Abstract
Cambridge II (A384S) is a highly prevalent antithrombin variant in the British population (1.14 per 1000) and predisposes carriers to a mild but significant increased risk of thrombosis. To determine if the association of Cambridge II with thrombophilia is due to a perturbation of the antithrombin inhibitory mechanism, we expressed and characterized the variant. Antithrombin Cambridge II was found to be normal in its affinity for heparin, its ability to form sodium dodecyl sulfate-stable complexes with factor Xa and thrombin, and its uncatalyzed stoichiometries and rates of inhibition. However, in the presence of full-length heparin there was a 3- and 7-fold increase in stoichiometry of inhibition of factor Xa and thrombin. The stoichiometries were not affected by pentasaccharides, indicating that the inhibitory mechanism of antithrombin Cambridge II is perturbed only in the presence of a bridging glycosaminoglycan. Thus, the vascular localization of antithrombin Cambridge II would render the carrier slightly thrombophilic. The high occurrence of this mutation and its possible propagation from a few founders suggests an evolutionary advantage, perhaps in decreasing postpartum bleeding. (Blood. 2003;102:4028-4034)
Introduction
Antithrombin is the major physiological inhibitor of factor Xa and thrombin, and to a lesser extent, the other coagulation proteases.1-3 It belongs to the serpin family of protease inhibitors, whose members include many of the key inhibitors involved in hemostasis (eg, the plasminogen activator inhibitors, heparin cofactor II, protein C inhibitor, and α2-antiplasmin4 ). The serpins function by forming covalent 1:1 complexes with proteases.5 After the substrate-like sequence in their protruding reactive center loop (RCL) is cleaved by the protease, the RCL integrates into the center of the dominant serpin structural feature, becoming strand 4 of the 6-stranded β-sheet A (Figure 1). The protease, which is still attached to the RCL by an ester bond, is thereby translocated to the other end of the inhibitor and concurrently inactivated by distortion of the active site architecture.6 The serpin mechanism can be thought of as a race between 2 competing events: RCL incorporation into β-sheet A and deacylation. For most reactions between serpins and target proteases the relative rates are such that the stoichiometry of inhibition is indistinguishable from one (one serpin molecule inhibits one protease molecule). Under certain conditions and for certain serpin variants the rate of loop insertion relative to deacylation is decreased resulting in stoichiometries of inhibition significantly greater than one, effectively rendering the serpin a substrate.7-11
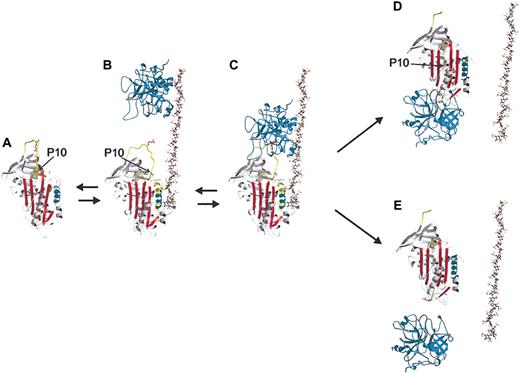
The heparin-dependent mechanism by which antithrombin inhibits coagulation proteases. Native antithrombin (ribbon diagram) circulates in a low activity state (panel A) until it encounters a specific sequence on heparin or heparan sulfate (ball-and-stick), which induces an activating conformational change (panel B). Heparin binding results in extensions (yellow) to helix D (cyan), and the expulsion of the reactive center loop (RCL, yellow coil) from β-sheet A (red). The release of the RCL reorients the reactive center P1 Arg side chain (red ball-and-stick) from contacts with the body of antithrombin for better recognition by proteases. The conformational change also increases the affinity of antithrombin for heparin by approximately 1000-fold through an induced-fit mechanism. Thrombin (cyan, top) interacts with heparin (panel B) and translates until it encounters the prebound antithrombin (panel C). This encounter, or Michaelis complex, then proceeds into the chemistry of proteolysis, resulting in either the translocation of thrombin to the opposite pole of antithrombin to form the inhibited complex (panel D) or the dissociation of the active protease from the cleaved serpin (panel E). The factor that determines the fate of the Michaelis complex is the ratio of the rate of incorporation of the cleaved RCL to the rate of deacylation of the protease acyl intermediate. In either case, the insertion of the RCL into β-sheet A reverses the induced fit mechanism causing the release of heparin. The mutation described in this work is in the part of the RCL, which inserts first the P10 residue of the hinge region and is shown as a green ball (also indicated by arrows). Due to the internal positioning of P10 in the RCL-inserted forms, the strand is transparent in panels D and E. This figure is based on the crystallographic structures of native antithrombin (1e05), pentasaccharide-activated antithrombin (1e03), cleaved bovine antithrombin (1att), the structure of thrombin in complex with heparin cofactor II (1jmo) and the final serpin-protease complex (1ezx). Other details are modeled.
The heparin-dependent mechanism by which antithrombin inhibits coagulation proteases. Native antithrombin (ribbon diagram) circulates in a low activity state (panel A) until it encounters a specific sequence on heparin or heparan sulfate (ball-and-stick), which induces an activating conformational change (panel B). Heparin binding results in extensions (yellow) to helix D (cyan), and the expulsion of the reactive center loop (RCL, yellow coil) from β-sheet A (red). The release of the RCL reorients the reactive center P1 Arg side chain (red ball-and-stick) from contacts with the body of antithrombin for better recognition by proteases. The conformational change also increases the affinity of antithrombin for heparin by approximately 1000-fold through an induced-fit mechanism. Thrombin (cyan, top) interacts with heparin (panel B) and translates until it encounters the prebound antithrombin (panel C). This encounter, or Michaelis complex, then proceeds into the chemistry of proteolysis, resulting in either the translocation of thrombin to the opposite pole of antithrombin to form the inhibited complex (panel D) or the dissociation of the active protease from the cleaved serpin (panel E). The factor that determines the fate of the Michaelis complex is the ratio of the rate of incorporation of the cleaved RCL to the rate of deacylation of the protease acyl intermediate. In either case, the insertion of the RCL into β-sheet A reverses the induced fit mechanism causing the release of heparin. The mutation described in this work is in the part of the RCL, which inserts first the P10 residue of the hinge region and is shown as a green ball (also indicated by arrows). Due to the internal positioning of P10 in the RCL-inserted forms, the strand is transparent in panels D and E. This figure is based on the crystallographic structures of native antithrombin (1e05), pentasaccharide-activated antithrombin (1e03), cleaved bovine antithrombin (1att), the structure of thrombin in complex with heparin cofactor II (1jmo) and the final serpin-protease complex (1ezx). Other details are modeled.
Antithrombin is a special serpin in that its mechanism can be modulated by the cofactor heparin. The inhibitory activity of antithrombin is improved approximately 1000-fold by heparin and the closely related heparan sulfate.2 Physiologically, free heparin is not found in the circulation, and it is probable then that heparan sulfate located on the vascular endothelium interacts with antithrombin (AT) in vivo.12 Different mechanisms underlie this heparin cofactor effect for thrombin and factor Xa, although a specific pentasaccharide sequence within heparin is required in both cases. In the case of thrombin, heparin molecules with a minimal chain length of approximately 18 saccharide units provide a common binding surface for AT and the enzyme, and an approximation effect appears to be mainly responsible for the heparin cofactor activity.13 In contrast, AT inhibition of factor Xa is accelerated approximately 300-fold by the pentasaccharide alone due to the induced conformational change in antithrombin.14 The recent crystal structures of native and pentasaccharide-activated antithrombins have revealed the structural changes associated with heparin activation.15-17 In its native state the RCL of antithrombin is constrained due to its partial incorporation into β-sheet A, and upon heparin binding the inserted portion (residues at P15 and P14, according to the substrate numbering scheme of Schechter and Berger18 ) is expelled (Figure 1A-B). This mechanism is somewhat counterintuitive since the full reinsertion of the RCL is required for efficient protease inhibition. The balance for antithrombin between loop expulsion and loop insertion has thus been precariously placed due to the biologic need of fine-tuned regulation of coagulation.
The physiological importance of antithrombin is emphasized by the recurrent episodes of thrombosis that individuals with deficient or functionally abnormal protein suffer. In the general population, the prevalence of antithrombin deficiency has been estimated to be between 1:200019 and 1:500020 and to affect 4% to 6% of individuals with thrombotic disease.21 A study on blood donors in western Scotland by Tait et al22 reported a surprisingly high frequency of type II antithrombin deficiency of 1:630 that was attributable to the finding of antithrombin Cambridge II (Ala384Ser). Excluding this variant, the prevalence of deficiencies in the same study reduced to 1:2300, in agreement with other studies. Although this common variant has been associated with cases of thrombotic disease,23,24 its clinical relevance often has been questioned. Although most type II antithrombin deficiencies are not associated with an increased risk of thrombosis, Cambridge II heterozygotes have a mild but significant increase in risk, with 21% of carriers experiencing a thrombotic episode.23,24 It is interesting to note that the variant has been reported only in individuals of British descent23-25 and that haplotype analysis on 18 affected families suggested an origin, or founder, effect.24 Since this mutation has been associated with an elevated risk of thrombotic episodes, there probably are some unknown benefits to balance its prothrombotic effects or the mutation would not have propagated to such high frequencies.
The effect of the conservative Ala-to-Ser amino acid substitution of antithrombin Cambridge II, mapping to the P10 proximal hinge region of antithrombin (Figure 1), on perturbation of the antithrombin inhibitory mechanism is unknown. We have expressed and characterized the Cambridge II variant and find a normal affinity for heparin and normal basal rates and stoichiometries of inhibition, but in the presence of full-length heparin antithrombin Cambridge II becomes a substrate of factor Xa and thrombin. We conclude that the heparin-induced substrate behavior of antithrombin Cambridge II is caused by a multiple allosteric effect.
Materials and methods
Human α-thrombin and factor Xa (predominantly α-form) were purchased from Enzyme Research Laboratories (Swansea, United Kingdom). The active protease concentrations were determined by active site titrations with known concentrations of plasma antithrombin. The synthetic heparin pentasaccharide corresponding to the antithrombin binding sequence (Fondaparinux) was generously provided by Dr Maurice Petitou (Sanofi Recherche, France), as was the fully O-methylated high-affinity pentasaccharide containing an extra sulfate group.26 A heparin fraction with high affinity for antithrombin was isolated from commercial heparin using an ultrafiltration method. Briefly, 4.8 μmol antithrombin was saturated with a solution of porcine mucosal heparin (Mr 12 000-15 000) in water, and the ionic strength was adjusted to 0.4 with NaCl. The resulting solution was spin concentrated repeatedly with fresh I = 0.4 buffer using 30-kDa cutoff filters. Full-length heparin containing high-affinity sites for antithrombin (FLH) was obtained by washing with 2 M NaCl and collecting the flow-through from the 30-kDa cutoff spin filter, and then desalting using a 10-kDa cutoff filter. The concentration of the resulting solution of FLH, as well as that of the pentasaccharides, was determined by a stoichiometric titration against a solution of known antithrombin concentration. The chromogenic substrates S-2222 and S-2238 were from Chromogenix. HiTrap-Q and Heparin Sepharose were from Amersham Pharmacia Biotech. Dulbecco modified eagle medium (DMEM), fetal bovine serum, 1 × trypsin ethylenediaminetetraacetic acid (EDTA) and penicillin-streptomycin for tissue culture were from Gibco-BRL (Carlsbad, CA), while methotraxate and geneticin (G418) were from Sigma (St Louis, MO).
Expression and purification of antithrombin
Recombinant expression was necessitated by the fact that the mutant and normal forms of the protein in the plasma of heterozygotes are of similar heparin affinity, thus making it difficult, if not impossible, to separate and purify them by conventional purification methods. The recombinant β-antithrombin control S137A was created by site-directed mutagenesis directly on the pMA-AT plasmid using the QuikChange site-directed Mutagenesis Kit (Stratagene, La Jolla, CA) following the manufacturer's instructions. Change from a Ser to Ala at the 137 position eliminates the N-linked glycosylation site at Asn 135. The advantage over true wild-type antithrombin is reduction in carbohydrate heterogeneity and consequently in heterogeneity of affinity for heparin. The Cambridge II (A384S) antithrombin mutation was created directly on the pMAAT-S137A background as above. The introduced mutation in each case was confirmed by plasmid DNA sequencing. The pMA-AT vectors were then cotransfected with plasmids pSV2dhfr and pRMH140 using the GenePorter transfection reagent (Gene Therapy Systems, San Diego, CA). The selection of transfected cells and the large-scale production of recombinant antithrombin was achieved as described previously,27 as was purification. As before, 2 peaks were obtained, and the high-affinity peak was used for this study. The low-affinity material has previously been shown to be due to core fucosylation.28 The protein was then concentrated to 1 mg/mL in 20 mM NaPi, 0.1 mM EDTA, 0.1% PEG 8000, pH 7.4, 0.1 M NaCl (I = 0.15 assay buffer) buffer. Plasma α-antithrombin was purified as previously described29 and exchanged into assay buffer as above. Antithrombin fractions were snap frozen in liquid nitrogen and stored at -70°C until use. The concentration of antithrombin in each case was determined with an extinction coefficient of 6.530 by measuring the absorbance at 280 nm. Recombinant antithrombin Cambridge II (S137A/A384S) is referred to in the text as Cambridge II and in the tables as A384S.
PAGE
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed in 10% (wt/vol) polyacrylamide. All samples for SDS-PAGE were boiled for 3 minutes under nonreducing conditions before electrophoresis. To verify that all the purified fractions contained fully active material, antithrombin and excess protease (thrombin) were incubated for 30 minutes at room temperature in the absence and presence of heparin and run on nonreducing SDS gels (Figure 2). The gels were stained with 0.25% Commassie Blue R250.
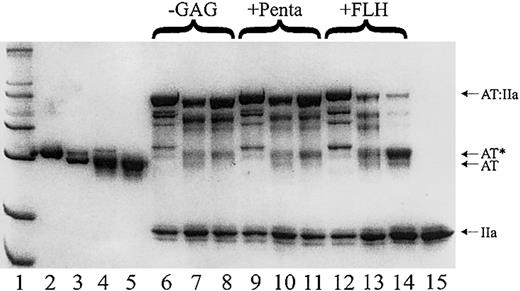
SDS-PAGE demonstrates that antithrombin Cambridge II is predominantly a substrate of thrombin in the presence of heparin. The electrophoretic mobility of plasma alpha (lane 2) and beta (lane 3) antithrombins compared to that of recombinant β-S137A (lane 4) and S137A, A384S (lane5) demonstrates the anticipated size of the recombinant β-antithrombin. An excess of thrombin (lane 15) was reacted with plasma alpha (lanes 6, 9, and 12), recombinant beta (lanes 7, 10, and 13), and the Cambridge II variant (lanes 8, 11, and 14) with and without heparins as indicated (-GAG for no glycosaminoglycan, +Penta for with the pentasaccharide, and +FLH for with full-length heparin). The gel clearly shows that nearly all of the antithrombin reacts with thrombin and either forms complex (high molecular weight bands) or cleaved antithrombin (AT*). The Cambridge II variant is mostly cleaved in the presence of FLH.
SDS-PAGE demonstrates that antithrombin Cambridge II is predominantly a substrate of thrombin in the presence of heparin. The electrophoretic mobility of plasma alpha (lane 2) and beta (lane 3) antithrombins compared to that of recombinant β-S137A (lane 4) and S137A, A384S (lane5) demonstrates the anticipated size of the recombinant β-antithrombin. An excess of thrombin (lane 15) was reacted with plasma alpha (lanes 6, 9, and 12), recombinant beta (lanes 7, 10, and 13), and the Cambridge II variant (lanes 8, 11, and 14) with and without heparins as indicated (-GAG for no glycosaminoglycan, +Penta for with the pentasaccharide, and +FLH for with full-length heparin). The gel clearly shows that nearly all of the antithrombin reacts with thrombin and either forms complex (high molecular weight bands) or cleaved antithrombin (AT*). The Cambridge II variant is mostly cleaved in the presence of FLH.
Stoichiometries of inhibition
The stoichiometry of protease inhibition by antithrombin was measured in the presence and in the absence of heparin by incubating 0.5 μM thrombin or factor Xa with plasma or recombinant antithrombin at concentrations ranging from 0 to 0.6 μM in I = 0.15 buffer as previously described.31 For the reactions carried out in the presence of heparin, 0.6 μM full-length heparin (FLH), high-affinity pentasaccharide (HAP), or physiological pentasaccharide (Penta) was used. Stoichiometries in the presence of FLH were also measured in 0.1 M NaCl, 0.02 M Tris [tris(hydroxymethyl)aminomethane]-HCl pH = 7.4, containing 1 mg/mL bovine serum albumin (BSA), 0.1% PEG 8000, and 0.1 mM EDTA. All reactions were carried out in 50-μL volumes at room temperature (23 ± 2°C). The residual protease activity was determined by diluting 20 μL of the incubated reaction mix into 200 μL assay buffer containing 0.2 mM S-2222 or S-2238 for factor Xa and thrombin, respectively, and the absorbance was read at 405 nm over 60 seconds on a Thermomax micropalte reader (Molecular Devices). The residual protease activity was plotted versus the molar ratio of antithrombin to protease, and the stoichiometry was taken as the x-intercept from the linear regression. When stoichiometries were significantly greater than one, higher antithrombin-protease ratios were used.
To determine the contribution of allostery in the absence of bridging, antithrombin preparations were mixed in a 1:1 molar ratio with the high-affinity pentasaccharide and samples incubated for 10 minutes to allow equilibration. A 4-mer or 14-mer heparin saccharide (Iduronics, Manchester, United Kingdom) was then mixed with thrombin in a molar ratio of 1:1.2 protease/heparin. These heparin saccharides were used, as they are not long enough to allow bridging13 and do not contain the antithrombin binding site.32 Antithrombin and thrombin were then mixed and the stoichiometries measured, as described above, after incubation of samples for 2 hours at room temperature. Reactions were carried out in 20 mM NaPi buffer containing 0.1 mM EDTA and 0.1% PEG 8000 pH 7.4 at different ionic strengths. Similar experiments were conducted without HAP.
The stoichiometries of thrombin inhibition were also investigated in the presence of high-affinity heparin as a function of ionic strength as described previously.33 Antithrombin was mixed in a 1:1 molar ratio with the full-length heparin and then reacted with a fixed amount of thrombin as described above.
Rates of protease inhibition
The rates of inactivation of thrombin and factor Xa by recombinant and plasma antithrombin in the absence of heparin were measured under pseudo first-order conditions using a discontinuous assay. Twenty-microliter samples containing 0.5 μM inhibitor and 50 nM protease were incubated in the presence of 0.05 mg/mL polybrene in I = 0.15 buffer at room temperature (23 ± 2°C). Reactions were quenched at various times by the addition of 1 mL of the appropriate substrate for each protease. The residual protease activity was measured from the initial rates of substrate hydrolysis monitored at 405 nm. For the heparin-catalyzed rates, 20-μL samples containing 0.5 μM antithrombin, 50 nM protease, and 0.5-4 nM FLH or 1-10 nM penta or HAP or the 14-mer heparin saccharide were incubated in the same buffer at room temperature, and the rates measured as before. The pseudo first-order rate constants (kobs) were obtained from the slopes of the plots of the natural logarithm of residual protease activity versus time of incubation. Second-order rate constants for the uncatalyzed reactions were obtained by dividing the observed pseudo first-order rate constant (kobs) by the inhibitor concentration. Second-order rate constants, kcat/Km, for the heparin-catalyzed reactions were taken as the slope of kobs versus the total heparin concentration, since the Kd under these conditions was always significantly less than the initial antithrombin concentrations.31 All measurements were carried out on a Kontron spectrophotometer.
Equilibrium binding studies
Equilibrium dissociation constants were determined for the physiological pentasaccharide binding to antithrombin by monitoring the increase in intrinsic tryptophan fluorescence with increasing amounts of oligosaccharide as described previously.31 All titrations were conducted at 25°C on a Perkin-Elmer LS 50B fluorimeter. Wavelengths and slits of 280 nm and 2.5 nm for excitation, and 340 and 5 nm for emission, respectively, were used. Solutions were made in buffers containing 20 mM NaPi, 0.1 mM EDTA, 0.1% PEG 8000, pH 7.4, with the ionic strength adjusted to 0.3 using NaCl. The data were fitted as previously by nonlinear least squares analysis using GraphPad Prism. All data are reported as the average ± standard deviation of at least 3 determinations.
Results
Initial characterization of the S137A and Cambridge II antithrombin variants
Recombinant antithrombin was expressed as the S137A background form in order to eliminate heparin-binding heterogeneity resulting from partial glycosylation at Asn135. Recombinant β antithrombins, S137A and S137A/A384S, comigrated with plasma β-antithrombin on SDS-PAGE (Figure 2). The Cambridge II variant eluted from a heparin-sepharose column at the same salt concentration as the S137A control antithrombin, suggesting that the proximal hinge mutation does not alter heparin binding affinity. This was subsequently confirmed by equilibrium binding studies (Table 1). Both the S137A and S137A/A384S variants were able to form SDS stable complexes with thrombin and factor Xa, and in the presence of excess protease the entire native band disappeared, indicating the absence of a significant fraction of unreactive material (Figure 2). For reactions conducted in the absence of heparin, most of the antithrombin was found in reduced mobility bands, indicating stoichiometric inhibition of the proteases. Similarly, in the presence of the heparin pentasaccharide, most of the antithrombin complexed factor Xa and thrombin. However, in the presence of full-length heparin, most of the antithrombin Cambridge II is cleaved, while the control antithrombins are still predominantly in complex with protease (Figure 2). These findings indicate an increase in the substrate behavior of antithrombin Cambridge II and suggest the dependence of substrate behavior on the presence of a bridging glycosaminoglycan.
Rates and stoichiometries of inhibition in the absence and presence of heparin
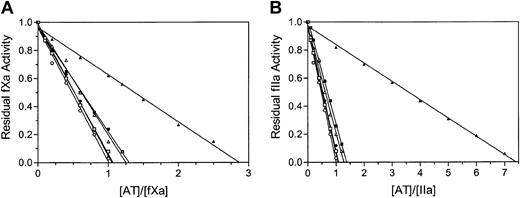
The rates of inhibition of factor Xa and thrombin in the absence and in the presence of heparin were determined (Table 2). The basal and pentasaccharide-activated rates of factor Xa and thrombin inhibition by antithrombin Cambridge II were similar to those of the recombinant and plasma controls, however, in the presence of full-length heparin, the second-order rate constants were consistently lower for antithrombin Cambridge II when compared with the controls. In order to determine if the apparent differences in rates of inhibition were caused by a change in the fraction of antithrombin Cambridge II acting as a substrate, stoichiometries of inhibition were determined for the reactions (Table 2). Antithrombin Cambridge II reacted with stoichiometries comparable to those of the controls in the absence of full-length heparin and were close to one in all cases. In the presence of full-length heparin, however, the stoichiometries determined for the Cambridge II variant were increased to 2.8 and 7.3 for factor Xa and thrombin, respectively (Figure 3 and Table 2). Rates and stoichiometries of inhibition of bovine factor Xa also were determined and were similar to those of human factor Xa inhibition (data not shown). These results demonstrate a heparin-dependent perturbation of the inhibitory mechanism of antithrombin Cambridge II, with the inhibitor in the presence of full-length heparin acting as a substrate for its target proteases. The substrate behavior of antithrombin Cambridge II is thus dependent on the presence of full-length heparin. Although the observed increase in stoichiometries of factor Xa and thrombin inhibition are partially responsible for the lower apparent rates of inhibition, there is still an approximate 3-fold and 10-fold slower rate of inhibition of factor Xa and thrombin by antithrombin Cambridge II in the presence of full-length heparin.
Stoichiometries of factor Xa and thrombin inhibition determined from residual protease activities with increasing antithrombin concentrations. The stoichiometries of inhibition of factor Xa (A) and thrombin (B) are taken as the x-intercept of the linear regression when residual protease activity is plotted against the ratio of antithrombin to protease. A value of one is normal for serpins and is found for the plasma alpha antithrombin (circles), recombinant beta antithrombin (squares), and recombinant antithrombin Cambridge II (triangles) in the absence of full-length heparin (open symbols). For all antithrombins there is an increase in stoichiometry in the presence of heparin (closed symbols), but this is exaggerated for the Cambridge II variant, which is predominantly a substrate of both factor Xa and thrombin.
Stoichiometries of factor Xa and thrombin inhibition determined from residual protease activities with increasing antithrombin concentrations. The stoichiometries of inhibition of factor Xa (A) and thrombin (B) are taken as the x-intercept of the linear regression when residual protease activity is plotted against the ratio of antithrombin to protease. A value of one is normal for serpins and is found for the plasma alpha antithrombin (circles), recombinant beta antithrombin (squares), and recombinant antithrombin Cambridge II (triangles) in the absence of full-length heparin (open symbols). For all antithrombins there is an increase in stoichiometry in the presence of heparin (closed symbols), but this is exaggerated for the Cambridge II variant, which is predominantly a substrate of both factor Xa and thrombin.
Effect of heparin affinity and bridging on stochiometries of inhibition
It has previously been shown that antithrombin behaves as a substrate for thrombin in the presence of high-affinity heparin under conditions of low ionic strength.33 The explanation at the time was that reducing the ionic strength increased the affinity of heparin for antithrombin, which slowed the conformational rearrangement required for inhibition. This explanation makes more sense now that we know that RCL expulsion is the result of heparin binding, but that RCL insertion is required for inhibition. To test the hypothesis that the affinity of antithrombin for heparin affects the rate of loop insertion, and thus the stoichiometry of inhibition, we evaluated the stoichiometries of inhibition for antithrombin in the presence of the high-affinity pentasaccharide at low ionic strength. The high-affinity pentasaccharide (HAP) binds to antithrombin approximately 100-fold more tightly than the physiological pentasaccharide (Penta)34,35 and normally a decrease in ionic strength from 0.15 to 0.01 would be associated with a 10 000-fold increase in heparin affinity.36 Thus, for our recombinant β-isoforms we would expect a Kd in the range of approximately 1 pM for the high-affinity pentasaccharide at I = 0.01. Contrary to expectation, the stoichiometries of inhibition were independent of the high-affinity pentasaccharide and ionic strength, and thus independent of strength of heparin binding to antithrombin (Table 3).
To directly test the effect of bridging on the substrate behavior of antithrombin Cambridge II toward thrombin, stoichiometric titrations were carried out in the presence of a synthetic bridging oligosaccharide containing an antithrombin binding sequence, a nonsulphated spacer, and a thrombin binding sequence.32 The resulting stoichiometries were comparable to those determined in the presence of full-length heparin (Table 2), supporting the importance of bridging for the substrate behavior of antithrombin Cambridge II.
Effect of thrombin allostery on stoichiometries of inhibition
Bridging is dependent on thrombin binding to the same heparin chain as antithrombin. The colocalization of thrombin and antithrombin on heparin is primarily responsible for the improved rate of reaction.13 In addition to colocalization is the allosteric effect on antithrombin and thrombin. The change in antithrombin conformation increases the rate of thrombin inhibition by approximately 2-fold, and heparin binding to thrombin also has been associated with an increase in catalytic activity through an allosteric mechanism.37 Since the stoichiometry of inhibition is dependent on the relative rates of RCL insertion and deacylation, it is likely that either one or both are affected through the binding of heparin. In order to distinguish between the increase in rate of deacylation due to thrombin allostery and the decrease in rate of thrombin translocation due to bridging, stoichiometries were determined after preincubating thrombin with small heparin fragments.
Preincubation of thrombin with 4- or 14-unit heparin chains, and then reacting this mix with antithrombin, alone or prebound to HAP, resulted in higher stoichiometries of inhibition (Table 3). The stoichiometries measured under these conditions were higher than those measured with HAP alone and without prebinding heparin to the thrombin. This demonstrates that there is a heparin-induced allosteric effect on thrombin that contributes to the increased stoichiometries measured in the presence of heparin. Bridging by the 14-mer heparin was ruled out since 14 saccharide units is insufficient to bridge thrombin and antithrombin, and since only a 2.4-fold increase in the rate of the antithrombin inhibition of thrombin was observed in the presence of the 14mer (data not shown). Furthermore, when antithrombin is prebound to HAP, binding of the 14-mer heparin to antithrombin is effectively blocked. The stoichiometries were, however, lower than those measured at the same ionic strength for antithrombin bound to full-length heparin (Table 2), demonstrating the need for bridging to obtain the full increase in stoichiometry of inhibition.
The effect of ionic strength on the stoichiometry of thrombin inhibition
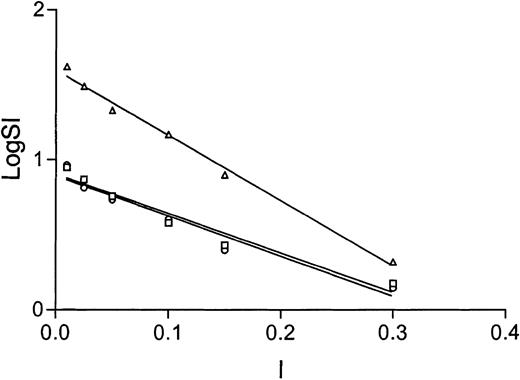
As mentioned above, the effect of ionic strength on the stoichiometry of thrombin inhibition by antithrombin in the presence of heparin has previously been reported. However, only 2 ionic strengths were evaluated, and the heparin concentration was fixed. We evaluated the effect of ionic strength on the stoichiometry of thrombin inhibition by antithrombin at 6 ionic strengths ranging from 0.01 to 0.3, for antithrombin Cambridge II, and the controls. We find that the effect is linear on a semilog plot (Figure 4), with a y-intercept corresponding to the log of the maximal SI and an x-intercept corresponding to the ionic strength where the SI would be one. Not surprisingly, the y-intercepts differ, yielding maximal SIs of 7, 8, and 40 for alpha, recombinant beta, and Cambridge II, respectively, however, nearly identical values for the x-intercept were found (0.33, 0.34, and 0.37).
The substrate behavior of antithrombin toward thrombin in the presence of full-length heparin is dependent on ionic strength. The linear fit of the semilog plot of stoichiometry of thrombin inhibition versus ionic strength demonstrates nearly identical x-intercepts for alpha (circles), recombinant beta (squares), and Cambridge II (triangles) antithrombins. The x-intercept corresponds to the ionic strength where the stoichiometry of inhibition will be one and suggests full inhibitory activity for Cambridge II if thrombin is not bound to heparin.
The substrate behavior of antithrombin toward thrombin in the presence of full-length heparin is dependent on ionic strength. The linear fit of the semilog plot of stoichiometry of thrombin inhibition versus ionic strength demonstrates nearly identical x-intercepts for alpha (circles), recombinant beta (squares), and Cambridge II (triangles) antithrombins. The x-intercept corresponds to the ionic strength where the stoichiometry of inhibition will be one and suggests full inhibitory activity for Cambridge II if thrombin is not bound to heparin.
Discussion
The finding of an increased stoichiometry of protease inhibition for antithrombin Cambridge II in the presence of full-length heparin is consistent with previous findings of immunological and functional assays on plasma samples from heterozygous carriers of the Cambridge II mutation. Such assays showed normal antigen levels and normal progressive antithrombin activities, indicating normal expression and function in the absence of heparin, but a consistent reduction of variable magnitude for antithrombin cofactor activities against both thrombin and factor Xa.23,24 Here we show that the conservative substitution of an alanine with a serine at position P10 in the RCL of antithrombin is sufficient to tip the balance from inhibitory to substrate behavior in the presence of heparin. It previously has been shown that antithrombin can be converted into a substrate of thrombin in the presence of heparin under low ionic strength conditions.33 The long-held explanation for this behavior was that the conformational rearrangement leading to the final antithrombin-thrombin complex was inhibited by the attachment of heparin (Figure 1). As heparin affinity for antithrombin increases with decreasing ionic strength, it follows that the rate of the conformational change (RCL incorporation into β-sheet A) would slow accordingly, resulting in higher stoichiometries of inhibition. This explanation is supported by the fact that RCL insertion reduces the affinity for heparin through a reversal of the induced fit binding mechanism.38 Thus, binding of heparin by antithrombin is strengthened 1000-fold by the expulsion of the hinge region during binding and is weakened 1000-fold by the reinsertion of the RCL upon formation of the final inhibitory complex (Figure 1). Although the rate of RCL insertion, and thus stoichiometry of inhibition, is probably slowed through the binding of heparin, our findings suggest that a contributing factor to the previously observed heparin-dependent substrate behavior of antithrombin is the interaction between thrombin and heparin. Thrombin activity is augmented by cofactor binding through increases in both acylation and deacylation rates. Thus, improvements in heparin binding by decreasing the ionic strength may increase the rate of deacylation and so increase the likelihood of dissociation of active thrombin before full translocation to the opposite pole of antithrombin can occur. Although heparin affinity for antithrombin is also affected by ionic strength, only a weak dependence of stoichiometry of thrombin inhibition on ionic strength is observed even when the high-affinity pentasaccharide is used. The x-intercept of the semilog plot of inhibition stoichiometry versus ionic strength for antithrombin and thrombin in the presence of full-length heparin corresponds to the ionic strength where the stoichiometry of inhibition will be one. For plasma alpha antithrombin, recombinant beta antithrombin, and for the Cambridge II variant the x-intercept is approximately 0.34. Since the dissociation constant for the binding of heparin by antithrombin at this ionic strength is still in the nanomolar range and alpha and beta glycoforms differ significantly in affinity for heparin, it is safe to conclude that this value does not correspond to the ionic strength at which antithrombin no longer binds efficiently to heparin. Rather, I = 0.34 is close to where thrombin elutes off heparin-sepharose39 and thus most likely corresponds to the ionic strength where thrombin no longer interacts with heparin. Consistent with this result is the observation that the related serpin, heparin cofactor II (L444R variant), inhibits thrombin with a high stoichiometry in the presence of heparin, but not dermatan sulphate.40 This can best be interpreted as an allosteric effect of heparin binding to thrombin since thrombin does not bind to dermatan sulfate with appreciable affinity.
We hypothesize that the effect of low ionic strength on the stoichiomentry of inhibition of thrombin by antithrombin in the presence of full-length heparin is the result of allostery: (1) the allosteric activation of antithrombin stabilizes the fully loop-expelled form, thus slowing the reincorporation of the RCL required for protease inhibition; (2) the allosteric activation of thrombin by heparin accelerates the rate of deacylation favoring release of the protease before full loop insertion is achieved; and (3) the stabilization of thrombin due to its interaction with heparin slows the final step of loop insertion by resisting the conformational destruction of thrombin required for full inhibition by antithrombin.
Antithrombin Cambridge II is also a substrate for factor Xa in the presence of full-length heparin, but less so than for thrombin. The relatively high factor Xa inhibitory activity of antithrombin Cambridge II suggests that screening patient plasma for anti-factor Xa activity, as opposed to antithrombin activity, would be more likely to missclassify the Cambridge II heterozygote as normal, as proposed previously.24 Somewhat paradoxically, the substrate behavior of Cambridge II toward factor Xa is not dependent on factor Xa binding to heparin, since neither the addition of CaCl2 or EDTA affected the stoichiometry of inhibition (data not shown). Although the explanation based on the substrate behavior of antithrombin to thrombin does not appear to apply to factor Xa, the effect of the Cambridge II mutation is consistent, since in a finely balanced system even a small perturbation can have great consequences. The consequence of the Cambridge II mutation is a normal circulating anticoagulant activity but a reduced activity in the microvasculature at the endothelial cell surface where antithrombin is bound to heparan sulfate.
The balance between substrate and inhibitor is thus precarious for antithrombin in the presence of heparin. In this context it is less surprising that a conservative Ala-to-Ser mutation at the P10 position in the RCL could tip the balance. The position of the mutation in the hinge region of the RCL will predictably slow the initial step of loop insertion because the serine side chain has to fit into the buried pocket evolved to fit an alanine side chain. Close evaluation of the structure of latent antithrombin, in which the RCL is fully inserted, reveals that the extra oxygen atom at position P10 cannot be accommodated without a buckling of the main chain. The serine would thus slow loop insertion up to P10 due to steric effects, but additionally it would also slow loop insertion beyond P10 due to the buckling of the strand.
The Cambridge II mutation is common in the British population, however, the reason for its high frequency is not known. A possible evolutionary advantage must exist to explain its perpetuation from a few founders24 to such high frequencies. Since the inhibitory mechanism of antithrombin Cambridge II is perturbed only in the presence of a bridging glycosaminoglycan, it follows that vascular localization of a proportion of the mutant protein in heterozygotes would render the carrier slightly thrombophilic. We propose that the mild prothombotic property of the Cambridge II variant, localized specifically to the microvasculature, may have provided a selective advantage in controlling intra/postpartum bleeding, or in embryonic implantation, as has been proposed for Factor V Leiden,41,42 thus allowing the propagation of this polymorphism. From a therapeutic standpoint, it also follows that administration of unfractionated heparin would be less effective in carriers of the Cambridge II mutation and that low molecular weight heparin should be considered as an alternative.
Prepublished online as Blood First Edition Paper August 7, 2003; DOI 10.1182/blood-2003-05-1560.
Supported by The Tesni-Parry Fund (A.M.), the Wellcome Trust (R.W.C.), and the United Kingdom Medical Research Council (MRC) and the National Institutes of Health (HL68629) (J.A.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to Professor John Gallagher for providing the size-fractionated heparins and to Maurice Petitou for providing the synthetic heparins.