Abstract
Mesenchymal stem cells (MSCs) are largely studied for their potential clinical use. Recently, they have gained further interest after demonstration of an immunosuppressive role. In this study, we investigated whether in vivo injection of MSCs could display side effects related to systemic immunosuppression favoring tumor growth. We first showed in vitro that the murine C3H10T1/2 (C3) MSC line and primary MSCs exhibit immunosuppressive properties in mixed lymphocyte reaction. We demonstrated that this effect is mediated by soluble factors, secreted only on “activation” of MSCs in the presence of splenocytes. Moreover, the immunosuppression is mediated by CD8+ regulatory cells responsible for the inhibition of allogeneic lymphocyte proliferation. We then demonstrated that the C3 MSCs expressing the human bone morphogenetic protein 2 (hBMP-2) differentiation factor were not rejected when implanted in various allogeneic immunocompetent mice and were still able to differentiate into bone. Importantly, using a murine melanoma tumor model, we showed that the subcutaneous injection of B16 melanoma cells led to tumor growth in allogeneic recipients only when MSCs were coinjected. Although the potential side effects of immunosuppression induced by MSCs have to be considered in further clinical studies, the usefulness of MSCs for various therapeutic applications still remains of great interest. (Blood. 2003;102:3837-3844)
Introduction
Mesenchymal stem cells (MSCs) are able to differentiate along multiple lineages such as chondrocytes, osteocytes, adipocytes, myocytes, and astrocytes.1 These cells, residents in the bone marrow, can be separated from hematopoietic cells by their adherence characteristics and expanded more than 104-fold in culture without loss of their multilineage differentiation potential.2 Phenotypically, MSCs are identified by the absence of the CD34 and CD45 hematopoietic cell markers and are positive for Thy-1 (CD90), endoglin (CD105), vascular cell adhesion molecule-1 (VCAM-1/CD106), SH2, and SH3 (for a review, see Noël et al3 ). MSCs express the major histocompatibility complex (MHC) class I but do not express MHC class II, B7-1, B7-2, CD40, or CD40L molecules. Furthermore, these stem cells secrete a number of cytokines and regulatory molecules that play important roles in the proliferation and maturation of hematopoietic stem cells.
A potential clinical application of MSCs is their use for enhancing hematopoietic stem cell engraftment. First, MSCs are capable of homing to the bone marrow and survive in the long term (> 1 year).4 Second, due to their particular function in the marrow microenvironment, it has been speculated that MSCs could improve allogeneic hematopoietic stem cell transplantation by replacing the damaged marrow stroma after myeloablative chemotherapy. Although several studies in animal models clearly showed that stromal cells could help in the hematopoietic recovery, controversial results have been found in humans. Indeed, following conventional bone marrow transplantation, 2 studies suggested that the marrow stroma belonged to the host although the allogeneic hematopoietic engraftment was successfully achieved.5,6 In another study, extensively T-cell-depleted marrow or mobilized blood was used and a low percentage (17%) of mixed chimerism at the stromal cell level could be detected, suggesting a limited but effective capacity of reconstituting the bone marrow microenvironment.7 It is likely that, along with the different sensitivity of the cell detection methods used, the low number of MSCs present in conventional allogeneic transplantations did not allow competition with residual host stroma and probably explains the low efficacy of stroma reconstitution.
It has been previously shown that MSCs could be used as precursor cells for tissue reconstitution. Indeed, infusion of MSCs into irradiated mice led to engraftment of cells not only into marrow but also various nonhematopoietic tissues, such as bone, cartilage, lung, and, less frequently, spleen, brain, and skin.8,9 Demonstration of engraftment of MSCs and their further differentiation into osteoblasts, competent for bone matrix production, was clearly demonstrated in mice.10 Furthermore, the therapeutic application of this approach has been tested in children with osteogenesis imperfecta. In this case, osteoblast engraftment (1.5%-2% donor cells) was detected after MSC transplantation and improvements were recorded in bone mineral content, growth velocity, and reduction of bone fracture frequencies.11
Recently, human and baboon MSCs have been shown to display immunosuppressive properties on T-lymphocyte proliferation induced by allogeneic cells.12,13 This phenomenon was reported to be mediated by the production of cytokines, in particular hepatocyte growth factor (HGF) and transforming growth factor-β1 (TGF-β1), and was not due to induction of apoptosis.13 In complement to the in vitro studies, in vivo intravenous administration of MSCs led to a modest but significant prolongation of skin graft survival with an efficacy comparable to the immunosuppressive agents currently being used clinically.12
The aim of this study was to investigate whether MSCs that are largely studied for their potential clinical use could display side effects related to systemic immunosuppression favoring tumor growth. First, using a mixed lymphocyte reaction (MLR) assay, we showed that the murine C3H10T1/2 mesenchymal stem cell line (C3 MSCs) induced the hyporesponsiveness of alloreactive T lymphocytes through production of soluble factors. Second, the immunoregulatory properties of MSCs favor the development of tumors as evaluated in allogeneic immunocompetent mice after coimplantation of MSCs and B16 melanoma cells locally or at distant sites. These results point to the notion that MSCs are powerful tools for tissue engineering in various non-MHC-matched individuals but, under certain circumstances, might exhibit adverse effects favoring tumor growth.
Materials and methods
Animals
Different strains of mice were used according to their MHC antigen disparity: C3H/He (H-2k haplotype), BALB/c (H-2d haplotype), DBA/1 (H-2q haplotype), C57 BL/6 (H-2b haplotype), OF1 (outbred mice), FVB (H-2q haplotype), NOD (H-2g7 haplotype), SWISS (H-2q haplotype), B10-BR (H-2k haplotype), and KRN (H-2b haplotype). Mice were housed in our facilities and cared for according to the Laboratory Animal Care guidelines. Adult animals, aged 8 to 16 weeks, were used.
Cell culture
The murine C3H10T1/2 MSC line (C3 MSC) was grown in complete Dulbecco modified Eagle medium (DMEM; Sigma, l'Isle d'Abeau, France) supplemented with 10% fetal calf serum (FCS) (Hyclone, Perbio, Bezons, France), 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Cergy, France). The C9 MSC clone derives from the C3 MSCs and expresses the human bone morphogenetic protein 2 (hBMP-2) gene under control of the inducible promoter TetOff.14 C9 cells were cultured in complete DMEM in the presence of 1 μg/mL doxycycline (Sigma). B16 melanoma cells, kindly given by J. Papon (INSERM U484, Clermont-Ferrand, France), were cultured in complete DMEM. Human primary MSCs (hMSCs) were obtained from bone marrow aspirates and cultured at low density in minimum essential medium α (MEMα; Invitrogen) supplemented with 10% FCS, 2 mM glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 1 ng/mL basic fibroblast growth factor (bFGF; R&D Systems, Lille, France). Confluent hMSCs were passaged at least twice to ensure homogeneous cell populations depleted of monocytes and macrophages. Before use, hMSC populations were phenotyped by flow cytometry (cells were negative for CD34 and CD45 and positive for CD90, CD105, CD73, and CD44). Mouse primary MSCs (mMSCs) were obtained from bone marrow aspirates (P.T. et al, manuscript in preparation) and cultured in complete DMEM. Human peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood by centrifugation on a Ficoll-Hypaque cushion. Murine bone marrow-derived dendritic cells (BM-DCs) were propagated from DBA1 bone marrow progenitor cells for 7 days as described elsewhere.15 Maturation of BM-DCs was obtained by harvesting DCs at day 6 and culture for 24 hours in the presence of 2 μg/mL lipopolysaccharides (Sigma).
MLR
Splenocytes were isolated from mouse spleen by disaggregation into 10 mL RPMI 1640 medium (Invitrogen). Erythrocytes were lysed with NH4Cl 0.84% and subsequently washed 3 times in RPMI 1640. Cell count and viability were assessed by trypan blue dye exclusion. Stimulator splenocytes (107 cells/mL) were treated with 50 μg/mL mitomycin C (Sigma) at 37°C for 45 minutes, followed by 5 extensive washes with FCS-containing RPMI 1640 medium. Responder splenocytes from BALB/c mice and stimulator splenocytes from different strains of mice were resuspended in RPMI 1640 containing 10% FCS (Hyclone), 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen), 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and 5 × 10-5 M 2-mercaptoethanol (Invitrogen). Each responder and stimulator cell population was seeded in triplicate at a concentration of 105 cells/100 μL/well, in 96-well round-bottom plates (BD Biosciences, Le Pont de Claix, France). MSCs (105 cells unless otherwise mentioned) were added to the MLR to obtain a 300-μL final volume. In mitogen proliferative assays, responder splenocytes were incubated with 5 μg/mL concanavalin A (ConA; Sigma). In the experiments using transwell chambers, MLR conditions were identical except that splenocytes were physically separated from C3 MSCs by a high-density pore membrane (1-μm pore size; BD Biosciences). Conditioned supernatants of C3 MSCs cultured in the presence of splenocytes were recovered after a 4-day incubation period and submitted to filtration through a 0.22-μm filter. After 4 to 5 days of incubation, 1 μCi/well (0.037 MBq/well) 3H-thymidine was added overnight and thymidine incorporation was measured using a β-scintillation counter. The data are presented as the percent of the relative proliferative response (unless otherwise mentioned), corresponding to the mean counts per minute (cpm) of a responder-stimulator pair in the absence of MSCs and was attributed a 100% value. All experiments were performed at least 3 times.
Depletion of CD8+ cells
CD8+ cells were depleted from total mouse splenocytes by incubation with Dynabeads mouse CD8 according to the manufacturer's recommendations (Dynal, Oslo, Norway). Absence of CD8+ cells in recovered fractions was tested using a FACScan laser flow cytometry system (BD Biosciences) and estimated to be 1.42% ± 0.18% in the different experiments. Cell suspensions containing either CD8-depleted cells or CD8+ cells were then used in the MLR assays.
Allogeneic implantation of MSCs
MHC-mismatched recipient mice were injected with C9 MSCs, originating from the C3H murine strain (H-2k haplotype). Different strains of mice were used in this study: BALB/c, DBA/1, NOD, B10-BR mice. Animals (5/group) were anesthetized using 0.01 mL/g body weight of a solution containing 0.1% xylazine (Bayer, Leverkusen, Germany) and 10 mg ketamine/mL (Rhône Mérieux, Lyon, France). Then, 106 C9 MSCs were administered into either the intra-articular space of knee joints or the tibialis anterior muscle of mice. To allow in vivo MSC differentiation through secretion of BMP-2, mice were not given doxycycline. On day 11 or 60 following cell injection, mice were humanely killed and the injected limbs were submitted to radiographic and histologic analysis.
Coloration CM-DiI
Stock solution of the fluorescent cell-tracer CM-DiI (Molecular Probes, Eugene, OR) was reconstituted at a concentration of 1 μg/μL in dimethyl sulfoxide (DMSO). Cells were trypsinized, washed with phosphate-buffered saline (PBS), and resuspended at the concentration of 107 cells/10 μg CM-DiI in 5 mL PBS, prepared extemporarily. Cells were labeled by an incubation at 37°C for 5 minutes followed by 15 minutes at 4°C, in the dark. Unincorporated fluorescent dye was then removed by centrifugation at 300g for 5 minutes and 2 washes in PBS. Cells were resuspended in PBS and maintained at 4°C until injection.
Melanoma murine tumor model
Eight- to 12-week-old C3H mice were anesthetized as described (“Allogeneic implantation of MSCs”). B16 cells, C3 MSCs, and mMSCs were prepared either as single-cell type suspensions (5 × 105 cells in 100 μL PBS) or a mix of cells (5 × 105 B16 cells and 5 × 105 MSCs, unless otherwise mentioned, in 100 μL PBS). Subcutaneous administration of B16 cells (alone or mixed with MSCs) was always performed in the abdominal area. Injection of MSCs at distant sites of B16 implantation was performed either subcutaneously at a distance of at least 2 cm or systemically by intravenous injection. Mice were examined 3 times a week and tumor growth was evaluated by measuring the length and width of tumor mass. At the end of the experiment, animals were killed and tumors were recovered. Tumor masses were weighed and analyzed by histology.
Histology
Tumor samples were fixed in 4% formaldehyde solution for several days and then processed for routine histology. Paraffin-embedded tissue sections (5 μm) were rehydrated through a gradient of toluene and alcohol and either stained with hematoxylin-eosin-safranin O before examination by light microscopy or mounted in fluorescent mounting medium (Dako, Trappes, France) for red fluorescence visualization.
Results
Proliferation of alloreactive T cells is inhibited by MSCs in a dose-dependent manner
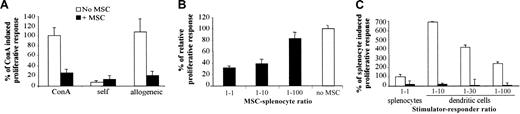
First, the immunologic properties of the murine mesenchymal progenitor cell line, C3 MSC, were tested in a proliferative assay using splenocytes of BALB/c mice as responding cells and ConA as mitogen. Addition of C3 MSCs inhibited the ConA-induced proliferative response of BALB/c splenocytes to a similar background level as that induced by autologous cells (Figure 1A). Using a MLR, C3 MSCs were also able to suppress the proliferation of responder BALB/c lymphocytes, elicited by allogeneic splenocytes of DBA1 mice (Figure 1A). Suppression of the proliferative response of allogeneic responder splenocytes occurred in a dose-dependent manner and was only partially reversed at the C3 MSC/responder cell ratio of 1:100, suggesting a potent effector mechanism (Figure 1B). We then tested the immunosuppressive effects of C3 MSCs on the proliferative response of BALB/c splenocytes when stimulated by mature BM-DCs. In these conditions, the proliferative activity of responding cells was much higher than that induced by allogeneic splenocytes and the C3 MSCs were still highly efficient in inhibiting the T-cell proliferation of lymphocytes (Figure 1C). Indeed, inhibition of T-cell proliferation induced by MSCs was observed whether the stimulation was triggered by a T-cell receptor (TCR)-independent mitogen, allogeneic cells, or professional antigen-presenting cells.
C3 MSCs inhibit an allogeneic proliferative response in a dose-dependent manner. Responding BALB/c splenocytes (105 cells) were incubated for 4 days with either 5 μg/mL ConA or mitomycin-treated DBA1 splenocytes (105) or various ratios of DCs, with or without C3 MSCs (105 cells or various ratios). (A) C3 MSCs inhibit the TCR-independent (ConA) and dependent (allogeneic) T-cell proliferative response. The proliferative response corresponding to the average cpm of triplicates of ConA-induced T-cell proliferation was assigned the value of 100% ± SD. (B) C3 MSCs induce a dose-dependent inhibition of allogeneic T-lymphocyte proliferation. Results are expressed as the percent of T-cell proliferation obtained in the absence of MSCs ± SD. (C) C3 MSCs repress T-cell proliferative activity induced by allogeneic professional antigen-presenting dendritic cells. The T-cell proliferation induced by allogeneic splenocytes was assigned the value of 100% ± SD.
C3 MSCs inhibit an allogeneic proliferative response in a dose-dependent manner. Responding BALB/c splenocytes (105 cells) were incubated for 4 days with either 5 μg/mL ConA or mitomycin-treated DBA1 splenocytes (105) or various ratios of DCs, with or without C3 MSCs (105 cells or various ratios). (A) C3 MSCs inhibit the TCR-independent (ConA) and dependent (allogeneic) T-cell proliferative response. The proliferative response corresponding to the average cpm of triplicates of ConA-induced T-cell proliferation was assigned the value of 100% ± SD. (B) C3 MSCs induce a dose-dependent inhibition of allogeneic T-lymphocyte proliferation. Results are expressed as the percent of T-cell proliferation obtained in the absence of MSCs ± SD. (C) C3 MSCs repress T-cell proliferative activity induced by allogeneic professional antigen-presenting dendritic cells. The T-cell proliferation induced by allogeneic splenocytes was assigned the value of 100% ± SD.
The immunosuppressive effect is effective regardless of the species of T cells or MSCs
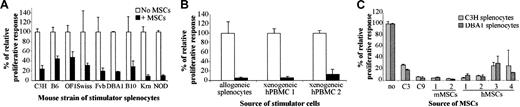
To investigate whether the immunosuppression mediated by C3 MSCs was associated with a particular mouse MHC haplotype, we used splenocytes obtained from various strains of mice. The BALB/c responder splenocytes were cultured with mitomycin-treated splenocytes originating from 9 different mouse strains in a MLR. In all cases, C3 MSCs clearly inhibited the T-lymphocyte proliferation between 52% to 91% (Figure 2A). Inhibition of the proliferative response of BALB/c splenocytes by C3 MSCs was also detected when xenogeneic human PBMCs (2 different sources) were used as stimulator cells (Figure 2B). Moreover, murine C3 MSCs could suppress the response of human responder T lymphocytes induced by ConA or allogeneic PBMCs, in the same range as that observed with murine T cells (data not shown).
Murine and human MSCs exhibit an immunosuppressive effect bypassing the species barrier. Responding BALB/c splenocytes (105 cells) were incubated for 4 days with 105 mitomycin-treated stimulator cells, in the presence or absence of MSCs (105 cells). The relative proliferative response corresponding to the mean cpm of a responder-stimulator pair in the absence of MSCs is attributed a 100% value ± SD. (A) C3 MSCs repress the proliferation of murine T lymphocytes independently of the haplotype of the stimulator splenocytes. Allogeneic stimulator splenocytes originated from 9 various mouse strains. (B) C3 MSCs inhibit murine T-cell proliferation induced by xenogeneic human peripheral blood mononuclear cells (hPBMCs). MSCs suppressed the proliferative response induced by 2 different sources of hPBMCs or allogeneic splenocytes. (C) MSCs from various species suppress the proliferative response to allogeneic stimulation. The response of murine T lymphocytes to allogeneic C3H and DBA1 splenocytes was inhibited in the presence of MSC lines (C3 and C9 cells), and primary mMSCs or hMSCs from 2 and 4 different samples, respectively.
Murine and human MSCs exhibit an immunosuppressive effect bypassing the species barrier. Responding BALB/c splenocytes (105 cells) were incubated for 4 days with 105 mitomycin-treated stimulator cells, in the presence or absence of MSCs (105 cells). The relative proliferative response corresponding to the mean cpm of a responder-stimulator pair in the absence of MSCs is attributed a 100% value ± SD. (A) C3 MSCs repress the proliferation of murine T lymphocytes independently of the haplotype of the stimulator splenocytes. Allogeneic stimulator splenocytes originated from 9 various mouse strains. (B) C3 MSCs inhibit murine T-cell proliferation induced by xenogeneic human peripheral blood mononuclear cells (hPBMCs). MSCs suppressed the proliferative response induced by 2 different sources of hPBMCs or allogeneic splenocytes. (C) MSCs from various species suppress the proliferative response to allogeneic stimulation. The response of murine T lymphocytes to allogeneic C3H and DBA1 splenocytes was inhibited in the presence of MSC lines (C3 and C9 cells), and primary mMSCs or hMSCs from 2 and 4 different samples, respectively.
We then tested whether the suppressive effects of mesenchymal progenitors were observed with various sources of MSCs. The genetically modified C9 MSCs (“Materials and methods”) and, more importantly, primary mMSCs (2 different origins) and primary hMSCs (4 different origins) were all able to inhibit the T-lymphocyte proliferation induced by allogeneic murine splenocytes (Figure 2C).
Altogether, these data demonstrated that the proliferation of T lymphocytes can be suppressed in an MHC-independent manner bypassing the species barrier, and the immunosuppressive effect observed was not restricted to the C3 MSCs but occurred with primary mMSCs and hMSCs.
The immunosuppressive properties of MSCs are mediated by an inducible soluble factor
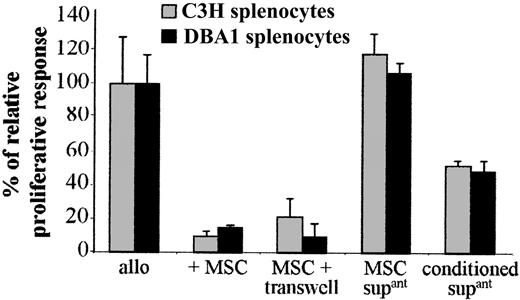
The possible involvement of a soluble mediator produced by C3 MSCs was tested using the transwell culture system. When responder and stimulator splenocytes were physically separated from C3 MSCs by the transwell membrane, the T-cell proliferation was inhibited to the same extent as that observed when MSCs were in direct contact with splenocytes (Figure 3). However, alloreactive splenocytes proliferated when incubated with supernatants from cultured C3 MSCs suggesting that “activation” of MSCs by the splenocytes was required for immunosuppression. To test this hypothesis, we used the conditioned supernatants obtained from MSCs cultured with murine splenocytes of MHC-mismatched pairs for 4 days and then passed through filter units of 0.22-μm pore size (Figure 3). In this case, the conditioned supernatant could suppress the proliferation of T cells in a second set of MLRs, although less efficiently than the coculture of the cell partners. These results strongly suggest that activation of MSCs by splenocytes was required for expression of any immunosuppressive factor.
A soluble factor secreted by MSCs on activation mediates the immunosuppressive effect. The proliferation of 105 BALB/c splenocytes triggered by 105 mitomycin-treated allogeneic C3H or DBA1 splenocytes (allo), in a 4-day MLR, was assigned a 100% value ± SD. The proliferative response was measured in the presence of 105 C3 MSCs, either in direct contact with splenocytes (+ MSC) or separated from splenocytes by a 1-μm transwell chamber (MSC + transwell). Supernatants from cultured C3 MSCs (MSC supant) or conditioned supernatants from C3 MSCs previously “activated” by a 4-day culture with allogeneic splenocytes (conditioned supant) were tested.
A soluble factor secreted by MSCs on activation mediates the immunosuppressive effect. The proliferation of 105 BALB/c splenocytes triggered by 105 mitomycin-treated allogeneic C3H or DBA1 splenocytes (allo), in a 4-day MLR, was assigned a 100% value ± SD. The proliferative response was measured in the presence of 105 C3 MSCs, either in direct contact with splenocytes (+ MSC) or separated from splenocytes by a 1-μm transwell chamber (MSC + transwell). Supernatants from cultured C3 MSCs (MSC supant) or conditioned supernatants from C3 MSCs previously “activated” by a 4-day culture with allogeneic splenocytes (conditioned supant) were tested.
Suppressive CD8+ cells are responsible for immunosuppression
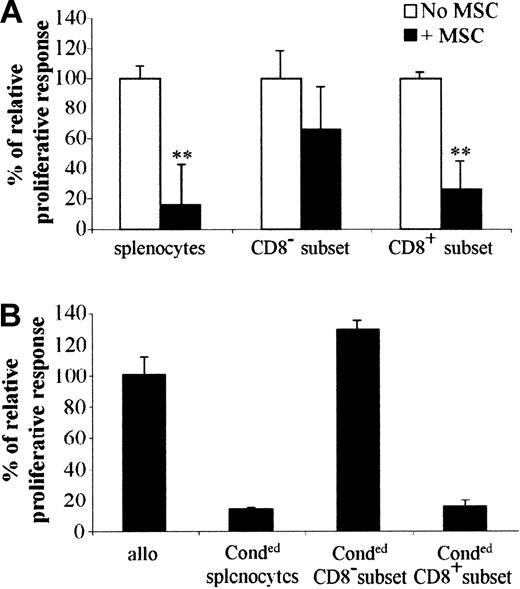
To investigate the mechanism involved in immunosuppression, we first determined whether a specific T-cell subset was inhibited to proliferate in the presence of MSCs. To address this question, splenocytes of BALB/c mice were incubated with anti-CD8-coupled magnetic beads and both CD8+ or CD8- subsets of cells were stimulated by allogeneic splenocytes. It is clearly shown that the proliferation of CD8+ cells was inhibited similarly to the total population of splenocytes when incubated with MSCs, whereas the CD8- subset was statistically not affected (Figure 4A). This result suggests that CD8+ cells were the main target cells that did not proliferate in the presence of MSCs within the entire population of splenocytes. In another experiment, we determined whether the inhibitory effect of MSCs was mediated by the emergence of regulatory T cells. To answer this question, we should be able to demonstrate that those regulatory T cells are able to induce tolerance in a secondary MLR using third-party splenocytes. We thus used splenocytes from BALB/c and DBA1 mice cocultured in MLRs in the presence of MSCs. After 4 days, these “conditioned” splenocytes were recovered and then depleted of CD8+ cells. The “conditioned” splenocytes, together with the CD8+ or CD8- subsets of “conditioned” cells, were used in a secondary MLR using proliferating splenocytes from C3H mice (Figure 4B). In these conditions, proliferation of C3H splenocytes was inhibited when the CD8+ subset of “conditioned” cells was added to the MLR, strongly suggesting that a population of regulatory CD8+ T cells was responsible for the immunosuppressive role induced by MSCs.
CD8+ regulatory cells are responsible for the immunosuppression induced by MSCs. (A) C3 MSCs inhibit the proliferation of the CD8+ subset in a direct MLR. BALB/c total splenocytes (splenocytes) were depleted of CD8+ cells (CD8- subset) using CD8+-coated beads (“Materials and methods”). Then 105 cells of both populations and the CD8+ splenocytes (CD8+ subset) were stimulated by 105 mitomycin-treated allogeneic splenocytes, with or without C3 MSCs. **P < .001. (B) The CD8+ subset suppresses the proliferative activity of murine lymphocytes stimulated by allogeneic splenocytes in a secondary MLR. BALB/c and DBA1 splenocytes were cultured in a primary MLR in the presence of C3 MSCs to obtain “conditioned” splenocytes. Then 105 total splenocytes (Conded splenocytes) or CD8+ (Conded CD8+ subset) or CD8- (Conded CD8- subset) cells were then used in a secondary MLR using 105 proliferative allogeneic splenocytes from C3H mice. Results are expressed as the percentage of the proliferative response ± SD.
CD8+ regulatory cells are responsible for the immunosuppression induced by MSCs. (A) C3 MSCs inhibit the proliferation of the CD8+ subset in a direct MLR. BALB/c total splenocytes (splenocytes) were depleted of CD8+ cells (CD8- subset) using CD8+-coated beads (“Materials and methods”). Then 105 cells of both populations and the CD8+ splenocytes (CD8+ subset) were stimulated by 105 mitomycin-treated allogeneic splenocytes, with or without C3 MSCs. **P < .001. (B) The CD8+ subset suppresses the proliferative activity of murine lymphocytes stimulated by allogeneic splenocytes in a secondary MLR. BALB/c and DBA1 splenocytes were cultured in a primary MLR in the presence of C3 MSCs to obtain “conditioned” splenocytes. Then 105 total splenocytes (Conded splenocytes) or CD8+ (Conded CD8+ subset) or CD8- (Conded CD8- subset) cells were then used in a secondary MLR using 105 proliferative allogeneic splenocytes from C3H mice. Results are expressed as the percentage of the proliferative response ± SD.
MSCs do not elicit any immune response when implanted into allogeneic recipients
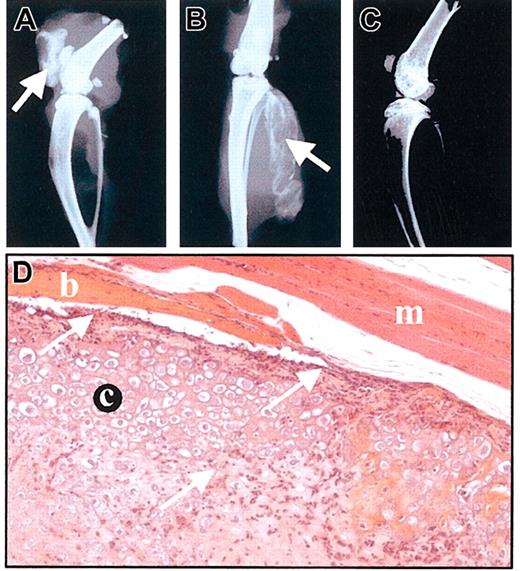
To assess the in vivo behavior of MSCs, we used the C9 MSC line that was shown to differentiate into bone after implantation in severe combined immunodeficiency (SCID) mice.14,16 C9 cells were implanted into the tibialis anterior muscles of 4 different immunocompetent murine strains (BALB/c, DBA1, NOD, and B10-BR) harboring different MHC haplotypes compared with C3H-derived C9 MSCs. We also tested the cell injection into the intra-articular space of knee joints of BALB/c mice. On day 11 after implantation, bone formation could be observed by x-ray in all the animals receiving C9 cells, whatever the site of injection (Figure 5A-B). On day 60, radiologic analysis showed that bone was still detected and histologic analysis of tissue sections revealed the presence of a hypertrophic cartilaginous matrix surrounded by bony tissue as previously observed.16 However, a lymphocytic infiltrate was present at the periphery of the neotissue and inside the cartilaginous matrix (Figure 5D). These results indicate that not only were MSCs not rejected by the immune system of the allogeneic animals but also they kept their potential to differentiate into bone.
MSCs are not rejected by allogeneic immunocompetent mice. (A-C) Radiologic analysis of mice injected with MSCs, on day 11. C9 MSCs (106 cells in PBS) were injected into BALB/c allogeneic mice either intra-articularly (A) or intramuscularly (B). Bone formation is indicated by arrows. A BALB/c mouse is shown as control (C). (D) Histologic analysis of muscle injected with C9 MSCs, on day 60. Hematoxylin-eosin-safranin O staining of injected muscle revealed presence of cartilage (c) and bone (b) inside the muscle (m). A lymphocytic infiltrate could be observed either lining or invading the neotissue (arrows). Original magnification, × 200.
MSCs are not rejected by allogeneic immunocompetent mice. (A-C) Radiologic analysis of mice injected with MSCs, on day 11. C9 MSCs (106 cells in PBS) were injected into BALB/c allogeneic mice either intra-articularly (A) or intramuscularly (B). Bone formation is indicated by arrows. A BALB/c mouse is shown as control (C). (D) Histologic analysis of muscle injected with C9 MSCs, on day 60. Hematoxylin-eosin-safranin O staining of injected muscle revealed presence of cartilage (c) and bone (b) inside the muscle (m). A lymphocytic infiltrate could be observed either lining or invading the neotissue (arrows). Original magnification, × 200.
The B16 melanoma cells are not rejected by allogeneic mice when coinjected with MSCs
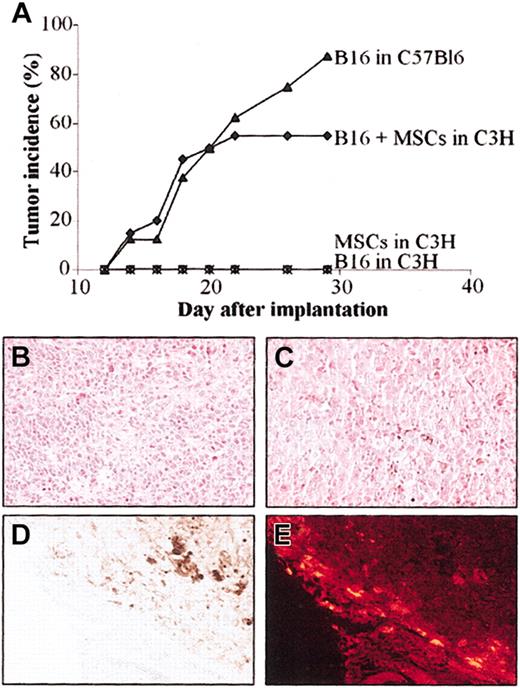
Because the B16 melanoma cells are poorly immunogenic, we implanted these tumor cells in allogeneic C3H mice, in the presence or absence of MSCs, to test the immunoregulatory properties of MSCs in vivo. The B16 melanoma cells, implanted subcutaneously in syngeneic C57BL/6 mice, rapidly develop tumors allowing reproducible and quantitative evaluation of tumor growth.17 In the first set of experiments, we injected subcutaneously a mix of 5 × 105 B16 cells and 5 × 105 C3 MSCs in MHC-mismatched C3H recipients. Tumor growth was compared to that of B16 cells implanted in syngeneic C57BL/6 animals. In both groups, tumor incidence was very similar during the first 22 days (Figure 6A). Tumor incidence increased to 90% on day 30 in the syngeneic group and remained stable at 57% in the allogeneic group. In control groups consisting of C3H animals receiving either the allogeneic B16 cells or the C3 MSCs alone, no tumor formation was observed.
Injection of MSCs induces tolerance of B16 melanoma cells in allogeneic mice. C3 MSCs (5 × 105) and 5 × 105 B16 melanoma cells (alone or as a mix) were injected subcutaneously into C3H mice and tumor development was monitored over time. (A) Tumor incidence was evaluated in the groups of mice either coinjected with B16 and MSCs in allogeneic C3H mice or injected with B16 melanoma cells in syngeneic C57Bl/6 mice. As negative controls, B16 cells or C3 MSCs alone were injected in C3H mice. (B-C) Hematoxylin-eosin staining of B16 melanoma tumor grown in allogeneic (B) or syngeneic (C) recipients. The brown pigment corresponding to the melanin produced by the melanocytes in both tumors is easily observed. (D-E) Distribution of CM-DiI-labeled C3 MSCs is shown by histology, at the periphery of the tumor in the stroma reaction where no melanocytes were observed (D) and by fluorescence microscopy as red fluorescent cells (E). Original magnification, × 200.
Injection of MSCs induces tolerance of B16 melanoma cells in allogeneic mice. C3 MSCs (5 × 105) and 5 × 105 B16 melanoma cells (alone or as a mix) were injected subcutaneously into C3H mice and tumor development was monitored over time. (A) Tumor incidence was evaluated in the groups of mice either coinjected with B16 and MSCs in allogeneic C3H mice or injected with B16 melanoma cells in syngeneic C57Bl/6 mice. As negative controls, B16 cells or C3 MSCs alone were injected in C3H mice. (B-C) Hematoxylin-eosin staining of B16 melanoma tumor grown in allogeneic (B) or syngeneic (C) recipients. The brown pigment corresponding to the melanin produced by the melanocytes in both tumors is easily observed. (D-E) Distribution of CM-DiI-labeled C3 MSCs is shown by histology, at the periphery of the tumor in the stroma reaction where no melanocytes were observed (D) and by fluorescence microscopy as red fluorescent cells (E). Original magnification, × 200.
Histologic analysis revealed similar anatomic-pathologic aspects in the 2 groups of mice developing tumors. Essentially, melanocytes were observed inside the tumor mass (Figure 6B-C) with some blood vessels. Brown staining, typical of the melanin pigment secreted by melanocytes, was also detected inside the cells. The CM-DiI-labeled C3 MSCs, coinjected with B16 cells, were easily observed in the sections as fluorescent red cells. They were localized mainly at the periphery of the tumor in the stroma reaction where no melanocytes were seen (Figure 6D-E). Thus, coinjection of MSCs with B16 cells permitted the proliferation and tumor growth of cells otherwise rejected.
Systemic immunosuppressive effect of MSCs in vivo
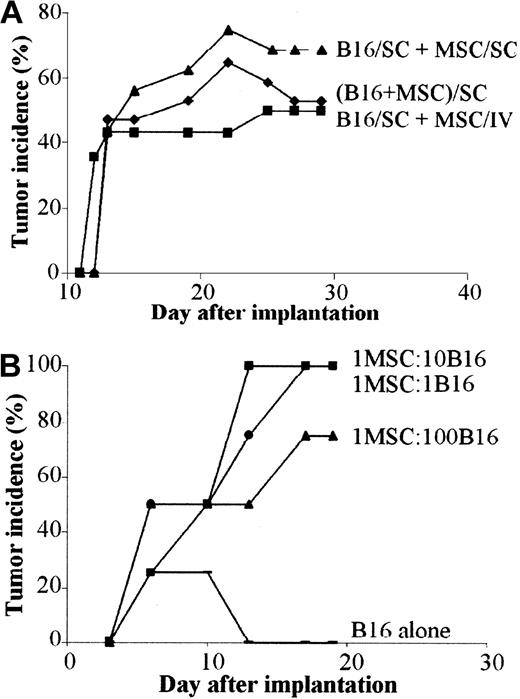
Because the immunosuppressive effect of MSCs is mediated, at least in a large part, by a soluble mediator, we wanted to investigate whether the immunosuppressive factor might be effective when secreted at sites distant from the tumor. To this aim, we implanted the B16 melanoma cells subcutaneously and C3 MSCs were injected either intravenously or subcutaneously apart from tumor cells. In both cases, tumor growth was observed with the same incidence and onset as the mix of B16 and C3 MSCs (Figure 7A). Tumor weights were very similar in the 3 groups (data not shown) and no labeled MSCs could be detected inside the tumor mass whatever the route of injection. Thus, as observed in vitro, direct contact between MSCs and B16 tumor cells was not necessary to promote tumorigenesis.
In vivo tolerance of B16 cells is induced by MSCs through a systemic and dose-independent immunosuppressive effect. (A) Tumor incidence of B16 melanoma cells in allogeneic mice is similar regardless of the route of MSC injection. B16 tumor cells (5 × 105) were injected subcutaneously and 5 × 105 C3 MSCs were injected either subcutaneously (SC) at a distant site (B16/SC + MSC/SC) or systemically in the tail vein of BALB/c mice (B16/SC + MSC/IV). As control, a mix of both cell types was injected subcutaneously (B16 + MSC/SC). (B) Incidence of B16 tumors is independent of the number of coinjected MSCs. C3 MSCs mixed with B16 tumor cells (5 × 105 cells) at various ratios (1:1; 1:10; 1:100, respectively) were implanted in subcutaneous locations and tumor growth was monitored at different time intervals.
In vivo tolerance of B16 cells is induced by MSCs through a systemic and dose-independent immunosuppressive effect. (A) Tumor incidence of B16 melanoma cells in allogeneic mice is similar regardless of the route of MSC injection. B16 tumor cells (5 × 105) were injected subcutaneously and 5 × 105 C3 MSCs were injected either subcutaneously (SC) at a distant site (B16/SC + MSC/SC) or systemically in the tail vein of BALB/c mice (B16/SC + MSC/IV). As control, a mix of both cell types was injected subcutaneously (B16 + MSC/SC). (B) Incidence of B16 tumors is independent of the number of coinjected MSCs. C3 MSCs mixed with B16 tumor cells (5 × 105 cells) at various ratios (1:1; 1:10; 1:100, respectively) were implanted in subcutaneous locations and tumor growth was monitored at different time intervals.
Because the immunosuppressive effect of MSCs was shown to be dose dependent in vitro, we tested whether the number of MSCs could play a role in tumor growth. We thus coimplanted various ratios of MSC/B16 melanoma cells subcutaneously in C3H mice. Similar onset and incidence were recorded for the first 10 days in the 3 groups of animals. Incidence reached 100% by day 19 for the highest MSC/B16 ratios and 75% for the lowest ratio (Figure 7B). Indeed, the ratio of 1 MSC for 100 B16 cells was still efficient to support tumor formation in allogeneic recipients.
Primary mMSCs favor tumor growth in vivo
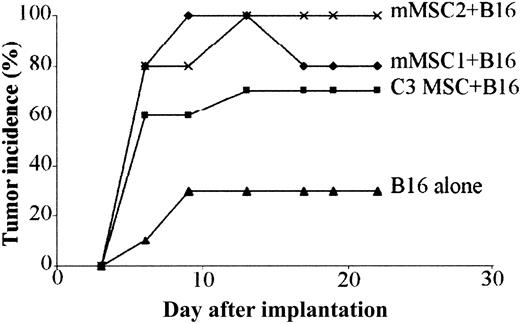
To investigate whether primary MSCs could display the same immunosuppressive properties as C3 MSCs in the melanoma allogeneic model, we subcutaneously coinjected B16 cells and 2 different populations of primary mMSCs. In both groups, onset and incidence of tumor formation were comparable and even higher than with mice injected with B16 cells and C3 MSCs (Figure 8). In this experiment, development of tumors was observed in the control group injected with the B16 allogeneic cells alone but with a low incidence, statistically highly significant (P < .0001 in Fisher exact test), as compared with the other experimental groups. These data obtained with primary cells further support the role of MSCs as immunosuppressive agents.
Primary mMSCs trigger tolerance of B16 melanomas. A mix of 5 × 105 B16 tumor cells and 5 × 105 mMSCs was implanted in subcutaneous sites of BALB/c mice and compared with the injection of B16 cells alone. C3 MSCs or 2 different sources of primary MSCs (mMSC1 and mMSC2) were used. The difference in tumor incidence between animals injected with B16 and MSCs and those receiving B16 alone was highly significant (P < .0001) using the Fisher exact test.
Primary mMSCs trigger tolerance of B16 melanomas. A mix of 5 × 105 B16 tumor cells and 5 × 105 mMSCs was implanted in subcutaneous sites of BALB/c mice and compared with the injection of B16 cells alone. C3 MSCs or 2 different sources of primary MSCs (mMSC1 and mMSC2) were used. The difference in tumor incidence between animals injected with B16 and MSCs and those receiving B16 alone was highly significant (P < .0001) using the Fisher exact test.
Discussion
The use of MSCs in regenerative medicine is of great interest and represents one major goal to achieve in the coming years. This interest resides in their ability to be easily isolated and expanded ex vivo and their multipotential differentiation capabilities together with their immunosuppressive properties. However, this last property could display adverse effects in certain circumstances such as the promotion of tumor growth. To investigate this hypothesis, we assessed the role of MSCs on the development of tumor by melanoma cells implanted in allogeneic mice.
In this paper, we explored in vitro the immunosuppressive mechanisms of MSCs and demonstrated that mMSCs suppress the proliferative activity of T lymphocytes when triggered by mitogen, allogeneic splenocytes, or professional antigen-presenting cells (DCs) in a dose-dependent manner. This study shows for the first time that: (1) inhibition of T-cell proliferation is not restricted by MHC because it occurred whatever was the species of MSCs or T cells, (2) suppression acts through a soluble factor that is secreted only after activation of MSCs by coculture with splenocytes, and (3) the immunosuppressive effect is mediated through the generation of CD8+ regulatory cells. Our data on the immunosuppressive role of mMSCs are in concordance with recent studies performed with human and nonhuman primate MSCs.12,13,18 Similar to those results, we showed that the inhibitory effect is mediated by a soluble factor and that T-cell suppression was comparable whether MSCs were in contact or separated from splenocytes by a transwell membrane. A very recent study reported contradictory data indicating that cell-to-cell contact was necessary for T-cell activation.19 This discrepancy may be explained by the fact that in their experiments, they used a 1:75 ratio of MSC/splenocytes versus 1:1 in our conditions. With a comparable cell ratio, 1:100, we showed only a slight inhibition (< 20%) of T-cell proliferation. Importantly, we demonstrated that the factor was secreted uniquely on MSC activation by splenocytes and could suppress a secondary MLR, further underlying the involvement of a soluble factor in the suppression of T-lymphocyte activation. The immunosuppressive HGF and TGF-β1 molecules have been proposed to be involved in this process using neutralizing monoclonal antibodies.13 However, we and others19 did not detect either of these molecules, or interleukin 10 (IL-10) or IL-4, in the MLR supernatants (data not shown). Although we could not exclude that an undetectable but still effective concentration of these factors might play a role in the immune suppression, it suggests that other factors, still to be identified, might be involved.
Inhibition of the T-lymphocyte proliferation occurred with both primary mMSCs that have been recently isolated and characterized (P.T., manuscript submitted) and the C3H10T1/2 MSC line that derives from C3H mouse embryos and is highly sensitive to postconfluence inhibition of cell growth.20 Importantly, no background of spontaneous transformation of these cells has been reported. Moreover, when these cells are injected into the knee joints of SCID mice, they form a mesenchymal-like tissue clearly detected after 2 months, but never form tumors (data not shown). Aside from its status of nontransformed cell line and its capability to differentiate into bone, cartilage, and muscle, the characteristic immune properties of this cell line further underline its similarity with primary MSCs and strengthen the relevance of its wide use both in vitro and in vivo.
The role of regulatory T cells in the control of immune responsiveness has been clearly identified in the prevention of allograft rejection, graft-versus-host disease, chronic inflammatory disease, and autoimmunity.21-23 Although most studies have focused on CD4+ regulatory T cells, CD8+ T cells with immunosuppressive properties have also been characterized.24 Our study highly suggests that such CD8+ T-regulatory cells are induced in the presence of MSCs and are at least in part responsible for the immunosuppressive activity observed in vitro, and maybe in vivo. This is further documented by a recent study that showed that CD4+/CD25+ T-suppressive cells were not required for the inhibitory activity mediated by MSCs.19 It should be mentioned that the possible involvement of CD8+ natural killer cells in the regulation of the immune response cannot be excluded at this time. The mechanism involved in the tolerance induced by CD8+ regulatory T cells is still unclear, although the role of soluble factors has been suggested. As an example, neonatal tolerance to mercuric salt-induced Th2 autoimmune disease was shown to be dependent on the generation of CD8+ Tc1-like regulatory cells producing interferon γ (IFN-γ) but neither IL-10 nor TGF-β.25 Whether such a mechanism may control the immunosuppression induced by MSCs has still to be demonstrated.
We also showed that mMSCs still display in vivo their immunosuppressive capacities because not only were BMP-2-expressing MSCs not rejected in allogeneic recipients but also they kept their potential to differentiate toward cartilage and bone. These genetically engineered MSCs were used because they can be easily and rapidly detected after implantation when they differentiate into bone.16 Moreover, because a cartilaginous matrix is formed only by day 5, potential recognition of MSCs by the host shortly after implantation might be sufficient to trigger an immune response. MSCs were not rejected either in the short term or at longer term. Indeed, 2 months after implantation, cartilaginous and bony tissues were still clearly detected. Although histologic analysis revealed the presence of an inflammatory infiltrate, localized principally at the periphery of the neotissue, this host reaction did not preclude MSC graft acceptance. Our study supports the relevance of recent allogeneic transplantation experiments such as a skin graft model12 or the MLR/lpr autoimmune mouse model of lupus26 and xenogeneic MSC transplantations, such those of hMSCs into the brain of rats27 or in utero in sheep.28 However, in these xenogeneic conditions, the graft tolerance could be partly explained by the fact that the targeted tissues are partially immune-privileged sites. Importantly, another study reported that after infusion into immunocompetent rats, mMSCs could be recruited from the bone marrow and participate in cardiac reconstitution after myocardial infarction.29 Altogether, these data demonstrate that in vivo, MSCs of various origins failed to trigger allogeneic and xenogeneic T-cell proliferation and induced immune tolerance in the host. Moreover, our work underlines the peculiar characteristics of the C3H10T1/2 cells, which can be used as universal donor cells for various transplantation studies in animal models.
The other important finding of our work is that the presence of MSCs may allow proliferation of allogeneic tumor cells otherwise rejected by immunocompetent recipients. This was obtained whether the mMSCs were primary cells or the C3H10T1/2 cell line. After coinjection of MSCs with melanoma cells, we observed that MSCs were essentially localized in the stroma surrounding the tumor. We previously showed that C3H10T1/2 MSCs secrete sustained levels of vascular endothelia growth factor (VEGF).16 So, in addition to their immunosuppressive role, MSCs may trigger angiogenesis through VEGF secretion and favor tumor development. Besides this, MSCs express the receptors for platelet-derived growth factor (PDGF), epidermal growth factor (EGF), insulin-like growth factor 1 (IGF-1), and nerve growth factor (NGF) and are dependent on growth factors for survival and proliferation.2 Those molecules secreted by the fibrous stroma at the tumor periphery might explain the preferential distribution of MSCs. Tumor development occurred whether MSCs were coinjected or injected systemically and subcutaneously at sites distant from tumor cells, with the same incidence and kinetics. Using a fluorescent tracer, we were unable to detect the labeled cells, when systemically injected, either in the stroma or inside the tumor, suggesting that acceptance of the tumor graft occurred through diffusion of the soluble factors and not homing of MSCs to the tumor. A recent study showed that MSCs, injected systemically, were able to target the tumor stroma in a melanoma lung tumor model.30 However, this occurred in 50% of the injected mice and only when the tumors were pre-established and repeated doses of MSCs were infused. It may be assumed that homing of MSCs into the tumor required both development of the neovascularization and secretion of various chemokines or growth factors, which were provided by the established tumor stroma. This has also been reported for inflammatory situations such as healing of bone fractures.31 In our situation, MSCs and melanoma cells were injected on the same day excluding the potential chemoattraction of MSCs by a well-organized tumor stroma. An alternative possibility is that the number of cells participating in the tumor stroma was too low to be detectable or MSCs lost their fluorescence intensity during in vivo cell division. Nevertheless, the immunosuppressive effect of MSCs was efficient enough to inhibit the host antitumor immune response because it was similar whatever the route of injection over time and occurred even at the ratio of 1 MSC to 100 melanoma cells. This effect and the fact that in some experiments a low incidence of tumor development occurred without the MSC counterpart might be related to the poorly immunogenic capacities reported for the B16 melanoma cell line.32 However, the incidence of melanoma was always statistically highly significant in mice coinjected with MSCs compared with basal allogeneic tumor acceptance.
In our model of allogeneic tumor cell implantation, the role of MSCs is comparable to their tolerigenic effect observed in an allogeneic skin graft model.12 Although implantation of the B16 melanoma cells does not obviously mimic the multiple events naturally occurring during in vivo autologous tumorigenesis, it points out the caution to the use of MSCs in the clinical setting. Whatever the therapeutic application, the injection of MSCs could suppress the patient's antitumor response. In allogeneic transplantations in which the graft-versus-tumor effect is expected, the presence of MSCs in the cell transplant might counteract the graft antitumor response. Furthermore, it has been clearly demonstrated that stromal cells in total bone marrow transplantation were responsible for the immunosuppressive effect in patients suffering from lupus and was shown, in that case, to be beneficial in treating the disease.26 Regardless of the mechanisms underlying the tolerigenic properties of MSCs, their role in cancer development, as well as in autoimmune diseases, remains unclear. This potential side effect has to be considered in further clinical trials and compared with the administration of various immunosuppressive agents currently used in therapeutic protocols such as total bone marrow transplantation. Indeed, the usefulness of MSCs for various therapeutic approaches of tissue engineering and hematopoiesis support still remains of great interest.
Prepublished online as Blood First Edition Paper, July 24, 2003; DOI 10.1182/blood-2003-04-1193.
D.N. and C.J. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Denis Greuet for excellent animal care, Michèle Radal (Centre de Recherches et de Lutte contre le Cancer, Val d'Aurelle, Montpellier, France) for histologic work, and the breast radiography group, headed by Prof Taourel (Lapeyronie Hospital, Montpellier, France), who performed the radiographies. We would like to thank J. Papon for providing the B16 cells, K. Tarte for providing human PBMCs, and D. Gazit for the kind gift of the C9 cells.