemias, whereas NST was used to treat mostly lymphoid malignancy. Second, NST patients were conditioned with fludarabine, a purine analog with profound suppressive effects on T-cell function. With a terminal plasma half-life of only 10 hours, fludarabine is unlikely to directly effect graft function. However, alemtuzumab has a half-life of 15 to 21 days in vivo in bone marrow transplant (BMT) recipients.22 The higher dose given in vivo as part of nonmyeloablative conditioning not only may have resulted in depletion of lymphocytes in the graft, but also could have effected the recovery of T lymphocytes because of its persistence in the circulation.
The impact of different doses of alemtuzumab on immune reconstitution and viral infections has been demonstrated by our group in larger cohorts. A high incidence of CMV reactivation (84.6%) was recorded in a large cohort of NST patients treated with alemtuzumab in vivo. Late recurrence of CMV infection was noted in 46.6% of these patients and this correlated with slow recovery of CD4+ T cells.1 On the other hand, the incidence of CMV reactivation was only 40% in patients that received alemtuzumab-treated grafts and the speed of immune recovery correlated with the in vitro dose of alemtuzumab.2 Similarly, the risk of adenovirus infection was high when 100 mg alemtuzumab was used in vivo, regardless of the intensity of the conditioning regimen, but was low if a lower dose of alemtuzumab was used in vitro to treat the graft. Lymphocyte recovery was slower in the group receiving alemtuzumab in vivo.23 Respiratory viruses occurred with a higher frequency in patients receiving alemtuzumab in vivo compared with those receiving alemtuzumab-treated grafts, irrespective of the intensity of conditioning.24 This pattern of high incidence and frequent recurrence of respiratory virus infections was consistent in a larger multicenter cohort of NST patients conditioned with alemtuzumab in vivo.25
In conclusion, this study for the first time addressed the issue of EBV-specific immunity following nonmyeloablative transplantation and has demonstrated a distinct difference in the pattern of EBV-specific immune reconstitution following the use of alemtuzumab in vivo and in vitro, in keeping with our previous observations on other virus infections and lymphocyte recovery. This underlies the importance of detailed studies on functional immune reconstitution in patients undergoing experimental conditioning strategies for allogeneic hematopoietic stem cell transplantation.
Supported by Locally Organised Research Fund (West Midlands) grant (A.R.). N.K. was supported by the Wellcome Trust. N.S. is a Medical Research Council Clinician Scientist Fellow.
One of the authors (G.H.) has declared a financial interest in a company (Millennium) whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the staff of the Therapeutic Antibody Centre, University of Oxford for their contributions to the production of CAM-PATH-1H antibody. Their work was supported by the United Kingdom Medical Research Council, Leukosite Inc, and the EP Abraham's Trust. We are very grateful to M. L. Billingham for advice on statistical methods and to S. Cassellas for technical support.