Imatinib mesylate (Gleevec), a small molecule inhibitor of abl, kit, and platelet-derived growth factor receptor (PDGFR) tyrosine kinases, has been reported to be effective in the treatment of hypereosinophilic syndrome (HES) and a rare eosinophilia-associated chronic myeloid disorder (eos-CMD) characterized by the t(5;12)(q33;p13) cytogenetic abnormality. In the current study, we sought to confirm the preliminary observations in HES as well as evaluate the therapeutic value of imatinib in eos-CMD that is not associated with t(5;12)(q33;p13). Five patients with HES (all men, median age = 46 years) and 2 with eos-CMD (both men, aged 45 and 58 years) were treated with imatinib at a starting dose of 100 to 400 mg/day. Cytogenetic studies showed no evidence of either the bcr-abl translocation or t(5;12)(q33;p13) in any patient. Screening of exons encoding the intracellular catalytic domains and extracellular ligand binding domains of PDGFRβ (exons 2-23) and c-kit (exons 1-21) in 6 patients demonstrated mostly previously known polymorphisms. At a median follow-up of 17 weeks (range, 10-33 weeks), 2 patients with HES and 1 with eos-CMD have achieved complete clinical remission and 1 additional patient with HES has achieved a partial remission. In contrast to previous observations, all 4 responding patients had elevated serum interleukin-5 levels. Although the drug was well tolerated in most patients, a previously unrecognized treatment toxicity of acute left ventricular dysfunction occurred in a responding patient with HES within the first week of treatment. Myocardial biopsy revealed eosinophilic infiltration and degranulation, and the cardiogenic shock was reversed with the prompt institution of corticosteroid therapy.
Introduction
Chronic, nonreactive eosinophilia characterizes the hypereosinophilic syndrome (HES) as well as a spectrum of other clonal hematopoietic diseases associated with eosinophilia. In clonal eosinophilic disorders, the eosinophils can be demonstrated to be either part of the malignant clone1,2 or produced as a result of cytokine production by the malignant clone.3-6Imatinib mesylate (Gleevec), a small molecule that inhibits signal transduction via tyrosine kinase oncoproteins including bcr-abl and receptors for stem cell factor (c-kit) and platelet-derived growth factor receptor (PDGFR), has recently been shown to be efficacious for the treatment of HES.7-9 In addition, imatinib has been shown to be effective in chronic myeloid disorders (CMDs) associated with eosinophilia and rearrangement of the PDGFRβ gene that results in constitutive activation of the PDGFRβ receptor.10 These findings suggest that eosinophilia in the setting of clinically and cytogenetically diverse malignant hemopathies may serve as a surrogate marker for treatment response to imatinib. To confirm this hypothesis and obtain preliminary information regarding the mechanism of action, we conducted a prospective pilot study using imatinib in 7 patients with persistent eosinophilia in whom reactive causes were ruled out.
Patients and methods
Patients
The current study population included 5 patients with HES and 2 patients with eosinophilia-associated CMD (eos-CMD) who were treated with imatinib and prospectively followed for response and toxicity assessments. Patients with one or more pathologic features typical of the clonal myeloproliferative disorders, including a clonal cytogenetic abnormality, bone marrow panhyperplasia, excess circulating or marrow blasts, or dysplastic changes in cells of noneosinophilic lineages, were classified as having eos-CMD. For the purposes of the current study, eosinophilia was defined as a peripheral blood eosinophil count of more than 1.5 × 109/L. Known causes of reactive eosinophilia were excluded in each instance. Only patients with symptomatic disease who were deemed to require cytoreductive treatment were accrued to the study, and all had pretreatment bone marrow examinations with cytogenetic studies. Patients were allowed to have received prior biologic therapies and/or chemotherapies, but they were required to be off of such therapy for at least 4 weeks prior to study enrollment. The prospective accrual of patient data and collection of biologic specimens was approved by Mayo Clinic's institutional review board, and written informed consent was obtained from all patients.
Study design
The primary end points were symptomatic improvement and a decrease in the peripheral eosinophil count by at least 50%, as manifest by monitoring of serial complete blood counts (CBCs). In addition, a follow-up, posttreatment bone marrow biopsy was performed for responding eos-CMD patients to evaluate morphologic changes in the noneosinophil cell lineages, as well as cytogenetic studies. Secondary objectives were assessment of safety and time to treatment failure.
All patients underwent baseline bone marrow biopsy with cytogenetics. Presence of the bcr-abl translocation was excluded by fluorescence in situ hybridization (FISH). Three of 5 patients with HES were screened for clonal T-cell–receptor (TCR) gene rearrangement by polymerase chain reaction (PCR) and/or Southern blot assays. Six of 7 patients were screened for mutations in coding exons of c-kit andPDGFRβ receptor tyrosine kinase genes, although the results were not used to exclude patients from study participation.
Patients were started at a dose of 100 to 400 mg imatinib orally, taken once a day with food. Although the starting dose was determined at the discretion of the treating investigator, all patients were treated at the 400 mg/day dose if no response was observed at lower doses.
Patients had regular physical examinations and evaluations of performance status, body weight, complete blood count, serum chemistry, chest X-ray, and cardiac echocardiography. Other imaging studies, including standard computed tomographic (CT) scans, radionuclide bone scans, and skeletal radiographic surveys, were performed as indicated, for assessing symptoms.
Mutation analysis
Genomic DNA isolated from either peripheral blood or bone marrow mononuclear cells as well as from purified eosinophil cell fractions from 6 patients was used in the mutational analysis (DNA was not available from case 4). PCR primers (sequences available on request) were designed to intronic sequence 50 to 100 bp away from the intron/exon boundary to detect any mutations that might affect splicing. All exons encoding the intracellular catalytic domains and extracellular ligand binding domains of PDGFRβ (exons 2-23) and c-kit (exons 1-21) were amplified from patient genomic DNA using Amplitaq Gold (Applied Biosystems, Foster City, CA). Products were then mixed with an equal quantity of amplified DNA from human placental DNA. The samples were denatured at 95°C and then slowly re-annealed by reducing the temperature by 1.5°C every minute to room temperature. The heteroduplexes of patient and normal DNA were then analyzed by denaturing high-performance liquid chromatography (dHPLC WAVE; Transgenomics, Crewe, United Kingdom). Melting temperatures for each amplicon were calculated using Transgenomic Wavemaker v4.1 software. The WAVE profiles for each patient were compared with the normal DNA, and any patients with extra peaks (suggesting mismatches) or shifted peaks (suggesting insertion or deletion of a few bases) were directly sequenced on an ABI 377 Prism DNA sequencer (Applied Biosystems).
Results
Hypereosinophilic syndrome
Case 1.
A 46-year-old man presented with a constellation of symptoms that included dyspnea, cough, night sweats, low-grade fever, skin lesions with pruritus, and patchy and migratory numbness in the lower extremities. Evaluation revealed leukocytosis (15.5 × 109/L) with eosinophilia (7.65 × 109/L) (Table 1), left tibial mononeuropathy, and duodenal eosinophilic enteritis. A bone marrow biopsy revealed increased numbers of mature eosinophils. He had previously received 2 courses of pulse steroids with partial transient symptomatic improvement. After discontinuation of corticosteroid therapy, he was started on imatinib at a daily dose of 100 mg.
The patient had a prompt and dramatic decrease in circulating eosinophils, which were undetectable after 10 days of imatinib therapy. He, however, developed progressive dyspnea and orthopnea in the interim from acute left ventricular dysfunction with cardiogenic shock and required intravenous pressor support. A chest X-ray revealed new bilateral pulmonary infiltrates, and an echocardiogram revealed new-onset severe generalized left ventricular (LV) hypokinesis (LV ejection fraction [LVEF] decreased from 71% to 10% after 8 days of therapy). Following emergent cardiac catheterization, which revealed normal coronary vessels, placement of an intra-aortic balloon pump (IABP) became necessary for hemodynamic support. A right ventricular endomyocardial biopsy was performed at the time of cardiac catheterization. The patient was started on high-dose steroids (1 g methylprednisolone given daily) with rapid improvement of hemodynamic parameters within a few hours of starting steroid therapy, and he was weaned off pressors after 72 hours. A follow-up echocardiogram at this time revealed that the LVEF had improved to 30%. Review of the endomyocardial biopsy specimen confirmed eosinophilic myocarditis with evidence of eosinophil degranulation and focal myocyte damage (Figure1). At the time of dismissal from the hospital (hospital day 7), he was significantly improved, and a follow-up echocardiogram revealed an LVEF of 46%. The peripheral eosinophil count was 290 × 109/L at dismissal. After LV function recovery (LVEF, 55%-60% at 4 weeks after imatinib discontinuation), the patient was rechallenged with imatinib (100 mg/day), with concurrent prednisone (60 mg/day on days 1-3, 20 mg/d on days 4-6, with slow taper thereafter), and close monitoring of cardiac function with serial echocardiograms. A symptomatic left lower extremity deep venous thrombus was noted at this visit for which anticoagulation was started. The patient tolerated imatinib retreatment well, without recurrence of cardiac toxicity. At last follow-up, the patient was asymptomatic, without evidence of peripheral eosinophilia (Table 1).
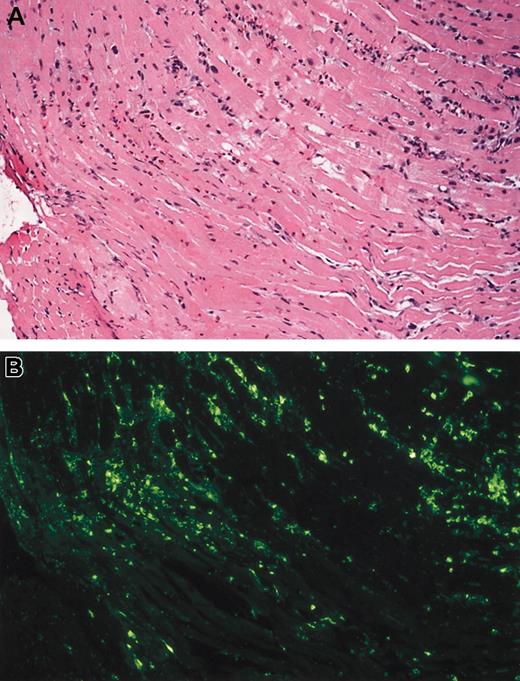
Eosinophil infiltrate and degranulation.
(A) Eosinophilic myocarditis (case 1). Low-power photomicrograph showing the presence of a patchy interstitial infiltrate of eosinophils. These infiltrates were focally associated with myocyte damage (hematoxylin-eosin). (B) Eosinophil major basic protein (MBP). Step section of microscopic field shown in (A) stained for eosinophil MBP. Note marked MBP deposition, much of it extracellular, indicating eosinophil degranulation (immunofluorescence). Original magnification for both panels, × 100.
Eosinophil infiltrate and degranulation.
(A) Eosinophilic myocarditis (case 1). Low-power photomicrograph showing the presence of a patchy interstitial infiltrate of eosinophils. These infiltrates were focally associated with myocyte damage (hematoxylin-eosin). (B) Eosinophil major basic protein (MBP). Step section of microscopic field shown in (A) stained for eosinophil MBP. Note marked MBP deposition, much of it extracellular, indicating eosinophil degranulation (immunofluorescence). Original magnification for both panels, × 100.
Case 2.
A 32-year-old man presented with a 24-month history of a progressive skin rash that began as small erythematous papules on the extremities but gradually evolved to a diffuse erythroderma associated with scaling. Response to pulse steroid therapy was transient with prompt relapse of the rash after steroid taper or cessation. Several months prior to presentation at Mayo Clinic, the patient developed intermittent and occasionally high-grade fevers, weight loss, generalized lymphadenopathy, splenomegaly, and abnormal liver function tests. A left axillary lymph node biopsy was consistent with CD20+, Epstein-Barr virus–associated B-cell lymphoproliferative disorder, and consequently chemo-immunotherapy with a combination of rituximab plus CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CHOP) was started. The patient received 8 courses of R-CHOP with reduction of skin rash, lymphadenopathy, and splenomegaly, but 1 month after completion of this therapy, the skin rash and lymphadenopathy recurred. At this time, leukocytosis with eosinophilia was noted (Table 1) as well as progressive upper-lobe pulmonary consolidation on chest X-ray. After recovery from aStaphylococcus aureus skin infection with septicemia that required 6 weeks of antibiotic therapy, the patient underwent fiberoptic bronchoscopy with bronchoalveolar lavage and transbronchial lung biopsy and right axillary lymph node biopsy. The lung biopsy was consistent with chronic eosinophilic pneumonia, and the lymph node biopsy revealed an atypical lymphohistiocytic infiltrate with eosinophils. Given the progressive increase in peripheral eosinophil count associated with infiltration of lungs, lymph nodes, and possibly skin with eosinophils, the diagnosis of hypereosinophilic syndrome was made, and therapy with imatinib was initiated at the dose of 400 mg/day.
The patient experienced prompt symptomatic improvement, with decreased cough and dyspnea, and decreased skin erythema and desquamation within the first week of imatinib therapy. By day 7 of therapy, both leukocyte and eosinophil counts had decreased from 17.8 × 109/L to 13.3 × 109/L and 10.0 × 109/L to 7.4 × 109/L, respectively, and a follow-up chest X-ray revealed significant clearing of his bilateral upper lobe pulmonary infiltrates. At last follow-up, 8 months after starting imatinib, the patient was in complete clinical remission and working full time. He had regained approximately 50 of the 70 lb of weight he had lost because of illness, and a follow-up chest X-ray and CT scan revealed complete resolution of the lung infiltrates. The dose of imatinib was empirically decreased to 300 mg/day at this time, although the patient tolerated the 400 mg/day dose without any toxicity.
Case 3.
A 24-year-old man presented with a long history of an acneiform skin rash involving his face and trunk and a several year history of relatively frequent upper respiratory tract, particularly sinus, infections, requiring antibiotic therapy. Three months prior to his Mayo Clinic presentation, leukocytosis and eosinophilia (Table 1) were incidentally noted during evaluation for an episode of “bronchitis.” He also had intermittent epigastric pain and low-grade fevers at this time. Evaluation revealed iron-deficiency anemia, but an esophagogastroduodenoscopy (EGD) with biopsies did not reveal evidence of eosinophilic gastritis or enteritis. A bone marrow aspirate and trephine biopsy confirmed absent iron stores and further revealed prominent eosinophilia, with mature eosinophils comprising 35% to 40% of all nucleated marrow cells. He was treated with prednisone at the dose of 20 mg/day for several weeks without any decrease in the peripheral eosinophil count. After tapering and discontinuation of corticosteroid treatment, the patient was started on imatinib at the dose of 100 mg/day.
A decline in peripheral leukocytosis and eosinophilia was promptly noted (within 1 week). The decline was gradual, but progressive, and at 8-week follow-up, the white blood count and eosinophil count had declined from 42.2 × 109/L to 22.4 × 109/L and from 32.5 × 109/L to 15.5 × 109/L, respectively. He was clinically stable and tolerating treatment well, and imatinib was escalated to 400 mg/day. He developed mild fatigue on the increased dose of imatinib, and at last-follow-up, while a further decline in the eosinophil count had been documented (Table 1), the higher dose of imatinib had only modest additional benefit.
Case 4.
A 50-year-old man developed progressive and eventually intractable, generalized skin pruritus that started 2 years prior to his Mayo Clinic presentation. The pruritus became more severe in the 6 months immediately prior to presentation, and, despite therapeutic trials with numerous agents, symptomatic benefit was consistently achieved only with prednisone. Unfortunately, however, the skin condition promptly relapsed on tapering of the corticosteroid, and he was consequently maintained on prednisone for a period of 9 months. In the year prior to presentation, eosinophilia had been noted (range, 8%-28% on the white blood count differential, while on prednisone therapy), but several skin biopsies had been unrevealing in terms of a specific diagnosis. The patient was noted to have excoriations and prurigo nodularis with pigmentation over the scalp, face, trunk, sacral area, buttocks, and proximal extremities on physical examination. Further evaluation revealed peripheral blood and marrow eosinophilia (Table 1), with maturing eosinophils and eosinophilic precursors accounting for 20% to 25% of bone marrow cellularity. A markedly elevated immunoglobulin E (IgE) level was also noted (9585.0 kU/L; mean, 13.2; +1 SD, 41.0; +2 SD, 127.0). The patient was started on imatinib at the dose of 100 mg/day.
Although an approximately 40% decline was noted in the peripheral eosinophil count (Table 1), the patient experienced profound fatigue (grade 3) and elected to stop imatinib after 4 weeks of therapy.
Case 5.
A 56-year-old man presented with a 5-year history of intensely pruritic skin nodules and intermittent debilitating headaches. The former initially appeared on the trunk and abdomen and later involved his proximal extremities. Peripheral eosinophilia was first noted 4 years ago (white blood cell count 16.3 × 109/L with 30% eosinophils), and a detailed evaluation, including bone marrow and skin biopsies, was unrevealing except for peripheral eosinophilia and elevated IgE (25 360 kU/L; mean, 13.2; +1 SD, 41.0; +2 SD, 127.0). The etiology of his headaches remained indeterminate despite multiple imaging studies. Three years prior to presentation, the patient was started on prednisone (80 mg/day), with prompt improvement of his skin lesions and headaches and a decrease in the peripheral eosinophil count. Unfortunately, however, both the skin lesions and headaches relapsed on tapering of the corticosteroid, and he has been consequently maintained on variable doses of prednisone since then. The patient also received interferon-α at the dose of 3 to 5 MU/day subcutaneously added to prednisone for a period of 9 months with little added benefit. A subsequent workup revealed persistent peripheral and bone marrow eosinophilia (Table 1; 15% of marrow cellularity) and the possible presence of a clonal T-cell population, both by PCR and Southern blot assays. Clinically, however, no evidence of a T-cell lymphoma was noted, and the interleukin-5 (IL-5) level was within normal range. The patient was started on imatinib at the dose of 100 mg/day.
After 4 weeks of therapy, he had derived modest (25%) symptomatic benefit from imatinib therapy, with decreased erythema and flattening of the skin nodules and decreased pruritus. Although no decrease was noted in the peripheral eosinophil count, given the symptomatic benefit noted until this time, the imatinib dose was escalated to 400 mg/day. Unfortunately, however, no additional clinical benefit was apparent in the subsequent 8 weeks of therapy at the higher dose, and the peripheral eosinophil count was unchanged, hence imatinib was decreased to 100 mg/day as maintenance dose, in the absence of other effective therapies.
Eosinophilia-associated chronic myeloid disorders (eos-CMD)
Case 6.
A 45-year-old man had a 20-year history of untreated eosinophilia, during which time his only complaints were a chronic cough, which responded to inhaled bronchodilator therapy, and gout, which required treatment with allopurinol and colchicine. In the year immediately preceding his presentation at Mayo Clinic, the patient had experienced a rapid clinical decline, with increasing fatigue, bone pain, weight loss (35 lb), and progressive anemia and splenomegaly (spleen crossed the midline by 3 cm at the umbilicus). The pain and fatigue had most recently left the patient largely bed-bound. Initial evaluation revealed anemia, thrombocytopenia, and leukocytosis with eosinophilia and a leukoerythroblastic picture (Table 1). The bone marrow aspirate and trephine biopsy revealed a hypercellular marrow with decreased numbers of erythroid precursors and megakaryocytes, but an expanded granulocytic pool with eosinophilia and left-shifted maturation. Reticulin fibers were modestly increased (grade 1+) and a slight increase in CD34+ blasts was noted. Cytogenetic studies revealed a normal karyotype. The aggregate findings were suggestive of an eos-CMD associated with myelofibrosis. The patient was started on imatinib therapy at the dose of 100 mg/day.
The patient had a dramatic response to therapy that was manifest both clinically, and by decreasing eosinophilia, within a week of starting treatment. At re-evaluation (12 weeks), the patient was clinically asymptomatic and reported a dramatic increase in his energy level and stamina. He had gained 9 kg in weight, and the spleen tip was no longer convincingly palpable on physical examination. Laboratory testing revealed improved hemoglobin concentration and platelet count, as well as complete resolution of the eosinophilia and leukoerythroblastic picture (Table 1). A follow-up bone marrow examination revealed a dramatic decrease in overall cellularity and resolution of eosinophilia (Figure 2). The patient at last follow-up continued to be in clinical and histologic remission.
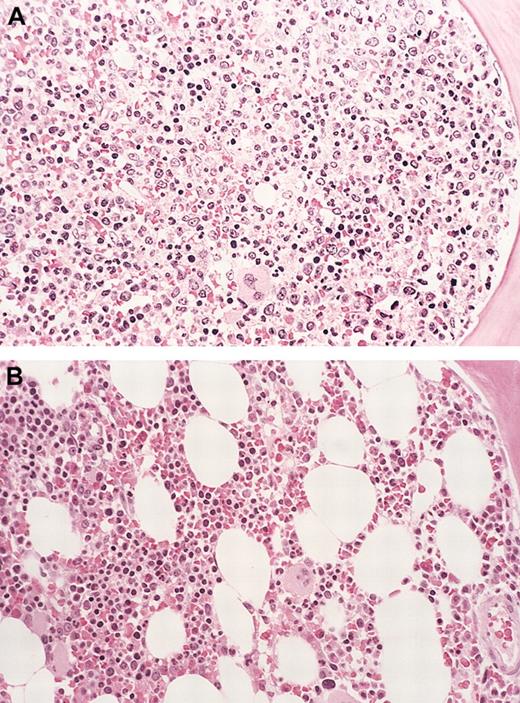
Bone marrow eosinophil cytoreduction with imatinib therapy (case 6).
Hematoxylin-eosin (H&E)–stained bone marrow biopsy tissue (A), showing markedly hypercellular marrow with left-shifted granulopoiesis and eosinophilia before treatment with imatinib. (B) Shows the dramatic reduction in marrow cellularity with normal trilineage hematopoiesis after imatinib therapy (at 16 weeks). Original magnification for both panels, × 160.
Bone marrow eosinophil cytoreduction with imatinib therapy (case 6).
Hematoxylin-eosin (H&E)–stained bone marrow biopsy tissue (A), showing markedly hypercellular marrow with left-shifted granulopoiesis and eosinophilia before treatment with imatinib. (B) Shows the dramatic reduction in marrow cellularity with normal trilineage hematopoiesis after imatinib therapy (at 16 weeks). Original magnification for both panels, × 160.
Case 7.
A 58-year-old man presented with a several-month history of progressive fatigue, weight loss, and abdominal pain from a rapidly enlarging spleen. He was found to have significant anemia, thrombocytopenia, a leukoerythroblastic picture (Table 1), and splenomegaly (spleen crossed the midline) on physical examination. An initial bone marrow biopsy was inadequate but was thought to be consistent with agnogenic myeloid metaplasia, and the patient received supportive care. He remained transfusion dependent despite erythropoietin support, and a presumptive diagnosis of pure red cell aplasia (PRCA) was made on the basis of absent erythroid precursors on the initial bone marrow study. The patient was started on hydroxyurea therapy for progressive symptomatic splenomegaly, and he did relatively well on this treatment (stable dose of 500 mg/day) for 3 to 4 months, at which time he had abrupt onset of fevers with rigors and developed acute pulmonary edema. Peripheral eosinophilia was noted at this time, and a repeat bone marrow aspirate and trephine biopsy showed a hypercellular marrow with increased granulocytic precursors and marked eosinophilia. Megakaryocytes were noted to be increased and present in clusters, and a focal increase in reticulin fibers was noted (grade 2+). Cytogenetic studies revealed trisomy 8, and a diagnosis of chronic eosinophilic leukemia with myelofibrosis associated with eosinophilic endomyocarditis was made. The patient was treated with pulse corticosteroids and continued on hydroxyurea (500 mg/day), which decreased the eosinophilia and spleen size. He did not, however, tolerate discontinuation of the steroids and was continued on a maintenance dose of 30 mg/day prednisone. The patient was also treated with cyclosporine for 3 months (in combination with steroids and hydroxyurea) on an empiric basis for PRCA, without response. Three weeks after the discontinuation of his ongoing therapies, the patient was started on imatinib at the dose of 400 mg/day.
The patient declined further clinically while on therapy with imatinib. He developed profound fatigue and lost more weight, and progressive hepatosplenomegaly was noted on physical examination. Similarly, there was no improvement in eosinophil count (Table 1); he remained transfusion dependent and was deemed to have failed imatinib therapy, and this agent was discontinued after 4 weeks of treatment.
Mutational analysis
Mutational analysis of c-kit and PDGFRβ genes for 6 patients revealed several previously described polymorphisms, and for case 3, a heterozygous C>G change at position 3496 (GenBank Accession no. J03278), which predicts Ser1047Cys. No other potentially pathogenic changes were found in either gene.
Discussion
We have previously reported the efficacy of imatinib mesylate for the treatment of 5 patients with HES.9 Here, we describe treatment responses of 5 additional patients with HES, and 2 patients with eos-CMD. This study confirms prior observations of the efficacy of imatinib for treatment of HES and potentially further extends the indications for use of this agent for therapy of select cases of eos-CMD. Furthermore, in contrast to a prior report,9 our results suggest that serum IL-5 levels are not useful in distinguishing responders from nonresponders. Finally, a previously unrecognized, potentially life-threatening toxicity of imatinib, which may be specific for HES/eos-CMD patients, is described. Two patients with HES had a prompt and dramatic resolution of peripheral eosinophilia and are in complete clinical remission, and another patient has experienced a gradual and sustained ongoing partial response in terms of a decrease in the eosinophil count. One patient with HES had no decrease in eosinophilia, and treatment was abandoned in another because of drug toxicity. Although 1 of the 2 patients with eos-CMD had progressive disease despite imatinib therapy, the second had a prompt and dramatic response to treatment and remains in complete clinical and histologic remission at last follow-up.
Constitutive activation of the PDGFRβ oncoprotein, commonly associated with the reciprocal translocation t(5;12)(q31-33;p13) and fusion of the ETV6 (TEL) and PDGFRβ genes, is frequently characterized by prominent eosinophilia and represents a relatively well-defined subtype of eos-CMD.11,12 This karyotypic abnormality, however, is an extremely rare occurrence, and in a retrospective review of 57 709 cytogenetic studies performed at the Mayo Clinic over a 14-year period, only 25 cases with t(5;12) were identified (prevalence = 0.04%) (P. T. Greipp, personal communication, December 2002). Eos-CMD syndromes are also associated with other specific cytogenetic abnormalities such as t(8;13)(p11;q12),13 t(8;9)(p11;q32-34),14 and t(6;8)(q27;p12),15 all of which are associated with rearrangement of the fibroblast growth factor receptor-1(FGFR1) gene to generate specific fusion genes that are likely oncogenic.16 Of note, no patients with cytogenetically cryptic rearrangements of either PDGFRβ orFGFR1 have been previously described. Other cytogenetic abnormalities reported to be associated with eos-CMD, where the molecular mechanisms for disease pathogenesis have not yet been elucidated, include t(8;9)(p22;p23), t(3;9;5)(q25;q34;q33),17 and trisomy 15.18-20Additionally, a few cases of eosinophilic-myelodysplastic syndrome, particularly therapy-related myelodysplastic syndrome, have also been described and are associated with the t(1;7) cytogenetic abnormality.21
In our study, despite careful evaluation of 6 of 7 patients by bone marrow (BM) cytogenetic analysis and screening of most coding exons of c-kit and PDGFRβ genes, none of the known mutations in cellular targets of imatinib (ie, bcr-abl, c-kit, or PDGFRβ tyrosine kinases) were identified. One patient (case 3) had a heterozygous change in PDGFRβ, which is predicted to substitute Ser1047Cys in the C-terminal domain of the protein. This change is not a known polymorphism and it occurs in a region that is highly conserved between the human, mouse, and rat. However, no functional importance has been ascribed thus far to the C-terminal domain, and so the significance of this change is unclear. The majority of responding patients demonstrated treatment responses at the dose of 100 mg/day, which is lower than the conventional dose of 400 to 600 mg/day used in other imatinib-responsive malignancies such as CML22-24and gastrointestinal stromal tumors (GISTs).25 In the phase 1 study that evaluated imatinib at doses from 25 to 1000 mg/day in chronic myeloid leukemia (CML), complete hematologic responses were consistently seen at a dose of 300 to 1000 mg/day.26 The above issues raise the possibility that a yet unidentified cellular tyrosine kinase that is inhibited by imatinib is implicated in the pathogenesis of HES and eos-CMD. However, despite an analysis of the pretreatment and posttreatment phosphoprotein complement of purified eosinophils from several patients, by probing whole cell lysates resolved by 1-dimensional sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) with antiphosphotyrosine antibodies, no candidate phosphoproteins targeted by imatinib treatment were identified (data not shown).
An alternative hypothesis is that aberrant production of cytokines such as IL-3, IL-5, and granulocyte-macrophage colony-stimulating factor (GM-CSF) by an occult T-cell clone or by phenotypically abnormal but polyclonal T lymphocytes has been implicated in eosinophilopoiesis and eosinophil activation.3-6 Three of the 5 patients with HES were tested for presence of an occult T-cell clone by T-cell receptor gene rearrangement studies. Of these, only 1 patient (case 5; nonresponder to imatinib with a normal serum IL-5 level) had a detectable occult T-cell clone, confirmed by testing on 2 separate occasions in a 2-year interval. In this study, the serum IL-5 level was elevated in 3 patients with HES (all 3 responding patients) and in the patient with eosinophilia-associated atypical chronic myeloproliferative disorder, who also responded to imatinib (Table 1). This finding refutes the earlier suggestion that elevated serum IL-5 levels may predict for poor responses to imatinib treatment in patients with HES.9 This observation is consistent with the previously reported dramatic response to treatment of a single patient with eosinophilia-associated chronic myeloproliferative disorder, who had the t(5;12) translocation and elevated IL-5 levels.10It should be stressed, however, that the demonstration of elevated serum IL-5 levels cannot be taken as conclusive evidence that eosinophilia is reactive, given the example of the above-mentioned case with elevated IL-5 levels in the presence of a clonal cytogenetic abnormality [t(5;12)] in myeloid cells. In addition, elevated levels of serum IL-6, another cytokine implicated in eosinophil production and activation, has been demonstrated in eos-CMD associated with specific clonal cytogenetic abnormalities.17 27
Although the exact role of c-kit–stem cell factor (SCF) signaling in the idiopathic hypereosinophilia syndromes is currently unclear, given the data that implicates this receptor-ligand pair in eosinophil adhesion, activation, and degranulation,28-30we examined c-kit expression on marrow eosinophils by CD117 immunostaining of bone marrow sections for 4 of the 7 patients (cases 1, 2, 4, and 5). C-kit expression was not detected on eosinophils in any of these cases.
Despite encouraging responses with imatinib in this study, and inearlier reports,7-9 the absence of both a specific cellular target for imatinib and a consistent cytogenetic abnormality has hindered the identification, in clinical practice, of those hypereosinophilic patients who are likely to benefit from imatinib therapy. Nevertheless, considering the commonly chronic and protracted clinical course of patients with primary eosinophilic disorders, and the relatively rapid decrease in the eosinophil count (days) in imatinib-responsive patients, a therapeutic trial of this drug is a useful intervention.
Imatinib was relatively well tolerated in this study and was transiently withheld in 1 patient (case 1) for a previously unrecognized fulminant acute cardiac toxicity that patients with HES may be specifically susceptible to, and was discontinued in another (case 4) because of patient preference following development of worsening fatigue. Occult infiltration of the endomyocardium with eosinophils, which is not apparent by echocardiography, probably occurs in at least a subset of patients with HES, who are otherwise asymptomatic from the cardiac standpoint. For case 1, the time course of development of acute LV dysfunction after starting imatinib, the rapid response to corticosteroid therapy, and the histologic findings on endomyocardial biopsy, all suggest that inflammatory response to degranulation and/or lysis of infiltrating eosinophils has the potential to cause acute and fulminant cardiogenic shock. It is imperative that this potentially life-threatening complication be recognized early, because, as shown for case 1, the acute LV dysfunction may be reversible with prompt institution of corticosteroid therapy. In our practice, we now routinely perform serial echocardiograms in the first week of imatinib therapy, particularly in those patients in whom the eosinophilia shows a rapid response, to screen for preclinical onset of cardiac dysfunction.
Although imatinib is clearly an active agent in HES and, as demonstrated in this report, other eosinophilia-associated chronic myeloid disorders, several crucial issues need further study. These issues include (1) the mechanism of imatinib activity at the molecular level remains unclear; and (2) the basis for heterogeneity of patients with HES, both in terms of the rate of decrease in eosinophil counts (eg, case 3 compared with cases 1 and 2) and the variable response to therapy, is also unclear.
We thank Gail Kephart for excellent technical assistance. We thank Novartis Pharmaceuticals (Basel, Switzerland) for providing the drug free of charge for patients on this study.
Prepublished online as Blood First Edition Paper, December 27, 2002; DOI 10.1182/blood-2002-10-3103.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ayalew Tefferi, Division of Hematology and Internal Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: tefferi.ayalew@mayo.edu.