Abstract
Acute myelogenous leukemia (AML) is a heterogeneous disease consisting of a variety of different leukemic subtypes. While acute promyelocytic leukemia displays marked sensitivity to the differentiating effects of trans-retinoic acid (tRA), other subtypes of AML display resistance. We now describe a novel compound (E)-4-[3-(1-adamantyl)-4-hydroxyphenyl]-3-chlorocinnamic acid (3-Cl-AHPC/MM002) that induces apoptosis in the tRA-resistant leukemia cell lines M07e, KG-1, and HL-60R, and in tRA-resistant patient leukemic blasts. The 3-Cl-AHPC totally inhibits leukemia colony formation at concentrations that inhibit committed human bone marrow stem cell proliferation, that is, granulocyte/macrophage colony-forming units (CFU-GMs) by only 30%. Exposure to 3-Cl-AHPC results in caspase activation and the cleavage of poly(adenosine diphosphate) (poly(ADP)) ribose polymerase. While activation of the extracellular signal-regulated kinase (ERK) and p38 pathways is not necessary for 3-Cl-AHPC-mediated apoptosis, maximal apoptosis requires c-Jun N-terminal kinase (JNK) activation. The 3-Cl-AHPC-mediated cleavage of the antiapoptotic B-cell leukemia XL (Bcl-XL) protein to a proapoptotic 18-kDa product is found in both the M07e cell line and patient leukemic blasts. The 3-Cl-AHPC treatment of mice bearing the AML 1498 cell line results in a 3.3-log kill in the leukemic blasts. While 3-Cl-AHPC does not activate retinoic nuclear receptors, it is a potent inducer of apoptosis in AML cells and may represent a novel therapy in the treatment of this disease. (Blood. 2003; 102:3743-3752)
Introduction
Acute myelogenous leukemia (AML) is a heterogeneous disease composed of numerous subclassifications displaying a wide spectrum of phenotypes.1,2 The major therapeutic approach to this disease has been the use of chemotherapeutic agents with associated life-threatening toxicity. Although nonspecific in their effects, these regimens have significantly increased the survival of AML patients.3-5 Recently, more targeted therapy has been developed. Treatment of acute promyelocytic leukemia (APL) patients with trans-retinoic acid (tRA) results in the differentiation of the leukemic cells, with 90% of the patients achieving a complete remission.6-8 The tRA exerts its effect by modulating gene expression through its role as a ligand to the retinoic acid nuclear receptors, RARs, with the subsequent binding of this complex to the retinoic acid response element (RARE) consensus sequences located in the regulatory regions of retinoid-responsive genes.9 The selective sensitivity of APL cells to tRA-mediated differentiation resides in their specific expression of a unique promyelocytic leukemia (PML)-RARα fusion product that results in the subsequent maturation arrest of these cells at the promyelocyte stage10-12 ; exposure of these cells to a micromolar concentration of tRA allows for the degradation of the PML-RARα fusion product, with subsequent maturation of the APL cells.13,14 Unfortunately, the other AML subtypes as classified by the French-American-British (FAB) classification demonstrate inherent resistance to tRA-mediated differentiation and induction of apoptosis.15,16
We and others have recently described a novel retinoid 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalenecarboxylic acid (AHPN/CD437), which is a potent inducer of apoptosis in a variety of cell types and which displays resistance to tRA-mediated differentiation and apoptosis.17-21 The mechanistic pathways by which AHPN induces apoptosis in malignant cells are not clearly defined. AHPN binds and transactivates both the RARβ and RARγ receptors.22,23 The roles of these retinoid nuclear receptors in AHPN-mediated apoptosis are controversial. While some studies have suggested that binding and transactivation of RARγ are essential for AHPN/CD437-mediated apoptosis,24,25 others have indicated that neither the RARs nor the retinoid X receptors (RXRs) to which AHPN does not bind or transactivate are involved.20,21,26
In the present study, we assessed the ability of the AHPN analog 4-[3-(1-adamantyl)-4-hydroxyphenyl]-3-chlorocinnamic acid (3-Cl-AHPC/MM002) to induce apoptosis in a number of human AML cell lines as well as in primary cultures of leukemic blasts obtained from patients. The 3-Cl-AHPC differs from AHPN in its inability to recruit RAR coactivators and transactivate the RARs (Zhang et al27 and data not shown). We found that 3-Cl-AHPC is a potent inducer of apoptosis in these cells, which display resistance to the antiproliferative/differentiating effects of tRA. The 3-Cl-AHPC-mediated apoptosis is preceded by activation of the p38, extracellular signal-regulated kinase 1/2 (ERK1/2), and c-Jun N-terminal kinase (JNK) protein kinases and accompanied by caspase-3-mediated cleavage of Bcl-XL to an 18-kDa proapoptotic form. The 3-Cl-AHPC is less toxic than AHPN/CD437 in vivo, and treatment of AML-bearing mice with 3-Cl-AHPC results in a significant leukemic cell kill and prolongation of survival.
Materials and methods
Materials
Recombinant interleukin 3 (IL-3), granulocyte-colony stimulating factor (G-CSF), granulocyte/macrophage colony-stimulating factor (GM-CSF), and stem cell factor (SCF) used in the human leukemia primary cell cultures were obtained from Biosource International (Camarillo, CA). Mouse recombinant GM-CSF was obtained from R&D Systems (Minneapolis, MN). JNK1 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The p38, phospho-p38, ERK, and phospho-ERK antibodies were obtained from Cell Signaling (Beverly, MA). Poly(adenosine diphosphate) [poly(ADP)], ribose antibodies were obtained from Pharmingen (San Diego CA).The ERK inhibitor PD98059, the p38 inhibitor PD169316, and the JNK inhibitor SP600125 were obtained from CalBiochem (San Diego, CA). The 3-Cl-AHPC was synthesized as we recently described.27
Cell growth
IL-3-transfected M07e cells as well as the KG-1 and HL-60R cells have been previously described.28-31 The cells were grown in RPMI supplemented with 5% heat-inactivated fetal bovine serum (FBS) and 25 μg/mL gentamicin in a 95% O2, 5% CO2, and 100% humidity environment. Leukemic cells were obtained from patients who met the criteria for the diagnosis of AML or chronic myelogenous leukemia (CML) in blast crisis (Table 1). Samples were obtained from patients at Harper Hospital (Detroit, MI) and the Southwestern Oncology Leukemia Cell Bank. Studies were approved by the Wayne State University Institutional Review Board (Detroit, MI). Informed consent was obtained from all patients. Briefly, leukemic blasts from patients were isolated by means of Ficoll hypaque. Cells at the interface between plasma and red blood cells were diluted with sterile phosphate-buffered saline (PBS) and then layered over Ficoll hypaque (1.077 density), and the leukemic cells were collected at the interface. Samples studied represented more than 90% blasts and were grown in RPMI medium supplemented with 10% FBS, gentamicin (25 μg/mL), IL-3 (20 ng/mL), SCF (25 ng/mL), GM-CSF (20 ng/mL), and G-CSF (20 ng/mL). Cell growth was assessed by means of a cell proliferation kit (Promega, Madison, WI) according to the manufacturer's directions.
Western blot
Western blots were performed according to our previously published protocol.32 Logarithmically growing cells were treated with 3-Cl-AHPC for various times, and cells were harvested and lysed in Laemmli lysis buffer (0.5 M Tris-HCI [tris(hydroxymethyl)aminomethane-HCI], pH 6.8, 0.002 M EDTA [ethylenediaminetetraacetic acid], 10% glycerol, 10% sodium dodecyl sulfate [SDS], and 5% β-mercaptoethanol). Protein lysates (50 μg per lane) were electrophoresed on 12% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were blocked with 5% nonfat dried milk in PBS/0.5% Tween 20 and then incubated with the appropriate antibodies. Horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin G (IgG) (Sigma, St Louis, MO) was used as the secondary antibody, and bands were developed by means of the Amersham (Arlington Heights, IL) electrochemiluminescence (ECL) nonradioactive method following the manufacturer's instructions.
Apoptosis quantification
Staining of apoptotic cells was performed as previously described.33,34 Briefly, after 3-Cl-AHPC treatment, cells were harvested, washed with PBS, and resuspended at 1 × 106/mL. Cell suspensions (50 μL) were stained with 5 μL acridine orange solution (100 mg/mL in PBS) in the dark. Cells displaying fragmented DNA were detected by means of a fluorescent microscope. Five hundred cells were screened. Apoptotic cells were also detected by means of the Apo-Direct Kit (Pharmingen) according to the manufacturer's directions. Cells that had incorporated fluorescein-labeled deoxyuridine triphosphate were detected by means of flow cytometry.
Flow cytometry
Flow cytometry was performed on a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA) equipped with an argon ion laser tuned to 15 mW at 488 nM for fluorescence excitation and light scattering, controlled by a Power Macintosh G3 (Apple Computer, Cupertino, CA) running CELLQuest software (BDIS). Fluorescein isothiocyanate (FITC) fluorescence was collected in the fluorescein 1 (FL1) detector by means of a 530/30-nm band pass filter, and propidium iodide (PI) fluorescence was reflected with a 560 short-pass dichroic filter to be collected by the FL2 detector by means of a 585/42-nm band pass filter. Electronic compensation for the spectral overlap of the fluorochromes was not used. The doublet discrimination module identified cell aggregates.35 Typically, 20 000 events of list mode data were saved and analyzed with CELLQuest software run on a Power Macintosh G3 computer. Routine quality control of the FACScan was performed by means of FACSComp software with CaliBRITE beads (BDIS) and CELLQuest software with DNA Quality Control particles (BDIS).
Caspase activation
Activation of caspases 1, 2, 3, 6, 8, and 9 was assessed by means of a caspase activation kit (BioVision, Palo Alto, CA).
Leukemia colony formation
Leukemic blasts (1 × 105 cells) from patients were isolated as described above and cultured in StemPro methylcellulose (Gibco BRL, Rockville, MD) supplemented with 20% FCS and SCF, IL-3, GM-CSF, and G-CSF, each at a final concentration of 10 ng/mL. The cells were then incubated in the presence and absence of various concentrations of 3-Cl-AHPC for 14 days at 37°C in a 5% CO2 humidified atmosphere, at which time colonies were counted.
CFU-GM colony formation
The granulocyte/macrophage colony-forming unit (CFU-GM) assay was performed as described by Parchment et al.36 Human mononuclear cells prepared from G-CSF-mobilized peripheral blood or bone marrow from certified donors were obtained from Poietics (Gaithersburg, MD). Murine mononuclear cells were isolated from the femurs of female severe combined immunodeficiency (SCID) and 6D2F1 mice immediately before use under a protocol approved by the Wayne State University Institutional Animal Care and Use Committee (IACUC). The 3-Cl-AHPC was added to the methylcellulose in such a way that the final concentration of the dimethyl sulfoxide (DMSO) vehicle was 0.05% vol/vol; this was also the DMSO concentration in the vehicle-treated controls and had no effect on human or murine CFU-GM colony formation.
p38 and ERK kinase activation
Activation of p38 and ERK kinase was assessed by determining phospho-p38 and phospho-ERK levels as we have previously described.27
JNK kinase assay
Activation of JNK was determined by assaying JNK kinase activity. Briefly, cells were lysed in buffer containing 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.4, 2 mM EGTA (ethylene glycol tetraacetic acid), 50 nM β-glycerolphosphate, 1 mM dithiothreitol (DTT), 1 mM Na3V04, 5 mM NaF, 1% Triton X-100, 10% glycerol, 10 μM leupeptin, 10 μM aprotinin, 0.1 mg/mL okadaic acid, and 400 μM phenylmethanesulfonyl fluoride (PMSF). Endogenous JNK was immunoprecipitated from cell lysates with anti-p46 JNK antibody. Beads were then washed 3 times with lysis buffer and then with kinase buffer (25 mM Tris, pH 7.5, 5 mM β-glycerolphosphate, 2 mM dithiothreitol, 0.1 mM Na3V04, 10 mM MgCl2). Pellets were resuspended with 20 μL × 1 kinase buffer containing 20 μM adenosine triphosphate (ATP), and 5 μCi (0.185 MBq) γ-[32P]ATP, and incubated for 30 minutes at 30°C with 1 μg c-Jun peptide (Santa Cruz Biotechnology). The reaction was terminated by the addition of 40 μL × 2 SDS sample buffer. The samples were boiled, and 10 μL sample was loaded and resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Phosphorylated c-Jun peptide was detected by autoradiography. JNK levels were detected by Western blot.
3-Cl-AHPC in vivo therapy
In vivo AHPN and 3-Cl-AHPC toxicity. Groups of 5 3CH3 mice (20 g, 4 to 6 weeks old) were injected by tail vein with varying total dose of AHPN or 3-Cl-AHPC. The total dose (milligrams per kilogram) was given over a 9-day period, and the mice were weighed daily.
In vivo therapy against AML 1498 cells. Murine AML 1498 cells were maintained in the mouse strain of origin (C57BL/6) and transplanted into this same inbred strain for the therapy trials. Individual mouse body weights at the start of therapy for each experiment were within 5 g, and all mice were more than 20 g (mean). The mice were supplied food and water ad libitum. Mice were randomly pooled into groups of 5 and implanted intravenously with varying numbers of AML 1498 cells (5 × 106, 5 × 104, or 5 × 102) prepared from mouse spleens demonstrating approximately 80% replacement of the spleen with leukemic cells. In all cases, treatment was started the day after implantation of the AML cells. The following quantitative end points were used to assess antitumor activity: (1) the percentage of increase in lifespan and (2) the tumor cell kill. The percentage of increase in lifespan (%ILS) was calculated as follows: %ILS = T - C/C × 100 where C is the the median day of death of the control group and T is the median day of death of the treated group. Survival was the end point, and moribund mice were killed. Cause of death was verified by necropsy. The tumor cell kill was calculated as follows: For leukemic survival trials, the log10 cell kill is calculated from as Log10 cell kill total (gross) = T - C/3.32 (Td), where T is the median day of death for the treated group and C is the median day of death for the control group.37-39 The Td (tumor doubling time) is determined from differences in the median days of death of the titered control groups.
Results
3-Cl-AHPC inhibition of leukemic cell growth
We initially assessed the ability of 3-Cl-AHPC to inhibit the growth of human myeloid leukemia cells using the human acute megakaryocytic leukemia cell line M07e. Exposure of these cells to various concentrations of 3-Cl-AHPC over time resulted in the progressive increase in the inhibition of proliferation (Figure 1A). Increase in growth inhibition was accompanied by the onset of apoptosis at 0.5 and 1.0 μM 3-Cl-AHPC (Figure 1B). While exposure to 0.2 μM 3-Cl-AHPC resulted in a minimal inhibition of growth, no significant increase in apoptosis was noted (Figure 1A-B). In contrast, tRA, a potent activator of the RARs, did not significantly inhibit M07e proliferation (Figure 1C) or induce their apoptosis (data not shown). Induction of apoptosis was noted in the KG-1 and HL-60R AML cell lines following exposure to 3-Cl-AHPC (Figure 1D-E). These cells demonstrated sensitivity to 3-Cl-AHPC, while tRA had no effect on the induction of apoptosis in these cell lines (Figure 1D-E).
3-Cl-AHPC and tRA inhibition of growth and the induction of apoptosis in human leukemia cell lines. M07e, KG-1, and HL-60R cells were seeded in RPMI 1640 supplemented with 5% FBS and incubated overnight; varying concentrations of 3-Cl-AHPC or tRA were added and the cells harvested at various times. The results represent the mean of 3 independent experiments, with the variation between the experiments being < 10% if error bars are not shown. Error bars represent standard deviations. (A) The 3-Cl-AHPC inhibition of proliferation of M07e cells. (B) The 3-Cl-AHPC induction of apoptosis in M07e cells. (C) The tRA inhibition of M07e proliferation. (D) The 3-Cl-AHPC (1 μM) and tRA (1 μM) induction of apoptosis in KG-1 cells. (E) The 3-Cl-AHPC (1 μM) and tRA (1 μM) induction of apoptosis of HL-60R cells.
3-Cl-AHPC and tRA inhibition of growth and the induction of apoptosis in human leukemia cell lines. M07e, KG-1, and HL-60R cells were seeded in RPMI 1640 supplemented with 5% FBS and incubated overnight; varying concentrations of 3-Cl-AHPC or tRA were added and the cells harvested at various times. The results represent the mean of 3 independent experiments, with the variation between the experiments being < 10% if error bars are not shown. Error bars represent standard deviations. (A) The 3-Cl-AHPC inhibition of proliferation of M07e cells. (B) The 3-Cl-AHPC induction of apoptosis in M07e cells. (C) The tRA inhibition of M07e proliferation. (D) The 3-Cl-AHPC (1 μM) and tRA (1 μM) induction of apoptosis in KG-1 cells. (E) The 3-Cl-AHPC (1 μM) and tRA (1 μM) induction of apoptosis of HL-60R cells.
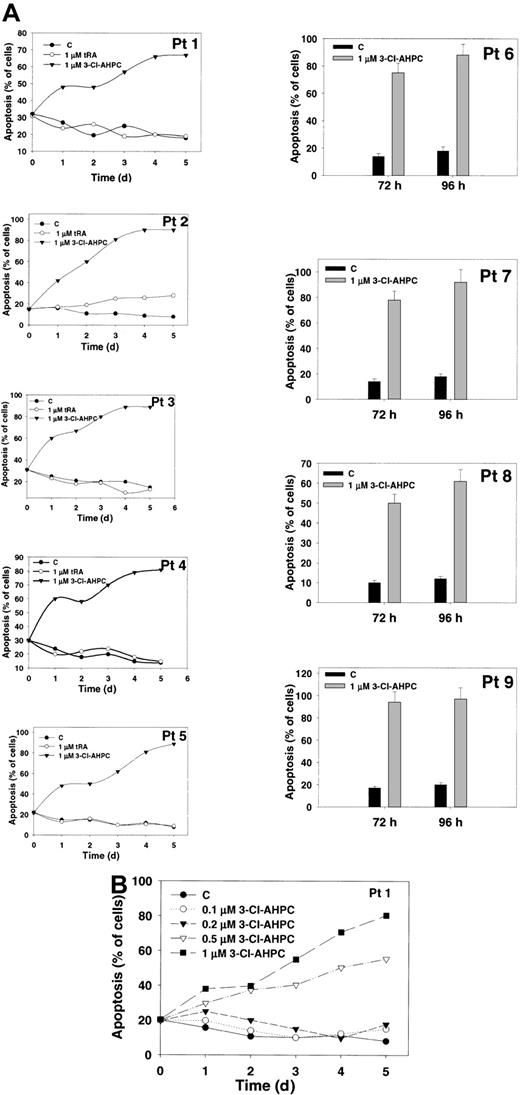
Breitman et al15 previously demonstrated that tRA treatment of primary AML cultures resulted in the differentiation of APL (M3) cells but had no effect on the other AML subtypes as classified according to the FAB classification system.16 Therefore, we examined whether 3-Cl-AHPC induced apoptosis in myeloid blasts of differing FAB classifications (Table 1). Exposure to 1 μM 3-Cl-AHPC resulted in the induction of apoptosis (greater than 80%) in all of the 13 primary cultures examined. Representative results are presented in Figure 2A. In contrast, exposure to tRA did not induce apoptosis in these cells (Figure 2A; also data not shown). The 3-Cl-AHPC inhibited the proliferation of the AML cultures from 70% to 90% (data not shown).
3-Cl-AHPC and tRA induction of apoptosis of leukemic blasts. Leukemia cells obtained from patients were cultured as described in “Materials and methods.” The results represent the mean of 3 independent experiments, with the variation between the experiments being less than 10% if error bars are not shown. Error bars represent standard deviations. (A) The 3-Cl-AHPC or tRA induction of apoptosis. The 3-Cl-AHPC and tRA were added to a final concentration of 1 μM; the cells were harvested at various times, and the percentage of apoptotic cells was assessed as described in “Materials and methods.” (B) Leukemic cells of patient 1 were exposed to varying concentrations of 3-Cl-AHPC for up to 5 days.
3-Cl-AHPC and tRA induction of apoptosis of leukemic blasts. Leukemia cells obtained from patients were cultured as described in “Materials and methods.” The results represent the mean of 3 independent experiments, with the variation between the experiments being less than 10% if error bars are not shown. Error bars represent standard deviations. (A) The 3-Cl-AHPC or tRA induction of apoptosis. The 3-Cl-AHPC and tRA were added to a final concentration of 1 μM; the cells were harvested at various times, and the percentage of apoptotic cells was assessed as described in “Materials and methods.” (B) Leukemic cells of patient 1 were exposed to varying concentrations of 3-Cl-AHPC for up to 5 days.
The ability of varying concentrations of 3-Cl-AHPC to induce apoptosis in the patient leukemic blasts was also examined (Figure 2B). The 3-Cl-AHPC (0.5 μM) induced apoptosis in 60% of the leukemic blasts, while minimal apoptosis was noted at 0.1 and 0.2 μM (Figure 2B). The 3-Cl-AHPC-mediated apoptosis in the myeloid blasts was further assessed by the transferase-mediated deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL) assay and flow cytometry. Treatment with 1 μM 3-Cl-AHPC for 24 hours resulted in 67% and 80% apoptotic cells in the leukemic cells from patient 1 (Figure 3A) and patient 10 (Figure 3B), respectively, whereas exposure to vehicle alone resulted in less than 10% apoptosis (Figure 3).
3-Cl-AHPC-mediated apoptosis in leukemic cells assessed by flow cytometry. Leukemia cells of patient 1 (A) and patient 10 (B) were treated with 1 μM 3-Cl-AHPC (Aii,Bii) or vehicle alone (Ai,Bi) for 24 hours and harvested; then the percentage of apoptotic cells was determined by means of an Apo-Direct Kit. The percentage of apoptotic cells is indicated in the right hand corner in the vehicle-treated control (labeled C) and 3-Cl-AHPC-treated cells.
3-Cl-AHPC-mediated apoptosis in leukemic cells assessed by flow cytometry. Leukemia cells of patient 1 (A) and patient 10 (B) were treated with 1 μM 3-Cl-AHPC (Aii,Bii) or vehicle alone (Ai,Bi) for 24 hours and harvested; then the percentage of apoptotic cells was determined by means of an Apo-Direct Kit. The percentage of apoptotic cells is indicated in the right hand corner in the vehicle-treated control (labeled C) and 3-Cl-AHPC-treated cells.
3-Cl-AHPC inhibition of leukemic cell colony formation
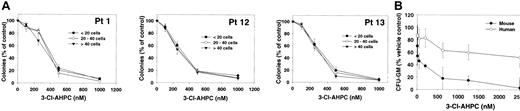
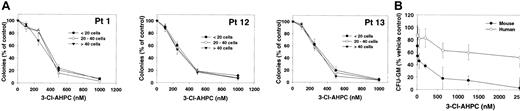
Leukemic cells obtained from patients were seeded in methylcellulose as described in “Materials and methods” in the presence and absence of varying concentrations of 3-Cl-AHPC. Small colony formation (fewer than 20 cells), cluster formation (colonies between 20 and 40 cells), and large colonies (more than 40 cells) were assessed after 14 days of growth (Figure 4A). Following the addition of varying concentrations of 3-Cl-AHPC, a dramatic fall in the number of all colony types was noted when 3-Cl-AHPC concentrations greater than 250 nM were used (effective dose [ED50] of approximately 375 nM). As demonstrated, 3-Cl-AHPC concentrations greater than 250 nM induce apoptosis in primary leukemia cells, suggesting that the decline in the colony numbers at 3-Cl-AHPC concentrations greater than 250 nM is secondary to the induction of apoptosis. More than 90% of leukemia colony formation was inhibited at 3-Cl-AHPC concentrations of 600 nM or more.
3-Cl-AHPC inhibition of leukemia and CFU-GM colony formation. (A) Leukemic colonies fewer than 20 cells (•), between 20 and 40 cells (○), and more than 40 cells (▾). Mean numbers of colonies in control cultures were as follows: Patient 1 (Pt 1), 205 ± 25, fewer than 20 cells; 100 ± 10, 20 to 40 cells; 45 ± 5, more than 40 cells. Patient 12 (Pt12), 370 ± 60, fewer than 20 cells; 125 ± 10, 20 to 40 cells; 50 ± 10, more than 40 cells. Patient 13 (Pt 13), 285 ± 20, fewer than 20 cells; 85 ± 10, 20 to 40 cells; 40 ± 5, more than 40 cells. (B) CFU-GM colonies were grown and their formation was assessed as described in “Materials and methods” in the presence and absence of varying concentrations of 3-Cl-AHPC. The results represent the mean and standard deviation of 3 separate experiments using different normal marrow and patient leukemia samples.
3-Cl-AHPC inhibition of leukemia and CFU-GM colony formation. (A) Leukemic colonies fewer than 20 cells (•), between 20 and 40 cells (○), and more than 40 cells (▾). Mean numbers of colonies in control cultures were as follows: Patient 1 (Pt 1), 205 ± 25, fewer than 20 cells; 100 ± 10, 20 to 40 cells; 45 ± 5, more than 40 cells. Patient 12 (Pt12), 370 ± 60, fewer than 20 cells; 125 ± 10, 20 to 40 cells; 50 ± 10, more than 40 cells. Patient 13 (Pt 13), 285 ± 20, fewer than 20 cells; 85 ± 10, 20 to 40 cells; 40 ± 5, more than 40 cells. (B) CFU-GM colonies were grown and their formation was assessed as described in “Materials and methods” in the presence and absence of varying concentrations of 3-Cl-AHPC. The results represent the mean and standard deviation of 3 separate experiments using different normal marrow and patient leukemia samples.
3-Cl-AHPC inhibition of CFU-GM colony formation
The effect of varying concentrations of 3-Cl-AHPC on the proliferation of committed marrow stem cells was examined with the use of CFU-GMs. The 3-Cl-AHPC concentrations, which completely inhibited leukemic colony formation, resulted in only a 30% inhibition of CFU-GM colony formation (Figure 4B). Murine CFU-GM cells were more sensitive than their human counterparts, with more than 80% inhibition of colony formation at 600 nM.
3-Cl-AHPC induction of caspase activity
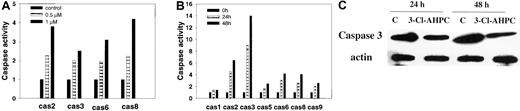
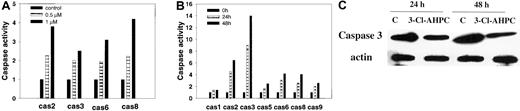
Apoptosis is associated with the activation of specific cysteine proteases referred to as caspases.40,41 We assessed whether 3-Cl-AHPC activated specific caspases during apoptosis of M07e and myeloid blasts and whether the same caspases were activated in these cells. Treatment of M07e with 1 μM 3-Cl-AHPC resulted in an approximately 4-fold increase in the activity of caspase-2 and caspase-8 and a 3- and 2.5-fold increase in caspase-6 and caspase-3 activities, respectively (Figure 5A). An approximately 2-fold increase in the activities of caspases 2, 3, 6, and 8 was noted at 0.5 μM 3-Cl-AHPC (Figure 5A). Activation of caspases was also noted when the patient leukemic cells were incubated with 1 μM 3-Cl-AHPC (Figure 5B). Caspase-3 displayed the greatest activation with 8-fold and 14-fold increases at 24 hours and 48 hours, respectively. Western blot revealed a 3- to 4-fold decrease in the inactive caspase-3 proenzyme level following 24- and 48-hour incubation with 3-Cl-AHPC (Figure 5C), which was coincident with the associated activation of caspase-3 (Figure 5B). As noted with M07e cells, exposure of the patient leukemic blasts to 3-Cl-AHPC resulted in activation of caspase-2 (6-fold), caspases 6 and 8 (4-fold), and caspases 5 and 9 (2-fold) (Figure 5B).
3-Cl-AHPC activates caspases. (A-B) M07e (A) and leukemic cells (patient 1) (B) were treated with 3-Cl-AHPC or vehicle alone, and caspase (cas) activation was assessed as described in “Materials and methods.” (C) The 3-Cl-AHPC activation of caspase-3. Leukemic cells obtained from patient 1 were treated with 1 μM 3-Cl-AHPC or vehicle alone, and the cells were harvested at 24 or 48 hours. Caspase-3 proenzyme levels were determined by means of Western blot as described in “Materials and methods.” Actin levels were used to assess loading. The results are representative of 2 independent experiments.
3-Cl-AHPC activates caspases. (A-B) M07e (A) and leukemic cells (patient 1) (B) were treated with 3-Cl-AHPC or vehicle alone, and caspase (cas) activation was assessed as described in “Materials and methods.” (C) The 3-Cl-AHPC activation of caspase-3. Leukemic cells obtained from patient 1 were treated with 1 μM 3-Cl-AHPC or vehicle alone, and the cells were harvested at 24 or 48 hours. Caspase-3 proenzyme levels were determined by means of Western blot as described in “Materials and methods.” Actin levels were used to assess loading. The results are representative of 2 independent experiments.
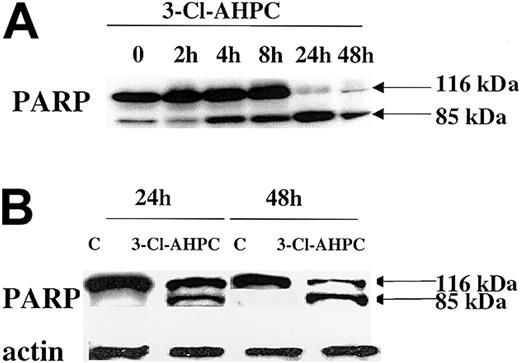
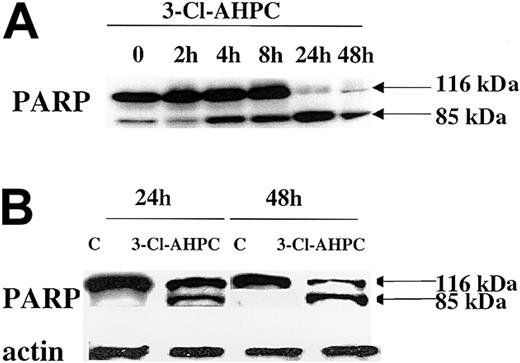
Caspase-mediated cleavage of proteins can result in either activation or inactivation.42 Poly(ADP) ribose polymerase (PARP), which plays an important role in both DNA synthesis and repair, is cleaved early in the apoptotic process induced by a number of agents.41-43 The 3-Cl-AHPC treatment of both M07e cells and patient leukemic blasts resulted in the rapid cleavage of the 116-kDa PARP and the generation of an 85-kDa fragment by 24 hours (Figure 6).
PARP cleavage. PARP cleavage occurs during 3-Cl-AHPC-mediated apoptosis. M07e (A) and leukemic cells (B) obtained from patient 10 were exposed to 1 μM 3-Cl-AHPC for indicated times; cells were harvested; and Western blots were performed as described in “Materials and methods.”
PARP cleavage. PARP cleavage occurs during 3-Cl-AHPC-mediated apoptosis. M07e (A) and leukemic cells (B) obtained from patient 10 were exposed to 1 μM 3-Cl-AHPC for indicated times; cells were harvested; and Western blots were performed as described in “Materials and methods.”
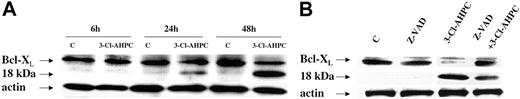
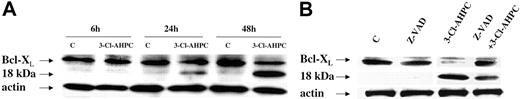
Apoptosis is a complex process, which is regulated at multiple levels by numerous mediators.26,44,45 The Bcl-2 family with its 19 members can exert either proapoptotic or antiapoptotic effects depending upon which member is playing a dominant role.46 Antiapoptotic Bcl-2 and the Bcl-2 family member Mcl-1 are both expressed in malignant hematopoietic cells and have been shown to play an important role in the survival of these cells.47-49 Both patient leukemic cells and M07e cells expressed Bcl-2 and Mcl-1; however, there was no modulation of their expression during 3-Cl-AHPC-mediated apoptosis (data not shown). The Bcl-X gene encodes for 2 proteins, termed Bcl-XL and Bcl-XS, through alternative splicing.50 While Bcl-XS is a potent inducer of apoptosis, Bcl-XL inhibits the apoptotic process.50 Western blots of M07e cells and patient leukemic cells demonstrated Bcl-XL expression while Bcl-XS expression could not be detected (Figure 7). Treatment of these cells with 1 μM 3-Cl-AHPC resulted in the cleavage of Bcl-XL to an 18-kDa product (Figure 7A). Previous studies in cell lines have found that cleavage of Bcl-XL and the subsequent generation of the 18-kDa product is the result of caspase-3 activation.51 Inhibition of caspase-3 activity by means of the caspase-3 inhibitor Z-VAD-fmk inhibited Bcl-XL cleavage (Figure 7B).
3-Cl-AHPC-mediated Bcl-XL cleavage. Leukemic cells obtained from patient 11 were incubated in the presence and absence of 1 μM 3-Cl-AHPC for varying periods of time (A) or in the presence of 1 μM 3-Cl-AHPC and in the presence and absence of the caspase inhibitor Z-VAD-fluormethylketone (Z-VAD-fmk) (50 μM) for 24 hours (B). Western blots were performed with the use of anti-Bcl-XL antibody as described in “Materials and methods.” Actin levels were used to assess loading.
3-Cl-AHPC-mediated Bcl-XL cleavage. Leukemic cells obtained from patient 11 were incubated in the presence and absence of 1 μM 3-Cl-AHPC for varying periods of time (A) or in the presence of 1 μM 3-Cl-AHPC and in the presence and absence of the caspase inhibitor Z-VAD-fluormethylketone (Z-VAD-fmk) (50 μM) for 24 hours (B). Western blots were performed with the use of anti-Bcl-XL antibody as described in “Materials and methods.” Actin levels were used to assess loading.
Activation of MAPK pathways during 3-Cl-AHPC-mediated apoptosis
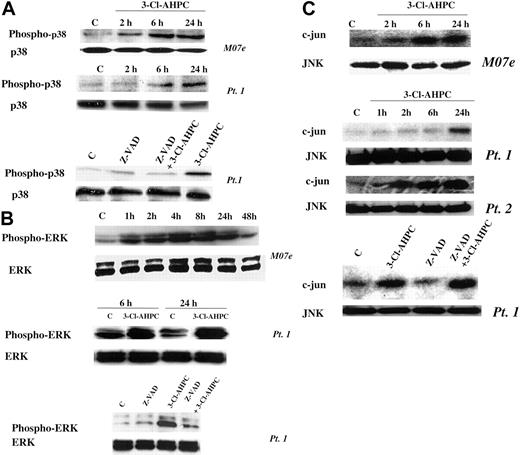
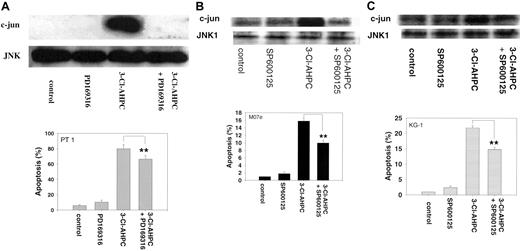
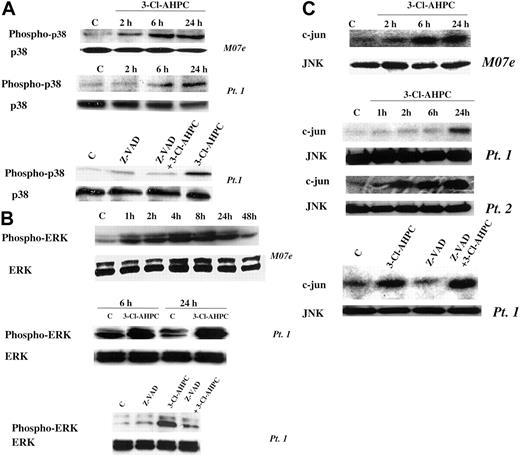
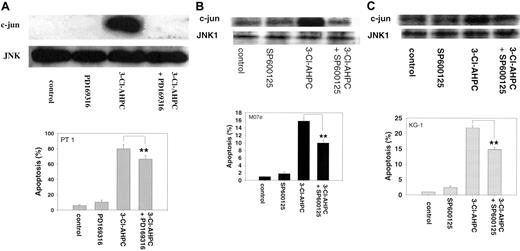
The mitogen-activated protein kinase (MAPK) pathways have been found to be associated with the induction of apoptosis in many cell types.52,53 Activation of p38 and the ERK and JNK protein kinases has been observed, but their roles in the apoptotic process remain unclear.54-56 We previously demonstrated that treatment of the HL-60R human leukemia cell line with AHPN/CD437 resulted in p38 and JNK activation.57 We, therefore, examined whether 3-Cl-AHPC activated any of these 3 MAPK pathways in M07e cells or the patient leukemic cells and their roles in 3-Cl-AHPC-mediated apoptosis. The 3-Cl-AHPC treatment resulted in the activation of the p38, ERK, and JNK kinases in both M07e and the patient leukemia cells as evidenced by increased phospho-p38 and phospho-ERK levels and enhanced JNK kinase activity (Figure 8). Activation of p38 and ERK required caspase activation as evidenced by the ability of the pancaspase inhibitor Z-VAD-fmk to prevent their activtion by 3-Cl-AHPC (Figure 8A-B). JNK activation was not inhibited by Z-VAD-fmk, suggesting that its activation was caspase independent (Figure 8C). The potential role of these kinases in 3-Cl-AHPC-mediated apoptosis was investigated. Inhibition of 3-Cl-AHPC-mediated p38 and ERK activation by the p38 inhibitor PD169316 and ERK inhibitor PD98059, respectively, did not inhibit or enhance 3-Cl-AHPC-mediated apoptosis in either M07e or the patient leukemia cells (data not shown). However, elevated levels of PD169316 (20 μM or higher) that inhibited both p38 and JNK activation inhibited 3-Cl-AHPC-mediated apoptosis by 20%, suggesting that JNK activation may be required for optimal 3-Cl-AHPC-mediated apoptosis of these leukemic cells (Figure 9A). To further assess the effect of JNK kinase inhibition, we exposed M07e and KG-1 cells to the specific JNK kinase inhibitor SP600125 in the presence and absence of 3-Cl-AHPC (Figure 9B-C). Interestingly, while SP600125 totally inhibited the 3-Cl-AHPC-mediated increase in JNK kinase activity, it inhibited 3-Cl-AHPC-mediated apoptosis only by approximately 25% (Figure 9).
3-Cl-AHPC activation of p38, ERK, and JNK. M07e and leukemic cells were exposed to 1 μM 3-Cl-AHPC or vehicle alone in the presence and absence of the capsase inhibitor Z-VAD-fmk (50 μM). Phospho-p38 (A) and phospho-ERK (B) levels were assessed with the use of anti-phospho-p38 and anti-phospho-ERK antibodies in conjunction with Western blots as described in “Materials and methods.” (C) JNK kinase activation was determined by measuring JNK kinase activity with c-Jun as the substrate, as described in “Materials and methods.”
3-Cl-AHPC activation of p38, ERK, and JNK. M07e and leukemic cells were exposed to 1 μM 3-Cl-AHPC or vehicle alone in the presence and absence of the capsase inhibitor Z-VAD-fmk (50 μM). Phospho-p38 (A) and phospho-ERK (B) levels were assessed with the use of anti-phospho-p38 and anti-phospho-ERK antibodies in conjunction with Western blots as described in “Materials and methods.” (C) JNK kinase activation was determined by measuring JNK kinase activity with c-Jun as the substrate, as described in “Materials and methods.”
Effect of inhibition of 3-Cl-AHPC-mediated JNK activation on 3-Cl-AHPC-mediated apoptosis. Patient 1 cells (A), M07e cells (B), or KG-1 cells (C) were exposed to 1μM 3-Cl-AHPC, the JNK kinase inhibitors PD169316 (20 μM) or SP600125 (30 μM), and the combination 3-Cl-AHPC (1 μM) and either PD169316 (20 μM) or SP600125 (30 μM). JNK kinase activity (upper panels) and the percentage of apoptotic cells (histograms, lower panels) were assessed as described in “Materials and methods.” **Significantly less than apoptosis mediated by 3-Cl-AHPC alone (P < .01). Error bars represent standard deviation of 3 separate experiments.
Effect of inhibition of 3-Cl-AHPC-mediated JNK activation on 3-Cl-AHPC-mediated apoptosis. Patient 1 cells (A), M07e cells (B), or KG-1 cells (C) were exposed to 1μM 3-Cl-AHPC, the JNK kinase inhibitors PD169316 (20 μM) or SP600125 (30 μM), and the combination 3-Cl-AHPC (1 μM) and either PD169316 (20 μM) or SP600125 (30 μM). JNK kinase activity (upper panels) and the percentage of apoptotic cells (histograms, lower panels) were assessed as described in “Materials and methods.” **Significantly less than apoptosis mediated by 3-Cl-AHPC alone (P < .01). Error bars represent standard deviation of 3 separate experiments.
3-Cl-AHPC is less toxic and inhibits AML growth in syngeneic mice
We tested the toxicity of AHPN/CD437 and 3-Cl-AHPC in female C3H mice, which were to be used in a mammary syngeneic model. Groups of C3H mice (5 mice per group) were treated with varying total doses of either AHPN or 3-Cl-AHPC, with the dose given over a 9-day period intravenously as described in “Materials and methods.” While AHPN/CD437 and 3-Cl-AHPC demonstrate similar in vitro activities against a number of breast, prostate, and leukemia cell lines, they differ significantly in their in vivo toxicity (Table 2). Treatment of C3H mice with AHPN/CD437 total dose of 150 mg/kg results in a 22% weight loss and 1 death in the group of 5 mice. Lowering the total dose to 130 mg/kg also results in a 14% weight loss. On the other hand, treatment of mice with 3-Cl-AHPC total doses of 178 mg/kg and 140 mg/kg results in only a 4% and 3% mean body weight loss, respectively, and no deaths (Table 2). Because we were unsuccessful in growing the M07e cell line in SCID mice, and we did not have access to an AML model in C3H mice, we used murine AML 1498 cells intravenously implanted in C57BL/6 mice to evaluate 3-Cl-AHPC inhibition of the in vivo growth of AML cells. The murine AML 1498/C57BL6 syngeneic model has been used as a valid screen to assess the activity of potential therapeutic agents against AML.58 Treatment of the mice with 3-Cl-AHPC at total dosage of either 140 or 115 mg/kg resulted in a 3.3-log cell kill with a doubling in the survival duration (Table 3). A total dose of 140 mg/kg 3-Cl-AHPC over 4 days resulted in a 17% weight loss and no animal deaths (Table 3) whereas the equally effective 115 mg/kg total dose led to a weight loss of 6%.
Discussion
The goals of the present study were to demonstrate the therapeutic potential of 3-Cl-AHPC in AML and investigate the pathways by which 3-Cl-AHPC may induce apoptosis in these cells. We used the growth factor-dependent M07e AML cell line as well as human primary AML and CML cells in blast crisis to assess the effect of 3-Cl-AHPC. The 3-Cl-AHPC inhibited the proliferation and induced apoptosis in patient leukemic cells and the M07e cell line as well as the KG-1 and HL-60R, all of which display resistance to tRA-mediated apoptosis induction. The AML cells from patients with a variety of FAB subtypes as well as the CML cells in blast crisis displayed sensitivity to 3-Cl-AHPC. To inhibit growth of M07e cells, 3-Cl-AHPC concentrations of 0.2 μM were required while 0.5μM concentrations were required to induce apoptosis in these cells. Similarly 0.5 μM 3-Cl-AHPC was required to induce apoptosis in the patient leukemic cells. The 3-Cl-AHPC also inhibited leukemia colony formation by the patient leukemic cells with an inhibitory concentration (IC50) of 375 nM and inhibited colony formation by 90% at 600 nM. We also assessed the ability of 3-Cl-AHPC to inhibit CFU-GMs cultured from human and murine bone marrows. We previously found that the interspecies difference in the IC90, the concentration of drug that inhibits in vitro CFU-GM colony formation by 90%, reflects the differences in tolerated exposure levels of the drug in vivo across species. In vitro, 3-Cl-AHPC demonstrated a 30-fold greater toxicity to mouse than to human CFU-GMs. The IC90 values in the mouse ranged from 315 to 794 nM whereas the human tolerated much higher levels of 3-Cl-AHPC (IC90 exceeding 15 000 nM). These data would strongly predict that 3-Cl-AHPC should be well tolerated in humans and display preferential toxicity in vivo against leukemia cells and significantly less toxicity to normal marrow.59
Exposure of M07e and patient leukemic cells to 3-Cl-AHPC resulted in apoptosis, as documented by a number of parameters. Staining of the cells with acridine orange following incubation with 3-Cl-AHPC revealed an intact plasma membrane but also nuclear fragmentation, which are characteristics associated with the process of apoptosis. Apoptosis was further documented by the end labeling of the DNA fragments generated following the addition of 3-Cl-AHPC to cells. Flow cytometry demonstrated that more than 80% of patient leukemic cells underwent apoptosis following exposure to 3-Cl-AHPC.
Activation of caspases through proteolysis of an inactive holoenzyme is associated with the induction of apoptosis60 ; whether the activation of these proteases is an absolute requirement for the onset of apoptosis is still unclear.61 In both M07e and patient leukemic cells, 3-Cl-AHPC activated a number of caspases. The most dramatic increases were seen in caspases 2, 3, 6, and 8 in both the patient leukemic and M07e cells. Caspase-2 and caspase-8 activation have been found to occur through the binding of ligands to the death receptors DR4 and DR5, leading to the recruitment of adaptor molecules.60 The ability of AHPN to enhance DR4 and DR5 expression was observed in 3 human non-small cell carcinoma cell lines.62 The addition of the DR4/DR5 ligand tumor-necrosis factor-related apoptosis-inducing ligand (TRAIL) enhanced AHPN-mediated apoptosis in these cells.63 Whether 3-Cl-AHPC also enhances DR4/DR5 expression with subsequent activation of caspases 8 and 2 is currently under investigation.
Caspase-3 activation was also noted following exposure of the cells to 3-Cl-AHPC. Caspase-3 has been found to be activated by a cytoplasmic protein complex consisting of apoptotic protease-activating factor 1 (APAF-1), cytochrome c, and activated caspase-9.64 The induction of mitochondrial transmembrane depolarization with the subsequent release of cytochrome c appears to be the major mechanism through which caspase-3 is activated. Once activated, caspase-3 has been found to be responsible for cleavage of a number of substrates including PARP,65 as was observed in the M07e and patient leukemic cells.
Treatment with 3-Cl-AHPC did not modulate the expression of either Mcl-1 or Bcl-2 in the M07e or patient leukemia cells, but did result in the cleavage of Bcl-XL with the generation of an 18-kDa cleavage product. A similar 18-kDa Bcl-XL product was identified in the WSU-CLL and ALL-REH cell lines following the addition of AHPN as well as following the induction of apoptosis in CTLL cells by depletion of the IL-3 growth factor.27,51 That the Bcl-XL fragment lacks the BH4 domain, which is located between amino acids 4 and 24, was demonstrated by the loss of a specific antibody epitope between residues 3 and 14.27,66 The BH4 domain of Bcl-XL has been shown to be essential for the antiapoptotic function of certain Bcl-2 family members and is responsible for the ability of Bcl-XL to bind and sequester APAF-1.66,67 Loss of the Bcl-XL BH4 domain results in a proapoptotic molecule.51,66
The role of the signal transduction pathways in the apoptotic process is controversial and appears to be pathway and cell-type dependent. Stress activation of the p38 MAP kinase has been well documented, but its role in stress-induced apoptosis remains unclear.53 Numerous studies have shown that p38 activation occurs on apoptosis induction but that inhibition of p38 activation does not inhibit apoptosis (Jarpe et al53 and references within Juo et al68 ). Other studies strongly suggest that the p38 kinase plays a vital role in the induction of apoptosis following nerve growth factor withdrawal from rat PC-12 pheochromocytoma cells, ultraviolet β radiation-induced apoptosis of the immortalized human keratinocyte HaCaT cell line, and chelerythrine-induced apoptosis of HeLa cells.69-71 We previously found that while the addition of AHPN to the HL-60R human leukemia cell line results in p38 activation, its activation is downstream of caspase activation and is not required for CD437-mediated apoptosis of this cell line.57 We now find that p38 activation occurs in both in the M07e cells as well as the patient leukemic cells following exposure to 3-Cl-AHPC. As in HL-60R cells, p38 activation in the M07e and patient leukemia cells required caspase activation, and inhibition of p38 activation did not block 3-Cl-AHPC-mediated apoptosis.
ERK activation has been commonly associated with protection against apoptosis induction by a variety of agents.72 While inhibition of the ERK pathway has been associated with the enhancement of apoptosis, its activation blocks cytosine arabinoside-induced and nerve growth factor withdrawal-mediated apoptosis.73-75 Recently, Wang et al54 reported that ERK activation plays an active role in mediating cisplatinum-induced apoptosis of HeLa cells and occurs upstream of caspase activation. Inhibition of ERK activation blocked cisplatinum-induced apoptosis.54 The 3-Cl-AHPC activates ERK1/2 in both M07e and patient leukemic cells. While inhibiting 3-Cl-AHPC-mediated ERK1/2 activation, the ERK1/2 inhibitor PD98059 had no effect on 3-Cl-AHPC induction of apoptosis, suggesting that ERK1/2 activation is not essential for 3-Cl-AHPC-induced apoptosis.
The 3-Cl-AHPC also activated JNK in the HL-60R and M07e cell lines as well as in all of the patient leukemic cell samples. We previously demonstrated that caspase activation is not required for JNK activation by AHPN.57 The lack of a requirement for caspase activation was also true for the patient cells treated with 3-Cl-AHPC. JNK activation has been found to be an essential component of the apoptotic process in a number of systems.53,76-78 JNK protein kinases are encoded by 3 genes.56 JNK1 and JNK2 are expressed ubiquitously while expression of JNK 3 is predominantly restricted to the brain; simultaneous loss of expression of JNK1 and JNK2 is associated with defects in neuronal apoptosis.55,56 The observation that high levels (20 μM) of the p38 kinase inhibitor PD169316 also inhibited JNK activation allowed us to assess whether JNK activation was necessary for 3-Cl-AHPC-mediated apoptosis in these leukemic cells. PD169316 inhibition of JNK activation significantly but minimally (20%) inhibited 3-Cl-AHPC induction of apoptosis. Similar results were obtained when the specific JNK kinase inhibitor SP600125 was used. Thus, JNK activation appears to be required for maximal 3-Cl-AHPC-mediated apoptosis in the AML cells and the leukemia cell lines. Interestingly, Ortiz et al79 found that in the Jurkat T-cell lymphoma cell line, inhibition of JNK and p38 activation blocked induction of apoptosis by the RARγ-selective compounds MX2870-1 and NX3350-1 by more than 80%.
The 3-Cl-AHPC demonstrates significantly less in vivo toxicity than AHPN, as was shown in C3H mice. We also find that 3-Cl-AHPC given intravenously to a total dose of 210 mg/kg over a 7-day period to nonobese diabetic-SCID (NOD-SCID) mice results in only a 10% weight loss and no deaths. Evidence that 3-Cl-AHPC demonstrated in vivo efficacy against AML was provided by the studies using the murine AML 1498 cells grown in C57BL/6 mice. Treatment of the leukemia-bearing mice with a 3-Cl-AHPC total dose of 140 mg/kg or 115 mg/kg over a 4-day period resulted in a 3.3-log cell kill and an 83% increase in survival. While treatment with a 140 mg/kg total dose resulted in a 17.3% weight loss, treatment with a 115 mg/kg total dose resulted in only a 5.8% weight loss. Even when the mice were treated with 3-Cl-AHPC at a total dose of 80 mg/kg, at which no toxicity (0% weight loss) was noted, a 2-log cell kill and 50% increase in survival were noted. The 3.3-log cell kill meets the National Cancer Institute criterion of a highly active drug.36 The 3-Cl-AHPC displayed a log cell kill similar to that noted with cytosine arabinoside (3.8) and daunomycin (3.8). This in vivo leukemia model is resistant to a number of chemotherapeutic agents including taxol, vinblastine, gemcitabine, and cisplatinum. Thus, the high sensitivity of these cells to 3-Cl-AHPC suggests a potential role for this agent in the treatment of AML. Additional studies assessing 3-Cl-AHPC pharmacokinetics as well as studies using different dosing schedules to further substantiate the efficacy of 3-Cl-AHPC in leukemia are underway.
Prepublished online as Blood First Edition Paper, July 31, 2003; DOI 10.1182/blood-2003-01-0108.
Supported by grants from the Medical Sevice of the Department of Veterans Affairs (J.A.F., A.K.R), the Leukemia Lymphoma Society of America (J.A.F.), and the National Institutes of Health (PO1 CA51993) (M.I.D., J.A.F., M.L., X.k.Z.)
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Joseph Kassab for his expertise in conducting the in vitro bone marrow toxicity studies, Donna Bennett for her secretarial assistance, and Bill Browning and the VA Medical Media for the preparation of the Figures.