Abstract
We evaluated the feasibility and efficacy of a reduced-intensity conditioning (RIC) regimen of fludarabine and melphalan to achieve rapid complete donor chimerism after allogeneic stem cell transplantation (SCT) in patients with metastatic solid tumors. Between January 1999 and January 2003, 8 patients with metastatic breast cancer (BC) and 15 with metastatic renal cell carcinoma (RCC) underwent allogeneic SCT after an RIC regimen of 5 days of fludarabine and 2 days of melphalan. Filgrastim-mobilized stem cells from HLA-identical related or unrelated donors were infused. Prophylaxis for graft-versus-host disease (GVHD) consisted of tacrolimus and methotrexate. All 22 evaluable patients had 100% donor chimerism at day 30 and at all measurement times thereafter. One patient died 19 days after SCT. Nine patients (39%) had grades II to IV acute GVHD and 10 patients (43%) had chronic GVHD. Five patients (22%) died of nonrelapse treatment-related complications. Treatment-related disease response was seen in 10 patients (45%), with 3 complete responses, 2 partial responses, and 5 minor responses. Fludarabine-melphalan is a feasible and effective RIC regimen for allogeneic SCT in metastatic BC and RCC. It induces rapid complete donor chimerism without the need for donor lymphocyte infusion. Tumor regression associated with GVHD is consistent with graft-versus-tumor effect. (Blood. 2003;102:3829-3836)
Introduction
Allogeneic hematopoietic stem cell transplantation (SCT) is well established as an effective treatment for hematologic malignancies.1-3 Antimalignancy effects result from cytoreduction by the pretransplantation preparative regimen and the immune-mediated graft-versus-tumor (GVT) effect. High-dose preparative regimens have been considered myeloablative because hematopoietic recovery does not occur without the infusion of hematopoietic stem cells. For many hematologic malignancies, the immune-mediated GVT effect plays a major role in eradicating the disease.4-29 For example, relapse rates have been significantly higher after T-cell-depleted transplantations or syngeneic transplantations than after non-T-cell-depleted or allogeneic transplantations, indicating the importance of T cells and allogeneic antigenic targets in this process.30-32 The importance of this GVT effect led to the development of nonmyeloablative allogeneic stem cell transplantation (NST) procedures in which less intense conditioning regimens are used to achieve engraftment of donor cells, allowing development of a GVT effect.33-36 These nonmyeloablative regimens have lesser regimen-related toxicity and are associated with lower rates of treatment-related mortality.37,38
A GVT effect against some solid tumors has recently been documented. Allogeneic hematopoietic SCT was evaluated in preliminary studies for metastatic breast cancer (BC) by Eibl et al,39 Ben-Yosef et al,40 and Ueno et al41 in the mid 1990s. In our study,41 a myeloablative conditioning regimen (high-dose cyclophosphamide, carmustine, and triethylenethiophosphoramide) followed by an allogeneic peripheral blood progenitor cell (PBPC) transplantation led to engraftment and regression of residual metastatic liver lesions associated with graft-versus-host disease (GVHD) in 2 patients. However, the myeloablative regimen also had substantial treatment-related toxicity and mortality.
NST has been studied for metastatic renal cell carcinoma (RCC). In this setting, the preparative regimen is intended to produce engraftment of donor-derived hematopoietic and immune cells, allowing development of a GVT effect. Childs et al42,43 first reported the use of NST with a conditioning regimen of fludarabine and cyclophosphamide. Of 19 patients in that study, 10 showed regression of disease. This truly nonmyeloablative fludarabine-cyclophosphamide preparative regimen typically produces initial mixed chimerism and little cytoreduction of the malignancy; disease often progresses during the early posttransplantation period. In Childs' study,43 the responses generally occurred after development of complete donor chimerism, typically later in the posttransplantation period, after either withdrawal of immunosuppressive therapy or development of GVHD.
Most institutions evaluating NST for solid tumors have used fludarabine and cyclophosphamide-based regimens.44-48 Other regimens include fludarabine combined with low-dose total body irradiation49,50 or fludarabine with busulfan.51-53 We chose a reduced-intensity conditioning (RIC) regimen consisting of fludarabine and melphalan. Previous evaluations of this regimen in hematologic malignancies revealed that it reliably allowed durable engraftment of HLA-matched related and unrelated donor hematopoietic transplants, and it generally produced complete donor chimerism 30 days after the transplantation, with relatively mild toxicity.34,54-61 For the study reported here, we hypothesized that a reduced-intensity fludarabine-melphalan preparative regimen would produce rapid complete donor chimerism, which in turn would enhance the antitumor response, in patients with metastatic BC or metastatic RCC.
Patients and methods
Patient eligibility
Between January 1999 and January 2003, 8 patients with metastatic BC and 15 patients with metastatic RCC were enrolled in prospective clinical trials of fludarabine and melphalan with allogeneic SCT at the University of Texas M. D. Anderson Cancer Center (Houston). All patients provided written informed consent to participate. Two protocols, one for each disease, were reviewed and approved by the Institutional Review Board of M. D. Anderson Cancer Center. For the BC group, eligibility requirements included age 60 years or younger and histologic confirmation of metastatic or recurrent invasive BC that had responded either completely or partially to standard-dose chemotherapy. Patients with bone involvement were eligible if they had stable disease that showed clinical improvement. For the RCC group, eligibility requirements included age 70 years or younger and histologic confirmation of metastatic disease, excluding pure sarcomatoid and pure transitional cell carcinoma types; the disease could have responded or progressed in response to previous treatments before the transplantation. Other requirements for all patients included having a donor who was related and HLA identical for the A and B antigens, and DR allele, or unrelated and HLA identical for the A, B, and C antigens and DR and DQ alleles; a Zubrod performance status of 0 or 1; and adequate organ function (creatinine ≤ 176.8 μM [2.0 mg/dL], bilirubin ≤ 34 μM [2.0 mg/dL], serum alanine aminotransferase level no more than 3 times the upper limit of normal, left ventricular ejection fraction of at least 50%, and forced expiratory volume in 1 second and diffusion capacity of carbon monoxide of at least 50% of predicted values). Exclusion criteria included evidence of chronic active hepatitis or cirrhosis, active infection, HIV infection, a history of prior allogeneic transplantation, or brain metastasis.
Preparative regimen and stem cell infusion
The RIC regimen consisted of 5 days of fludarabine and 2 days of melphalan. For patients with BC, fludarabine was given intravenously at 30 mg/m2 daily from day -6 to day -2 (total dose 150 mg/m2), and melphalan was given intravenously at 70 mg/m2 daily from day -3 to day -2 (total dose 140 mg/m2). The regimen was the same for patients with RCC except that the dose of fludarabine was lower (25 mg/m2 daily; total dose 125 mg/m2) to lessen the chance of renal toxicity, because these patients had previously undergone nephrectomy. Donor PBPCs or bone marrow cells were infused on day 0, after premedication with 100 mg intravenous hydrocortisone and 25 mg intravenous diphenhydramine.
Stem cell collection
HLA-identical sibling donors were given subcutaneous granulocyte colony-stimulating factor (filgrastim) 6 μg/kg twice a day to mobilize PBPCs, which were collected by apheresis beginning on day 4 and repeated daily until at least 3 × 106 CD34+ cells/kg recipient body weight were collected. No positive selection or T-cell-depletion procedures were performed. The PBPCs were cryopreserved by programmed freezing in 5% dimethyl sulfoxide and thawed for infusion on the day of the transplantation. Bone marrow cells were used if donors were unrelated. For the marrow collection, at least 3 × 108 nucleated cells/kg recipient body weight were harvested under general anesthesia. Bone marrow cells were infused freshly on the day of the transplantation.
GVHD prophylaxis and supportive care
Tacrolimus and methotrexate were administered as immunosuppressive therapy after the transplantation procedure for the prevention of GVHD. Patients were given tacrolimus at a dose of 0.03 mg/kg/d as a continuous, 24-hour intravenous infusion from day -2 until they were able to take oral medications. At that time, the intravenous tacrolimus was discontinued and oral tacrolimus was given instead (0.12 mg/kg/d, given in 2 divided doses). The serum tacrolimus level was monitored regularly to maintain a blood level of 5 to 15 ng/mL. The tacrolimus was generally continued for at least 3 months after transplantation for patients without disease progression, after which the dose was tapered by 20% every week until discontinuation if GVHD did not occur. Methotrexate was administered intravenously at 5 mg/m2 on days 1, 3, and 6, and an additional dose was given on day 11 if the donors were unrelated. Patients who developed grade II or greater acute GVHD were given methylprednisolone 2 mg/kg/d in divided doses that were tapered as tolerated. Patients were also offered the option of participating in other protocols for the treatment of acute GVHD being conducted at M. D. Anderson.
All patients were given quinolones, fluconazole, and antiviral agents (acyclovir or valaciclovir) for infection prophylaxis during admission until their absolute neutrophil counts exceeded 0.5 × 109/L. The fluconazole and the antiviral agents were continued until day 100. Patients were monitored for cytomegalovirus antigenemia and were preemptively treated with ganciclovir if antigenemia was detected. Patients with active GVHD received prophylactic ganciclovir. Patients also were given anti-Pneumocystis carinii prophylaxis with trimethoprim/sulfamethoxazole, atovaquone, or aerosolized pentamidine for one year after the transplantation. Those with chronic GVHD received prophylactic antibiotics.
All patients were given 5 μg/kg subcutaneous filgrastim daily from day 0 until the absolute neutrophil count exceeded 1.5 × 109/L for 3 days. Irradiated and filtered blood products were given to maintain a hemoglobin level of more than 80 g/L (8 g/dL) and a platelet count of higher than 10 × 109/L.
Treatment of residual or recurrent disease
Patients with fulminant progressive disease or recurrent disease after day 30 or residual disease at day 100 were weaned from the tacrolimus if no signs of GVHD were present. If GVHD appeared, patients were given either topical corticosteroids or systemic methylprednisolone and observed for tumor response. If the tumor did not respond in 6 weeks and GVHD was not present, a donor lymphocyte infusion (DLI) was given consisting of 1 × 107 CD3+ cells/kg. If no response was seen over the next 6 weeks, a second DLI was administered at a dose of 5 × 107 CD3+ cells/kg. Similarly, if no response was seen after the next 6 weeks, a third DLI consisting of 1 × 108 CD3+ cells/kg was given. DLI was not given if symptomatic GVHD was present. Mononuclear cells were collected for DLI by leukapheresis without filgrastim priming from the same donors as those for the original transplant.
Posttransplantation evaluation, chimerism, and response
Toxicity was assessed according to the National Cancer Institute Common Toxicity Criteria version 2.0 (National Cancer Institute, Bethesda, MD). Hematopoietic chimerism was evaluated in bone marrow and peripheral blood samples on day 30, day 100, then every 3 months after transplantation by quantitative microsatellite polymorphism gene scanning using established techniques.62-64 For the bone marrow sample, only total nucleated cells were used for the determination. For the peripheral blood sample, T cells and myeloid cells were sorted out separately by the use of RosetteSep Lymphoid Cell Enrichment kit and RosetteSep Myeloid Cell Enrichment kit (Stem Cell Technologies, Vancouver, BC, Canada), respectively. Complete donor chimerism was defined as 100% DNA-donor cells. Any less than 100% DNA-donor cells was defined as donor mixed chimerism.
Acute and chronic GVHD were evaluated and scored according to standard criteria.65
Response to therapy was evaluated at 3-month intervals. Evaluations included physical examination, blood counts, complete blood chemistry, bone marrow aspiration and biopsy if the bone marrow was involved, chest x-ray, skeletal scintigraphy, and chest and abdominal/pelvic computed tomography. Tumor markers (carcinoembryonic antigen and CA 27-29) were measured in the patients with BC.
Complete response (CR) indicates disappearance of all disease and symptoms related to the tumor for more than 4 weeks; partial response (PR), more than 50% reduction in the sum of the products of the diameters of each measurable lesion for more than 4 weeks; minor response (MR), a reduction in measurable lesions that was too small to qualify as a partial response; stable disease (SD), no change in tumor size; and progressive disease (PD), the appearance of new lesions or a more than 25% increase in the sum of the products of diameters of any measurable lesions.
Statistical considerations
Survival was defined as the interval from the day of SCT until death or the end of the follow-up period, and it was estimated using the Kaplan-Meier product-limit method. All data were updated through April 30, 2003.
Results
Patient characteristics
Patient characteristics are summarized in Table 1. In the BC group, the median age of the 8 women was 44.5 years (range, 36-53 years) and the median follow-up was 470.5 days (range, 239-1119 days). Six patients had bone involvement. The median number of metastatic sites was 1 (range, 1-2). Seven patients had previously undergone adjuvant chemotherapy, and all but one (patient no. 6) had undergone at least one course of salvage chemotherapy before the transplantation. The median number of salvage chemotherapy regimens received was 2 (range, 1-4). All patients had previously received anthracycline or taxane-containing chemotherapy. Three patients had undergone palliative local radiation therapy. Two patients had previously undergone high-dose chemotherapy and autologous hematopoietic transplantation. All 8 patients received PBPCs from a genotypically HLA-identical sibling donor.
Patient characteristics
Patient no. . | Age, y . | Sex . | Tumor histology . | Estrogen receptor . | Metastatic sites . | Prior surgery . | Prior treatment . |
|---|---|---|---|---|---|---|---|
| BC group | |||||||
| 1 | 38 | F | Inv ductal | Positive | Regional nodes | Mastectomy | FAC, paclitaxel, ASCT, tamoxifen, anastrozole, XRT |
| 2 | 48 | F | Inv ductal | Positive | Bone | None | AC, docetaxel |
| 3 | 50 | F | Inv lobular | Negative | Breast, lymph nodes | Mastectomy | AC, paclitaxel, vinorelbine, capecitabine, trastuzumab, XRT |
| 4 | 42 | F | Inv ductal | Positive | Bone, bone marrow | Mastectomy | AC, paclitaxel, vinorelbine, trastuzumab |
| 5 | 36 | F | Inv ductal | Positive | Bone, bone marrow | Lumpectomy | AC, CMF, paclitaxel, vinorelbine, tamoxifen, trastuzumab, XRT |
| 6 | 53 | F | Inv lobular | Positive | Bone | Lumpectomy | AC, tamoxifen, anastrozole, XRT |
| 7 | 47 | F | Inv ductal | Positive | Bone | Mastectomy | FAC, CMF, docetaxel, anastrozole gemcitabine, tamoxifen, XRT, ASCT |
| 8 | 40 | F | Inv ductal | Negative | Bone, liver | Mastectomy | FAC, docetaxel, vinorelbine, trastuzumab, XRT |
| RCC group | |||||||
| 9 | 50 | M | Clear cell | Lung, liver, spleen, lymph nodes | Nephrectomy | None | |
| 10 | 54 | M | Granular cell | Lung, liver | Nephrectomy | IL-2, IFN-α, thalidomide | |
| 11 | 45 | M | Clear/granular cell | Lung, lymph nodes | Nephrectomy | IL2, IFN-α | |
| 12 | 52 | M | Clear cell | Renal fossa | Nephrectomy | XRT | |
| 13 | 57 | M | Clear cell | Pleura, lung, | Nephrectomy | IL-2, IFN-α | |
| 14 | 49 | F | Clear cell | Lymph nodes | Nephrectomy, parotidectomy | IL-2, XRT | |
| 15 | 45 | M | Clear cell | Liver, spleen, Lymph nodes | Nephrectomy, Liver resection | IL-2, 5-FU, gemcitabine, thalidomide | |
| 16 | 53 | M | Clear cell | Lung, renal fossa | Nephrectomy | IFN-α, 5-FU, gemcitabine, cis-retinoic acid | |
| 17 | 53 | M | Clear cell | Lung, bone | Nephrectomy | IL-2, XRT | |
| 18 | 64 | M | Clear cell | Pleura, bone, skin | Nephrectomy | IFN-α, 5-FU, gemcitabine, capecitabine | |
| 19 | 57 | M | Clear cell | Lung, bone | Nephrectomy, hemipelvectomy | IL-2, IFN-α, 5-FU, gemcitabine, thalidomide, XRT | |
| 20 | 53 | F | Clear cell | Lung, lymph nodes | Nephrectomy | IL-2 | |
| 21 | 19 | F | Papillary | Lung, liver, bone | Nephrectomy | cisplatin, gemcitabine, carboplatin, ifosfamide, IL-2, IFN-α, 5-FU, thalidomide, topotecan vinblastine, capecitabine, XRT | |
| 22 | 31 | F | Clear cell | Lung, lymph nodes | Nephrectomy | IL-2, IFN-α, capecitabine | |
| 23 | 48 | M | Granular | Bone | Nephrectomy, RPLND | XRT |
Patient no. . | Age, y . | Sex . | Tumor histology . | Estrogen receptor . | Metastatic sites . | Prior surgery . | Prior treatment . |
|---|---|---|---|---|---|---|---|
| BC group | |||||||
| 1 | 38 | F | Inv ductal | Positive | Regional nodes | Mastectomy | FAC, paclitaxel, ASCT, tamoxifen, anastrozole, XRT |
| 2 | 48 | F | Inv ductal | Positive | Bone | None | AC, docetaxel |
| 3 | 50 | F | Inv lobular | Negative | Breast, lymph nodes | Mastectomy | AC, paclitaxel, vinorelbine, capecitabine, trastuzumab, XRT |
| 4 | 42 | F | Inv ductal | Positive | Bone, bone marrow | Mastectomy | AC, paclitaxel, vinorelbine, trastuzumab |
| 5 | 36 | F | Inv ductal | Positive | Bone, bone marrow | Lumpectomy | AC, CMF, paclitaxel, vinorelbine, tamoxifen, trastuzumab, XRT |
| 6 | 53 | F | Inv lobular | Positive | Bone | Lumpectomy | AC, tamoxifen, anastrozole, XRT |
| 7 | 47 | F | Inv ductal | Positive | Bone | Mastectomy | FAC, CMF, docetaxel, anastrozole gemcitabine, tamoxifen, XRT, ASCT |
| 8 | 40 | F | Inv ductal | Negative | Bone, liver | Mastectomy | FAC, docetaxel, vinorelbine, trastuzumab, XRT |
| RCC group | |||||||
| 9 | 50 | M | Clear cell | Lung, liver, spleen, lymph nodes | Nephrectomy | None | |
| 10 | 54 | M | Granular cell | Lung, liver | Nephrectomy | IL-2, IFN-α, thalidomide | |
| 11 | 45 | M | Clear/granular cell | Lung, lymph nodes | Nephrectomy | IL2, IFN-α | |
| 12 | 52 | M | Clear cell | Renal fossa | Nephrectomy | XRT | |
| 13 | 57 | M | Clear cell | Pleura, lung, | Nephrectomy | IL-2, IFN-α | |
| 14 | 49 | F | Clear cell | Lymph nodes | Nephrectomy, parotidectomy | IL-2, XRT | |
| 15 | 45 | M | Clear cell | Liver, spleen, Lymph nodes | Nephrectomy, Liver resection | IL-2, 5-FU, gemcitabine, thalidomide | |
| 16 | 53 | M | Clear cell | Lung, renal fossa | Nephrectomy | IFN-α, 5-FU, gemcitabine, cis-retinoic acid | |
| 17 | 53 | M | Clear cell | Lung, bone | Nephrectomy | IL-2, XRT | |
| 18 | 64 | M | Clear cell | Pleura, bone, skin | Nephrectomy | IFN-α, 5-FU, gemcitabine, capecitabine | |
| 19 | 57 | M | Clear cell | Lung, bone | Nephrectomy, hemipelvectomy | IL-2, IFN-α, 5-FU, gemcitabine, thalidomide, XRT | |
| 20 | 53 | F | Clear cell | Lung, lymph nodes | Nephrectomy | IL-2 | |
| 21 | 19 | F | Papillary | Lung, liver, bone | Nephrectomy | cisplatin, gemcitabine, carboplatin, ifosfamide, IL-2, IFN-α, 5-FU, thalidomide, topotecan vinblastine, capecitabine, XRT | |
| 22 | 31 | F | Clear cell | Lung, lymph nodes | Nephrectomy | IL-2, IFN-α, capecitabine | |
| 23 | 48 | M | Granular | Bone | Nephrectomy, RPLND | XRT |
Inv indicates invasive; FAC, 5-fluorouracil (5-FU), doxorubicin, and cyclophosphamide; ASCT, autologous stem cell transplantation; XRT, radiation therapy; AC, doxorubicin cyclophosphamide; CMF, cyclophosphamide, methotrexate, and 5-fluorouracil; IL-2, interleukin-2; IFN-α, interferon-α; and RPLND, retroperitoneal lymph node dissection.
In the RCC group, the median age of the 11 men and 4 women was 52 years (range, 19-64 years) and the median follow-up was 229 days (range, 19-1015 days). The median number of metastatic sites was 2 (range, 1-4), with the most common site being the lung. All patients had undergone cytoreduction nephrectomy and 4 had had a metastasectomy. Twelve patients had undergone prior treatment with, and did not respond to, interferon-α or interleukin-2. Thirteen of the 15 patients with RCC received PBPCs from an HLA-identical sibling; one patient received marrow cells from an unrelated donor matched for HLA-A, -B, -C, -DQ and -DR and one patient received marrow cells from an unrelated donor with one mismatched at a single HLA-C locus.
Engraftment and donor chimerism
Twenty-two of the 23 patients achieved engraftment and hematopoietic recovery. Patient no. 18 (with RCC) died on day 19 after the transplantation, before hematologic recovery, and thus engraftment could not be evaluated. The median time to reach an absolute neutrophil count of 0.5 × 109/L was 12 days (range, 10-17 days), and the median time to achieve a platelet count higher than 50 × 109/L was 17.5 days (range, 10-75 days). All 22 evaluable patients showed 100% (complete) donor chimerism of T cells and myeloid cells at day 30, at day 100, and at subsequent evaluations after the transplantation. No DLI or change in immunosuppressive therapy was done to enhance donor engraftment.
Toxicity and infections
Fourteen patients experienced reversible grade III nonhematopoietic toxicity, including nausea, vomiting, pancreatitis, neutropenic fever, renal insufficiency, and headache, within the first year after the transplantation (Table 2). Two incidents of thrombotic thrombocytopenic purpura (TTP) occurred, one on day 152 in patient no. 14, which resolved after treatment with plasma exchange, and the other on day 533 in patient no. 11, who died of complications from TTP. The incidence of infection is also summarized in Table 2. Ten cases of cytomegalovirus antigenemia occurred, all of which resolved with preemptive antiviral treatment.
Toxicity and infection at 1 year after transplantation
Effect . | All patients, n (%) . | BCC group, n (%) . | RCC group, n (%) . |
|---|---|---|---|
| Grades III-IV toxicity | |||
| Cardiac | 3 (13) | 1 (13) | 2 (13) |
| Pulmonary | 3 (13) | 0 | 3 (20) |
| Gastrointestinal | 9 (39) | 3 (38) | 6 (40) |
| Renal | 3 (13) | 0 | 3 (20) |
| Neurologic | 3 (13) | 0 | 3 (20) |
| Metabolic | 2 (9) | 1 (13) | 1 (7) |
| Fever/flulike symptoms | 7 (30) | 3 (38) | 4 (27) |
| Coagulopathy | 1 (4) | 0 | 1 (7) |
| Infection | |||
| Bacterial | 4 (17) | 2 (25) | 2 (13) |
| Fungal | 3 (13) | 1 (13) | 2 (13) |
| Acid-fast bacillus | 1 (4) | 0 | 1 (7) |
| Cytomegalovirus | 10 (43) | 5 (63) | 5 (33) |
| Herpes simplex/herpes zoster virus | 1 (4) | 1 (13) | 0 |
| Other virus | 3 (13) | 1 (13) | 2 (13) |
Effect . | All patients, n (%) . | BCC group, n (%) . | RCC group, n (%) . |
|---|---|---|---|
| Grades III-IV toxicity | |||
| Cardiac | 3 (13) | 1 (13) | 2 (13) |
| Pulmonary | 3 (13) | 0 | 3 (20) |
| Gastrointestinal | 9 (39) | 3 (38) | 6 (40) |
| Renal | 3 (13) | 0 | 3 (20) |
| Neurologic | 3 (13) | 0 | 3 (20) |
| Metabolic | 2 (9) | 1 (13) | 1 (7) |
| Fever/flulike symptoms | 7 (30) | 3 (38) | 4 (27) |
| Coagulopathy | 1 (4) | 0 | 1 (7) |
| Infection | |||
| Bacterial | 4 (17) | 2 (25) | 2 (13) |
| Fungal | 3 (13) | 1 (13) | 2 (13) |
| Acid-fast bacillus | 1 (4) | 0 | 1 (7) |
| Cytomegalovirus | 10 (43) | 5 (63) | 5 (33) |
| Herpes simplex/herpes zoster virus | 1 (4) | 1 (13) | 0 |
| Other virus | 3 (13) | 1 (13) | 2 (13) |
Values in the table are presented for all patients (n = 23), the BC group (n = 8), and the RCC group (n = 15).
Nine patients (39%) developed grade II or higher acute GVHD; 8 responded to methylprednisolone therapy, but one patient with RCC (no. 18) had grade IV acute GVHD and died on day 19 after the transplantation. Ten patients (43%) developed chronic GVHD, which was controlled by further immunosuppressive therapy.
In all, 5 (22%) of the 23 patients died of nonrelapse treatment-related complications, patient no. 10 of an acute myocardial infarction on day 76 after the transplantation, patient no. 12 of acute respiratory distress syndrome on day 85, and patient no. 14 of complications of pneumonia on day 175 (Table 3). As noted previously, patient no. 18 died on day 19 of GVHD, and patient no. 11 died on day 590 of complications of TTP.
Clinical outcome
Patient no. . | Status at study entry . | Time and site of PD . | Site of grades II-IV acute GVHD . | Site of chronic GVHD . | No. of DLIs . | Overall best response . | Outcome . |
|---|---|---|---|---|---|---|---|
| BCC group | |||||||
| 1 | PR | —† | — | — | — | CR | Died in PD, d 245 |
| 2 | SD | — | — | Eye, mouth | — | CR | Alive in CR, d 1119 |
| 3 | PR | d 43; skin | Skin, GI | GI | — | SD | Died in PD, d 239 |
| 4 | SD | d 310; bone, liver | — | Skin, GI | — | SD | Died in PD, d 537 |
| 5 | SD | d 84; bone | GI | GI | — | SD | Alive in SD, d 633 |
| 6 | SD | d 59; bone | — | — | 3 | MR | Alive in SD. d 646 |
| 7 | SD | d 166; bone | — | Mouth | 2 | SD | Died in PD, d 404 |
| 8 | PR | — | — | Eye, mouth, liver | — | SD | Alive in SD, d 345 |
| RCC group | |||||||
| 9 | PD | d 160; liver | Skin, GI | Skin, eye | — | CR | Alive in CR, d 1015 |
| 10 | PD | — | Skin, GI | — | — | SD | Died in SD, d 76 (acute ML) |
| 11* | PD | d 370; lung | GI | Skin, mouth | — | PR/CR‡ | Died in CR, d 590 (TTP) |
| 12 | PD | d 27; renal fossa | — | — | — | SD | Died in SD, d 85 (ARDS) |
| 13 | PD | d 94; lymph nodes | — | — | 1 | PD | Died in PD, d 245 |
| 14 | PD | d 32; renal fossa | GI | — | — | MR | Died in SD, d 175 (pneumonia) |
| 15 | PD | d 153; abdominal mass | — | — | 2 | MR | Died in PD, d 374 |
| 16 | PD | d 50; renal fossa | — | — | 1 | PD | Died in PD, d 221 |
| 17 | PD | d 78; bone, lung | — | Skin | — | PD | Died in PD, d 262 |
| 18 | PD | — | Skin (IV) | — | — | ED | Died, d 19 (GVHD) |
| 19 | PD | d 176; lung, soft tissue mass | — | Skin | — | MR | Alive in SD, d 405 |
| 20 | PD | d 28; bone | — | — | 4 | MR | Alive in PD, d 319 |
| 21* | PD | d 29; lung, liver | Liver | — | — | SD | Died in PD d 123 |
| 22 | SD | — | — | — | — | PR | Alive in PR, d 229 |
| 23 | PR | — | Liver | — | — | CR§ | Alive in CR, d 97 |
Patient no. . | Status at study entry . | Time and site of PD . | Site of grades II-IV acute GVHD . | Site of chronic GVHD . | No. of DLIs . | Overall best response . | Outcome . |
|---|---|---|---|---|---|---|---|
| BCC group | |||||||
| 1 | PR | —† | — | — | — | CR | Died in PD, d 245 |
| 2 | SD | — | — | Eye, mouth | — | CR | Alive in CR, d 1119 |
| 3 | PR | d 43; skin | Skin, GI | GI | — | SD | Died in PD, d 239 |
| 4 | SD | d 310; bone, liver | — | Skin, GI | — | SD | Died in PD, d 537 |
| 5 | SD | d 84; bone | GI | GI | — | SD | Alive in SD, d 633 |
| 6 | SD | d 59; bone | — | — | 3 | MR | Alive in SD. d 646 |
| 7 | SD | d 166; bone | — | Mouth | 2 | SD | Died in PD, d 404 |
| 8 | PR | — | — | Eye, mouth, liver | — | SD | Alive in SD, d 345 |
| RCC group | |||||||
| 9 | PD | d 160; liver | Skin, GI | Skin, eye | — | CR | Alive in CR, d 1015 |
| 10 | PD | — | Skin, GI | — | — | SD | Died in SD, d 76 (acute ML) |
| 11* | PD | d 370; lung | GI | Skin, mouth | — | PR/CR‡ | Died in CR, d 590 (TTP) |
| 12 | PD | d 27; renal fossa | — | — | — | SD | Died in SD, d 85 (ARDS) |
| 13 | PD | d 94; lymph nodes | — | — | 1 | PD | Died in PD, d 245 |
| 14 | PD | d 32; renal fossa | GI | — | — | MR | Died in SD, d 175 (pneumonia) |
| 15 | PD | d 153; abdominal mass | — | — | 2 | MR | Died in PD, d 374 |
| 16 | PD | d 50; renal fossa | — | — | 1 | PD | Died in PD, d 221 |
| 17 | PD | d 78; bone, lung | — | Skin | — | PD | Died in PD, d 262 |
| 18 | PD | — | Skin (IV) | — | — | ED | Died, d 19 (GVHD) |
| 19 | PD | d 176; lung, soft tissue mass | — | Skin | — | MR | Alive in SD, d 405 |
| 20 | PD | d 28; bone | — | — | 4 | MR | Alive in PD, d 319 |
| 21* | PD | d 29; lung, liver | Liver | — | — | SD | Died in PD d 123 |
| 22 | SD | — | — | — | — | PR | Alive in PR, d 229 |
| 23 | PR | — | Liver | — | — | CR§ | Alive in CR, d 97 |
— indicates not applicale; GI, gastrointestinal; MI, myocardial infarction; ARDS, acute respiratory distress syndrome; and ED, early death.
Patient received bone marrow from a matched but unrelated donor.
Patient lost follow-up after day 95.
Patient underwent metastasectomy and became surgical CR.
Patient underwent RTX to site of evaluable disease before transplantation and is not included as CR in analysis.
Antitumor response
Antitumor responses of the 22 evaluable patients are listed in Table 3. Of the 8 patients with BC, 3 were in PR and 5 had SD after standard-dose chemotherapy at the time of study entry. After transplantation, patient nos. 2 and 8 had no disease progression. Patient no. 2 initially had SD after the transplantation. Later, in the 13 months after the procedure, the disease resolved completely after she had developed chronic GVHD at 10 months after the transplantation. Patient no. 8 was alive with SD at day 345. She also had chronic GVHD, which was controlled with methylprednisolone. Patient nos. 3, 5, and 6 had disease progression within 100 days after the transplantation and were treated by early withdrawal of tacrolimus immunosuppressive therapy. Patient no. 3 developed acute skin GVHD that was resolved by methylprednisolone, but her disease continued to progress. Patient no. 5 developed gastrointestinal GVHD that was resolved by methylprednisolone. She had SD associated with a long period of chronic gastrointestinal GVHD. Patient no. 6 continued to have PD despite the withdrawal of tacrolimus. However, she had an MR from DLI at 17 months with no further progression. Three other patients (nos. 1, 4, and 7) experienced disease progression after the first 100 days after the transplantation. Patient no. 1 had a CR at 3 months but the response did not last, and she eventually died of PD. Patient no. 4 had PD at day 310; despite the development of a mild chronic gastrointestinal GVHD, her disease continued to progress and she died on day 537. Patient no. 7 died of PD on day 404 despite development of chronic GVHD and 2 DLIs.
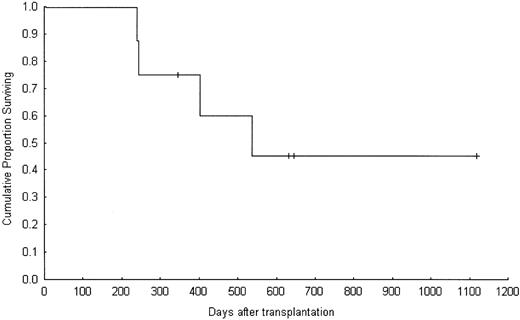
Overall, the best responses among the 8 patients with BC were 2 CRs and one MR (37.5%; Table 3). Four patients were alive at a median follow-up of 639.5 days (range, 345-1119 days), for an actuarial 1-year overall survival rate of 75%. The median survival time was 493 days (range, 239-1119 days). Overall survival is shown in Figure 1. The number of BC patients was not sufficient to make any conclusion in survival different between patients with a tumor response and those without.
Of the 15 patients with RCC, 13 had PD, 1 had SD, and 1 had PR at the time of study entry. After the transplantation, 3 patients (nos. 10, 22, and 23) had no disease progression. Patient no. 10 had SD but died of an acute myocardial infarction. Patient no. 22 underwent withdrawal of tacrolimus as scheduled at 3 months; at 7 months, she had a PR with no signs of GVHD. Patient no. 23 had minimal disease in the spine (by magnetic resonance imaging) before transplantation, which had been treated with, and was responding to, radiation therapy. He developed acute liver GVHD at 7 weeks. Five weeks later, he achieved a CR, confirmed by magnetic resonance imaging. Because his only site of evaluable disease had received prior local therapy, it was not possible to categorize the patient as transplant-related CR. Seven patients (nos. 12, 13, 14, 16, 17, 20, and 21) had disease progression within 100 days after the transplantation and were treated by early withdrawal of tacrolimus immunosuppressive therapy. Three of them (nos. 12, 14, and 20) had disease controlled after withdrawal of tacrolimus. Patient no. 12 developed grade I skin GVHD and had SD, but he died from acute respiratory distress syndrome. Patient no. 14 developed acute gastrointestinal GVHD that was resolved with methylprednisolone. Six weeks later, she had an MR but died of pneumonia. Patient no. 20 had an MR after withdrawal of tacrolimus at 3 months with no GVHD. However, she later had PD that did not respond to DLI. Four patients (nos. 13, 16, 17, and 21) died of PD despite the withdrawal of tacrolimus and DLI given to 2 of them (nos. 13 and 16). Four other patients (nos. 9, 11, 15, and 19) also had disease progression 100 days after the transplantation. Patient no. 9 initially had a PR after the development of acute GVHD at 2 months. After the resolution of acute GVHD, his disease progressed again at day 160. Immunosuppression was stopped and his disease again showed a PR. After 7 months, he developed chronic GVHD, which was well controlled but not completely resolved with immunosuppressive therapy. At 28 months, his chronic GVHD had become quiescent and did not require immunosuppression; this patient eventually had a CR and is currently alive at day 1015. Patient no. 11 had an MR at 2 months after developing acute gastrointestinal GVHD, which resolved completely. He then developed chronic GVHD and the disease slowly became a PR. After the chronic GVHD resolved, disease progressed at the right apex of the lung at 12 months after the transplantation. Because this was the only site of disease progression, he underwent complete surgical resection of the metastatic site. Six months after the surgery, he remained in CR with no evidence of disease. Patient no. 15 had grade I acute skin GVHD at 1 month that later resolved and an MR that lasted 4 months. However, disease subsequently progressed and did not respond to the withdrawal of tacrolimus and DLI. Patient no. 19 developed chronic GVHD after disease progression. Four weeks after resolution of the GVHD, he had an MR at 8 months, which persists until the present.
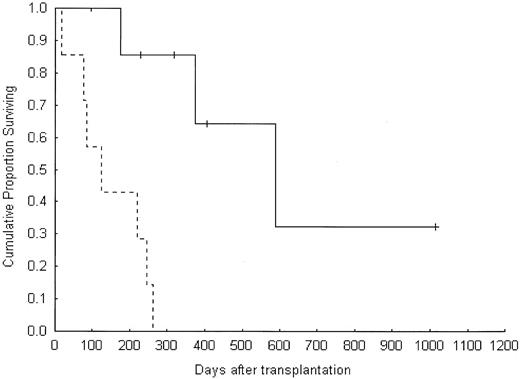
Overall, the best responses among the 14 evaluable RCC patients were one CR, 2 PRs and 4 MRs (50%; Table 3). Five patients were alive at a median follow-up of 319 days (range, 97-1015 days). The actuarial 1-year overall survival rate was 31%, with a median survival time of 245 days (range, 19-1015 days). Overall survival of patients with RCC who experienced a tumor response was better than that of patients who did not (log-rank test = 0.006, Cox F test = 0.0008; Figure 2).
Overall survival in the RCC group according to disease response. Solid line indicates response; dotted line, no response.
Overall survival in the RCC group according to disease response. Solid line indicates response; dotted line, no response.
Discussion
NST has been proposed as a means of inducing a GVT effect against selected solid tumors. This immune-mediated GVT phenomenon can be considered a form of adoptive immunotherapy. The goal of the preparative regimen in NST is to ensure durable engraftment of the donor cells. Because antitumor responses generally occur after development of complete donor chimerism, we sought to identify a preparative regimen that produces complete chimerism at the time of initial engraftment. In the study reported here, we evaluated a reduced-intensity fludarabine-melphalan conditioning regimen for this purpose.
Complete donor chimerism was achieved at 30 days after transplantation in all evaluable patients. Moreover, neither DLI nor early withdrawal of immunosuppression was needed to achieve this complete donor chimerism. Mixed donor chimerism has been associated with a lower rate of GVHD and alloreactivity.66-68 Lower alloreactivity, however, could affect the immune-mediated GVT effect. Prolonged mixed donor chimerism may increase the risk of repopulation by recipient cells and subsequent graft failure.67,69-72 Early graft manipulation, either by DLI or by early withdrawal of immunosuppression, can convert mixed to complete donor chimerism, but it has the risk of inducing acute GVHD. Besides, DLI has its limitations, among which are its limited availability from unrelated donors and its contraindication in the presence of active GVHD. Although early complete donor chimerism could also increase the risk of acute GVHD, our study revealed that the rate of acute GVHD was not much higher than that in other recent reports—9 (39%) of our 23 patients developed grades II to IV acute GVHD, and 5 patients (22%) had grades III to IV acute GVHD. Several different preparative regimens have been tested with allogeneic transplantation for solid tumors, with various rates of mixed and incomplete donor chimerism (Table 4). Some regimens were truly nonmyeloablative but others including our fludarabine-melphalan regimen were reduced-intensity only. The findings in Table 4 indicate that the intensity of the conditioning regimen is related to the achievement of complete donor engraftment.
Conditioning regimens used for allogeneic transplantation for solid tumors
Authors . | Nonmyeloablative conditioning regimens . | Chimerism results . |
|---|---|---|
| Childs et al43 | Fludarabine 25 mg/m2 × 5 d, cyclophosphamide 60 mg/kg × 2 d | 8 of 19 patients needed DLI for T-cell chimerism, disease control or both |
| Rini et al45 | Fludarabine 30 mg/m2 × 5 d, cyclophosphamide 2 g/m2 × 2 d | 5 of 12 patients had achieved 100% donor chimerism on d 100 |
| Pedrazzoli et al46 | Fludarabine 30 mg/m2 × 4 d, cyclophosphamide 30 mg/kg × 2 d | 13 of 17 patients had achieved 100% donor chimerism on d 90 |
| McDermott et al48 | Fludarabine 25 mg/m2 × 5 d, cyclophosphamide 60 mg/kg × 2 d | 6 of 7 patients had achieved > 95% T-cell chimerism on median d 75 |
| Sandmaier et al49 | Fludarabine 30 mg/m2 × 3 d, total body irradiation 2 Gy × 1 d | 1 of 5 patients had graft rejection; 4 of 5 patients had a median T-cell chimerism rate of 73% on d 84 |
| Hentschke et al50 | Fludarabine 30 mg/m2 × 3 d (related donor) or 30 mg/m2 × 5 d (unrelated donor), total body irradiation 2 Gy × 1 d, ATG 2 mg/kg × 2 d (unrelated donor) | 12 of 18 patients had complete donor chimerism, 2 had graft failure, 1 had marrow aplasia, and 3 had mixed chimerism |
| Bregni et al47 | Fludarabine 30 mg/m2 × 2 d, cyclophosphamide 30 mg/kg × 2 d, thiotepa 10 mg/kg × 1 d (for BC) or 5 mg/kg × 1 d (for RCC) | 12 of 13 patients had > 80% donor chimerism on d 60 |
| Hori et al52 | Fludarabine 30 mg/m2 × 6 d, busulfan 4 mg/kg × 2 d, ATG 2.5 mg/kg × 2 d | All 8 patients had 100% complete donor chimerism |
| Makimoto et al51 | Fludarabine 30 mg/m2 (cladribine 0.11 mg/kg for the first 3 patients) × 6 d, busulfan 4 mg/kg × 2 d, ATG 2.5 mg/kg × 2 d | 13 of 14 patients had complete donor chimerism |
| Blaise et al53 | Fludarabine 180 mg/m2 (total dose), busulfan 8 mg/kg (total dose), ATG 2.5-10 mg/kg | 80% of evaluable patients had full donor chimerism |
| Ueno et al (current study) | Fludarabine 30 mg/m2 × 5 d (for BC) or 25 mg/m2 × 5 d (for RCC), melphalan 70 mg/m2 × 2 d | 1 of 23 patients had early death; 22 of 23 patients had 100% donor chimerism on d 30 and d 100 |
Authors . | Nonmyeloablative conditioning regimens . | Chimerism results . |
|---|---|---|
| Childs et al43 | Fludarabine 25 mg/m2 × 5 d, cyclophosphamide 60 mg/kg × 2 d | 8 of 19 patients needed DLI for T-cell chimerism, disease control or both |
| Rini et al45 | Fludarabine 30 mg/m2 × 5 d, cyclophosphamide 2 g/m2 × 2 d | 5 of 12 patients had achieved 100% donor chimerism on d 100 |
| Pedrazzoli et al46 | Fludarabine 30 mg/m2 × 4 d, cyclophosphamide 30 mg/kg × 2 d | 13 of 17 patients had achieved 100% donor chimerism on d 90 |
| McDermott et al48 | Fludarabine 25 mg/m2 × 5 d, cyclophosphamide 60 mg/kg × 2 d | 6 of 7 patients had achieved > 95% T-cell chimerism on median d 75 |
| Sandmaier et al49 | Fludarabine 30 mg/m2 × 3 d, total body irradiation 2 Gy × 1 d | 1 of 5 patients had graft rejection; 4 of 5 patients had a median T-cell chimerism rate of 73% on d 84 |
| Hentschke et al50 | Fludarabine 30 mg/m2 × 3 d (related donor) or 30 mg/m2 × 5 d (unrelated donor), total body irradiation 2 Gy × 1 d, ATG 2 mg/kg × 2 d (unrelated donor) | 12 of 18 patients had complete donor chimerism, 2 had graft failure, 1 had marrow aplasia, and 3 had mixed chimerism |
| Bregni et al47 | Fludarabine 30 mg/m2 × 2 d, cyclophosphamide 30 mg/kg × 2 d, thiotepa 10 mg/kg × 1 d (for BC) or 5 mg/kg × 1 d (for RCC) | 12 of 13 patients had > 80% donor chimerism on d 60 |
| Hori et al52 | Fludarabine 30 mg/m2 × 6 d, busulfan 4 mg/kg × 2 d, ATG 2.5 mg/kg × 2 d | All 8 patients had 100% complete donor chimerism |
| Makimoto et al51 | Fludarabine 30 mg/m2 (cladribine 0.11 mg/kg for the first 3 patients) × 6 d, busulfan 4 mg/kg × 2 d, ATG 2.5 mg/kg × 2 d | 13 of 14 patients had complete donor chimerism |
| Blaise et al53 | Fludarabine 180 mg/m2 (total dose), busulfan 8 mg/kg (total dose), ATG 2.5-10 mg/kg | 80% of evaluable patients had full donor chimerism |
| Ueno et al (current study) | Fludarabine 30 mg/m2 × 5 d (for BC) or 25 mg/m2 × 5 d (for RCC), melphalan 70 mg/m2 × 2 d | 1 of 23 patients had early death; 22 of 23 patients had 100% donor chimerism on d 30 and d 100 |
ATG indicates antithymocyte globulin.
Another potential advantage of using the fludarabine-melphalan regimen described here is the success in achieving engraftment with cells from unrelated donors, a practice that carries a higher risk of graft rejection after NST. This success increases the fraction of patients who could undergo this treatment modality. Moreover, stem cells from an unrelated donor could increase the chance of a GVT effect because of the genetic disparities between unrelated persons. In our study, patient no. 11, who received bone marrow stem cells from an unrelated donor, was in PR for about 1 year until the disease progressed at a single site. When a truly nonmyeloablative regimen was used in allogeneic SCT using an unrelated donor, additional immunosuppressive agents such as antithymocyte globulin were occasionally added to prevent graft rejection. In our study, the fludarabine-melphalan regimen was able to achieve successful engraftment using cells from unrelated donor without addition of other immunosuppressive agents.
Of the 22 evaluable patients in this study, 3 with BC (nos. 1, 2, and 6) and 7 with RCC (nos. 9, 11, 14, 15, 19, 20, and 22) experienced tumor response, with 3 CRs (nos. 1, 2, and 9), 2 PRs (nos. 11 and 22), and 5 MRs (nos. 6, 14, 15, 19, and 20) after the transplantation. The overall response rate was 45%. Among those 10 patients, 7 (nos. 2, 9, 11, 14, 15, 19, and 23) had disease response associated with acute or chronic GVHD, and 2 (nos. 20 and 22) showed disease response after the withdrawal of immunosuppressive therapy. One patient (no. 6) had disease response after DLI. The median time at response among these 10 patients was 7.5 months (range, 1-33 months). These features of tumor response were all consistent with the GVT phenomenon. GVT effects have been noted to occur after complete donor chimerism, in association with GVHD, after the withdrawal of immunosuppressive therapy, after DLI, and late after transplantation.43 Of these features, the achievement of complete donor chimerism seems essential and is clearly related to the intensity of the conditioning regimen. Although a more intense conditioning regimen could produce more treatment-related toxicity and mortality, our fludarabine-melphalan regimen treatment profile seemed acceptable, because none of the reversible grades III to IV toxic effects in our study occurred in more than 50% of the patients (Table 2). The nonrelapse treatment-related mortality rate at day 100 was 13% (3 of 23 patients), and the overall nonrelapse treatment-related mortality rate was 22% (5 of 23 patients). These nonrelapse treatment-related mortality rates are comparable to those found in other studies, which range from 11% to 33%.43,45-47,50
The ultimate goal of using NST to treat solid tumors is to induce an immune-mediated GVT effect. Although data to support this strategy are limited, a GVT effect is more likely to occur after complete donor chimerism. After the fludarabine-cyclophosphamide regimen used by Childs et al,43 mixed chimerism was generally present soon after the transplantation and complete donor chimerism was achieved after withdrawal of immunosuppression or DLI. We used a more intensive regimen to achieve rapid complete donor chimerism and reserved graft manipulations for use later if they were needed to control disease. In this study, this strategy led to tumor response in 10 of 23 patients after transplantation, with 7 durable responses (nos. 2, 6, 9, 11, 14, 19, and 22). Six patients received DLI for disease control with one response, but none of those patients developed GVHD after DLI. The median time of the first DLI was 214 days after transplant (range, 116-422 days). This delay in use of DLI seemed to be safe but produced no GVHD, which may have affected the overall tumor response rate.
A unique characteristic of the patients with BC in our study was that most of them had only bone disease. Of the 3 BC patients who experienced disease response, 2 (nos. 2 and 6) had only bone disease. Bone disease in BC is difficult to assess and can show a delayed response to chemotherapy. In our study, all bone disease was assessed by plain radiography, skeletal scintigraphy, and tumor marker measurements if those markers were elevated before the transplantation. Patient no. 2 had abnormalities on skeletal scintigraphy for 13 months after transplantation, and those abnormalities resolved completely 3 months after she developed chronic GVHD. Patient nos. 2 and 6 demonstrated tumor response at 13 and 17 months, respectively, after the transplantation. This delayed response was also seen in most of the patients with RCC who experienced tumor response. Because the fludarabine-melphalan RIC regimen tested here produces little cytoreduction in metastatic BC or RCC, the disease response was unlikely to be related to the preparative chemotherapy. More than half of our patients showed disease progression soon after the transplantation, possibly because of posttransplantation immune deficiency and immunosuppressive therapy to prevent GVHD. GVT effects typically become evident later, after sufficient time has elapsed to allow maturation of the allogeneic donor cells and withdrawal of immunosuppression. Our CR plus PR rate in the RCC groups was 20%. This response rate was lower than that found by Childs et al,43 which probably reflected variations in patient characteristics and treatment approach between the 2 studies. Most of our RCC group (13 of 15) had had PD before the transplantation and the median time from the diagnosis of metastatic disease until the SCT was 272 days, which means that most of our patients had far advanced disease. Also, our patients had been on immunosuppressive therapy for longer period of time to prevent severe GVHD. Therefore, longer follow-up may allow additional objective responses to appear, because the GVT effect usually occurs late after the transplantation or after the discontinuation of immunosuppressive therapy. In our study, the median follow-up for living RCC patients was only 319 days.
Further evaluations of this reduced-intensity fludarabine-melphalan conditioning regimen for the treatment of metastatic BC and metastatic RCC by allogeneic SCT are needed. The optimal conditioning regimen and the optimal strategy for limiting treatment-related morbidity and mortality remain to be identified. Our findings indicate the potential of immune GVT effects to control advanced solid tumors. RIC regimens may provide a platform for antigen-specific immunotherapy directed against relevant tumor-associated antigens. Controlled trials are required to definitively assess the role of allogeneic hematopoietic SCT for individual solid tumors and to compare alternative transplantation strategies for achieving complete donor chimerism and GVT effects.
Prepublished online as Blood First Edition Paper, July 24, 2003; DOI 10.1182/blood-2003-04-1022.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Christine F. Wogan for her excellent help in developing this manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal