Approximately 40% of children with acute myeloid leukemia (AML) who respond to initial therapy subsequently relapse. Multidimensional flow cytometry employing a standardized panel of monoclonal antibodies enables the detection of small numbers of occult leukemic cells that persist during therapy using technology adaptable by most clinical laboratories. We performed a prospective, blinded evaluation of bone marrow specimens obtained from 252 pediatric patients with de novo AML to determine whether detection of occult leukemia defined as more than or equal to 0.5% blasts with aberrant surface antigen expression as determined by flow cytometry was predictive of subsequent relapse. Occult leukemia was detected in 41 (16%) of the 252 patients who responded to initial induction therapy. In time-dependent multivariate analyses that controlled for allogeneic marrow transplantation, variable intervals between sample submission, age, sex, white blood cell count at diagnosis, presence of splenomegaly or hepatomegaly, and presence of more than 15% blasts in the marrow after the first course of induction, patients harboring occult leukemia were 4.8 times more likely to relapse (95% confidence interval [CI] = 2.8 to 8.4,P < .0001) and 3.1 times more likely to die (95% CI; 1.9 to 5.1, P < .0001) than those lacking leukemia detectable by flow cytometry. In this analysis, flow cytometric evidence of leukemia after the initiation of therapy emerged as the most powerful independent prognostic factor associated with poor outcome. Among patients in whom a marrow sample was available for analysis at the end of consolidation therapy, overall survival at 3 years was 41% versus 69% for patients with and without occult leukemia, respectively (P = .0058).
Introduction
Recently, several cooperative groups have characterized highly predictive parameters associated with poor outcome in acute myeloid leukemia (AML).1,2 Such features are now being used to identify high-risk patients in remission who might derive the greatest benefit from treatment intensification. Similarly, these parameters are being used to define a lower-risk population for whom allogeneic transplantation in first remission might offer minimal clinical advantage. Using parameters including diagnostic cytogenetics and morphologic response to the first course of induction therapy, almost half of patients in first remission can be categorized as good or poor risk.1 There remains however an unmet need to develop and evaluate assays that predict relapse in the other half of patients who carry a 50% risk of leukemia recurrence.
Detecting leukemia cells that remain after delivery of intensive induction chemotherapy is one of the most promising means of identifying patients who will likely relapse. Most AML minimal residual disease studies have employed assays that identify occult leukemia by detection of unique fusion genes or patterns of aberrant cell surface antigen expression. Detection of the characteristic t(15;17) chromosomal abnormality by reverse transcription and the polymerase chain reaction (PCR) appears particularly useful as a means of predicting relapse for the small subset of AML patients with acute promyelocytic leukemia (APL).3-5 In contrast, the fusion genes expressed in the core binding factor leukemias t(8;21) and inv(16) may remain detectable by PCR in patients who have remained in long-term remissions.6,7 While newer, quantitative PCR assays8 9 might enable accurate prediction of relapse in these patients, the marked heterogeneity of cytogenetic abnormalities in AML precludes the general use of PCR-based assays for detecting residual leukemia.
Because normal surface antigen expression during differentiation is uniform and tightly regulated, normal cells demonstrate characteristic antigen expression patterns that are reliably observed in healthy individuals.10 Leukemic blast cells display inappropriate cell surface antigen patterns that differ considerably from normal expression patterns and can thus be distinguished from normal hematopoietic elements.11-14 Multiple fluorescence signals corresponding to the expression of various cell surface antigens can be measured simultaneously to identify and quantitate leukemic cells using multidimensional flow cytometry.
Assays using either standardized or patient-specific antibody combinations have been studied as a means of detecting occult leukemia in patients with AML actively receiving therapy. There are 2 groups that have prospectively employed patient-specific cocktails of antibody combinations to identify residual leukemia in the portion of adult patients with AML in whom aberrant immunophenotypes were identified at diagnosis. San Miguel et al have provided elegant data defining 4 risk categories for subsequent relapse among the 70% to 80% of 126 adult patients with AML bearing sufficiently aberrant phenotypic abnormalities amenable to their detection strategy.15Among those patients harboring levels of residual leukemia greater than 1% in the first marrow aspirate obtained during morphologic remission, 85% subsequently relapsed. Venditti et al reported similar results in another study of adult patients with AML.16 These 2 studies of adult AML populations suggest that many patients continue to harbor detectable levels of leukemia during remission, and might benefit from augmented therapy.
In these 2 studies, up to one quarter of adult patients with AML were not evaluable for residual disease using patient-specific antibody cocktails because sufficiently aberrant immunophenotypes were not identified at diagnosis. These detection strategies also required custom antibody panels that might be difficult to employ in busy clinical laboratories. With the intent of efficiently evaluating all patients with de novo AML, we employed a standardized panel of antibody combinations to identify leukemic blast cells based on a difference from antigen expression that characterizes normal hematopoiesis. The antibody combinations were selected to cover a broad range of antigens expressed on myeloid cells both in the neutrophil and monocyte lineages. Because the intensities of these antigens change in a well-characterized manner as these cells mature, inappropriate relationships in expression enable tumor detection.17 In a retrospective study of 35 pediatric patients with AML, we found that patients harboring residual leukemia during remission were approximately 3 times more likely to relapse than those lacking detectable leukemia.18 To confirm and extend our previous findings in a much larger pediatric population, we evaluated marrow specimens from 252 patients with de novo AML who were responsive to treatment with intensively timed chemotherapy on 2 nearly identical Children's Cancer Group AML treatment protocols.
Patients and methods
Study patients
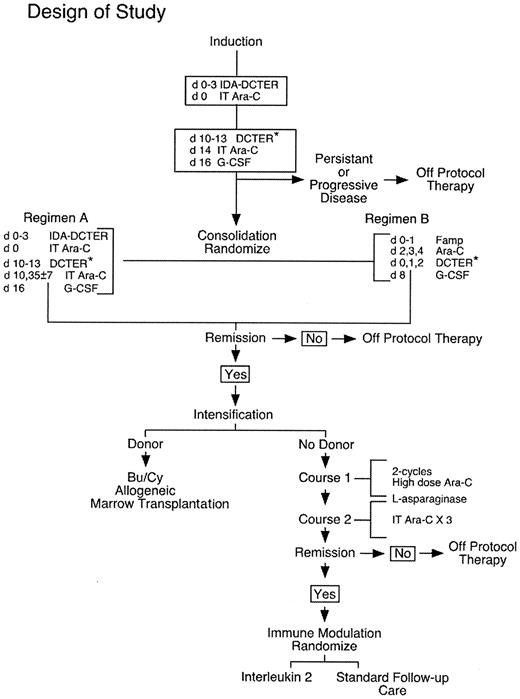
From 1995 to 2000, 469 children with AML and myelodysplastic syndrome (MDS) were enrolled in Children's Cancer Group (CCG) Study B-942, “Detection of Minimal Residual Disease in Children Receiving Therapy for AML or MDS” and were concurrently enrolled in prospective groupwide treatment studies CCG-2941 or CCG-2961 for patients with newly diagnosed AML or MDS. Figure1 shows the treatment schema for the randomized, phase 3 CCG-2961 study. CCG-2941 was a smaller pilot study of the idarubicin-containing induction regimen given prior to regimen B as noted in Figure 1. CCG-2961 differed from CCG-2941 in the following manner: daunorubicin replaced idarubicin in the second cycle of induction therapy and 2 consolidation regimens and postintensification interleukin 2 (IL-2) were evaluated in randomized comparisons. Recognizing the limitations of conventional microscopy of marrow aspirates to define remission, therapy responsive disease was defined by the CCG-2961 study as less than 30% blast cells by morphologic examination of a marrow aspirate after one course of chemotherapy. Among 469 enrolled patients, 371 were categorized by the treatment protocols as having responsive disease and were thus eligible to receive consolidation therapy. The evaluable study group consisted of those 252 of 371 responding patients in whom a bone marrow specimen was submitted to the CCG AML Reference Laboratory at least 14 days after the initiation of chemotherapy and at any time prior to any evidence of leukemic relapse.
Design of Children's Cancer Group AML Treatment Study 2961.
Marrow samples were aspirated fresh and submitted to the AML Reference Laboratory at diagnosis, and at the end of induction, consolidation, and intensification therapies. “DCTER*” refers to dexamethasone, cytosine arabinoside, 6-thioguanine, etoposide, and rubidamycin (daunomycin). Idarubicin replaces daunomycin in “IDA-DCTER.”
Design of Children's Cancer Group AML Treatment Study 2961.
Marrow samples were aspirated fresh and submitted to the AML Reference Laboratory at diagnosis, and at the end of induction, consolidation, and intensification therapies. “DCTER*” refers to dexamethasone, cytosine arabinoside, 6-thioguanine, etoposide, and rubidamycin (daunomycin). Idarubicin replaces daunomycin in “IDA-DCTER.”
Leukemic relapse was defined in the treatment protocols as (1) 2 sequential morphologic marrow evaluations greater than one week apart showing more than or equal to 30% blasts, (2) emergence of more than or equal to 30% blasts after less than 5% blasts were previously identified by morphologic evaluation of marrow, or (3) emergence of extramedullary disease after induction therapy. Characteristics of this study group are summarized in Table 1. In aggregate, 549 bone marrow specimens were evaluated for occult leukemia. The median number of analyses per evaluable patient was 2 with a range from 1 to 7 analyses. Median follow-up time for the 252-patient study cohort was 34.6 months. The institutional review board of each participating institution approved the study and informed consent for study participation was obtained from each patient or legal guardian.
Comparison of demographic and clinical characteristics of 252 patients who achieved remission and were evaluated for occult leukemia by flow cytometry
| . | Flow+ N = 41 . | Flow− N = 211 . | P . | ||
|---|---|---|---|---|---|
| . | n . | % . | n . | % . | |
| Age, in years | .765 | ||||
| 0-2 | 9 | 22 | 44 | 21 | |
| 2-10 | 12 | 29 | 65 | 31 | |
| 10-21 | 20 | 49 | 102 | 48 | |
| Sex | .719 | ||||
| Male | 24 | 59 | 114 | 54 | |
| Female | 17 | 41 | 97 | 46 | |
| Race | .328 | ||||
| White | 26 | 65 | 148 | 70 | .490 |
| Black | 7 | 18 | 15 | 7 | .060 |
| Hispanic | 4 | 10 | 28 | 13 | .796 |
| Asian | 1 | 3 | 6 | 3 | > .99 |
| Other | 2 | 5 | 13 | 6 | > .99 |
| WBC count, median | 15.45 | 18.75 | .839 | ||
| Hemoglobin level, median | 7.5 | 8.2 | .504 | ||
| Platelet count, median | 60.0 | 46.5 | .249 | ||
| FAB | .082 | ||||
| M0 | 2 | 5 | 5 | 2 | .309 |
| M1 | 10 | 25 | 31 | 15 | .159 |
| M2 | 5 | 13 | 69 | 33 | .370 |
| M4 | 11 | 28 | 49 | 23 | .549 |
| M5 | 5 | 13 | 32 | 15 | .810 |
| M6 | 2 | 5 | 3 | 1 | .181 |
| M7 | 2 | 5 | 13 | 6 | > .99 |
| MDS (NOS) | 0 | 0 | 1 | 0 | > .99 |
| RAEB | 0 | 0 | 2 | 1 | > .99 |
| RAEB-T | 2 | 5 | 6 | 3 | .617 |
| Other | 1 | 3 | 0 | 0 | .159 |
| Splenomegaly | 12 | 29 | 63 | 30 | .940 |
| Hepatomegaly | 12 | 29 | 62 | 29 | .988 |
| Cytogenetics* | .066 | ||||
| Normal | 9 | 38 | 24 | 19 | .057 |
| t(15;17) | 1 | 4 | 0 | 0 | .158 |
| t(8; 21) | 1 | 4 | 33 | 26 | .017 |
| Abnormal 16 | 2 | 8 | 16 | 13 | .740 |
| Abnormal 11 | 5 | 21 | 32 | 25 | .799 |
| t(6;9) | 1 | 4 | 2 | 2 | .405 |
| −7/7q- | 0 | 0 | 2 | 2 | > .99 |
| +8 | 2 | 8 | 5 | 4 | .305 |
| +21 | 0 | 0 | 1 | 1 | > .99 |
| Pseudodiploid | 3 | 13 | 8 | 6 | .381 |
| Hyperdiploid | 0 | 0 | 5 | 4 | > .99 |
| . | Flow+ N = 41 . | Flow− N = 211 . | P . | ||
|---|---|---|---|---|---|
| . | n . | % . | n . | % . | |
| Age, in years | .765 | ||||
| 0-2 | 9 | 22 | 44 | 21 | |
| 2-10 | 12 | 29 | 65 | 31 | |
| 10-21 | 20 | 49 | 102 | 48 | |
| Sex | .719 | ||||
| Male | 24 | 59 | 114 | 54 | |
| Female | 17 | 41 | 97 | 46 | |
| Race | .328 | ||||
| White | 26 | 65 | 148 | 70 | .490 |
| Black | 7 | 18 | 15 | 7 | .060 |
| Hispanic | 4 | 10 | 28 | 13 | .796 |
| Asian | 1 | 3 | 6 | 3 | > .99 |
| Other | 2 | 5 | 13 | 6 | > .99 |
| WBC count, median | 15.45 | 18.75 | .839 | ||
| Hemoglobin level, median | 7.5 | 8.2 | .504 | ||
| Platelet count, median | 60.0 | 46.5 | .249 | ||
| FAB | .082 | ||||
| M0 | 2 | 5 | 5 | 2 | .309 |
| M1 | 10 | 25 | 31 | 15 | .159 |
| M2 | 5 | 13 | 69 | 33 | .370 |
| M4 | 11 | 28 | 49 | 23 | .549 |
| M5 | 5 | 13 | 32 | 15 | .810 |
| M6 | 2 | 5 | 3 | 1 | .181 |
| M7 | 2 | 5 | 13 | 6 | > .99 |
| MDS (NOS) | 0 | 0 | 1 | 0 | > .99 |
| RAEB | 0 | 0 | 2 | 1 | > .99 |
| RAEB-T | 2 | 5 | 6 | 3 | .617 |
| Other | 1 | 3 | 0 | 0 | .159 |
| Splenomegaly | 12 | 29 | 63 | 30 | .940 |
| Hepatomegaly | 12 | 29 | 62 | 29 | .988 |
| Cytogenetics* | .066 | ||||
| Normal | 9 | 38 | 24 | 19 | .057 |
| t(15;17) | 1 | 4 | 0 | 0 | .158 |
| t(8; 21) | 1 | 4 | 33 | 26 | .017 |
| Abnormal 16 | 2 | 8 | 16 | 13 | .740 |
| Abnormal 11 | 5 | 21 | 32 | 25 | .799 |
| t(6;9) | 1 | 4 | 2 | 2 | .405 |
| −7/7q- | 0 | 0 | 2 | 2 | > .99 |
| +8 | 2 | 8 | 5 | 4 | .305 |
| +21 | 0 | 0 | 1 | 1 | > .99 |
| Pseudodiploid | 3 | 13 | 8 | 6 | .381 |
| Hyperdiploid | 0 | 0 | 5 | 4 | > .99 |
RAEB indicates refractory anemia with excess blasts; RAEB-T, refractory anemia with excess blasts in transformation.
For cytogenetics: N = 24 for Flow+; N = 128 for Flow−.
Bone marrow specimens
The diagnosis of AML or MDS and remission classification of bone marrow aspirates were established independently by the patient's oncologist or the institutional pathologist following review of the Wright-Giemsa–stained slide, enzyme histochemistry, and standard immunophenotype performed initially at the institution where the patient was treated. These data were entered into case report forms and submitted to the CCG Operations Office for inclusion in the study data set.
Bone marrow specimens aspirated from the iliac crest were requested at diagnosis and after each course of induction, consolidation or intensification therapy in accordance with the treatment protocols. Specimens were shipped overnight to the CCG AML Reference Laboratory in Seattle, WA, at ambient temperature. The majority of specimens underwent flow cytometric analysis 48 to 72 hours after the time of aspiration.
Multidimensional flow cytometric analysis
The procedure for labeling cells has been previously described.19 In short, a working dilution of antibodies (Table 2) titered for maximum fluorescence, CD45 peridinin-chlorophyll-a protein (CD45 PerCP), and 0.1 mL of well-mixed heparinized bone marrow were incubated for 20 minutes at room temperature in the dark. The erythrocytes were lysed by adding 3 mL NH4Cl (0.83%, buffered with KHCO3, pH 7.2) for 5 minutes at 37°C. The cells were then pelleted, washed once in phosphate buffered saline (PBS)/2% fetal calf serum/0.1% sodium azide solution, then pelleted again and resuspended in PBS with 1% paraformaldehyde.
Antigen combinations assessed by multidimensional flow analysis
| PE signal* . | FITC signal† . | PerCP signal‡ . |
|---|---|---|
| Negative control for IgG1 | Negative control for IgG2 | CD45 |
| CD11b | HLA DR | CD45 |
| CD19 | CD5 | CD45 |
| CD36 | CD38 | CD45 |
| CD13 | CD16 | CD45 |
| CD34 | CD15 | CD45 |
| CD33 | CD14 | CD45 |
| CD56 | CD7 | CD45 |
| PE signal* . | FITC signal† . | PerCP signal‡ . |
|---|---|---|
| Negative control for IgG1 | Negative control for IgG2 | CD45 |
| CD11b | HLA DR | CD45 |
| CD19 | CD5 | CD45 |
| CD36 | CD38 | CD45 |
| CD13 | CD16 | CD45 |
| CD34 | CD15 | CD45 |
| CD33 | CD14 | CD45 |
| CD56 | CD7 | CD45 |
Phycoerythrin (PE) monoclonal antibody conjugates.
Fluorescein isothiocyanate (FITC) monoclonal antibody conjugates.
Peridinin-chlorophyll-a protein (PerCP) monoclonal antibody conjugate.
Specimens were analyzed on a Cytoron Absolute (Ortho Diagnostic Systems, Raritan, NJ) or a FACS Calibur (Becton Dickinson Immunocytometry Systems, San Jose, CA) flow cytometer. For each combination of antibodies, a total of 10 000 events was recorded. List mode data were analyzed with WinList software (Verity Software, Topsham, ME) in accordance with previously published techniques.18-20 For each sample, a computerized region was created as a means of limiting the analysis to viable cells with a forward and right-angle light scatter pattern characteristic of lymphocytes, monocytes, maturing monocytoid or myeloid elements, and blast-sized cells. Secondary gating was performed to limit further analyses to myeloblasts based on characteristic CD45 versus log side scatter properties. Antigenic expression patterns of these gated myeloblasts were then evaluated in 2-dimensional histograms displaying fluorescence intensity from each of 2 additional monoclonal antibodies (Table 2). This expression pattern was compared with patterns identified during normal differentiation and, when available, to those of the patient's diagnostic leukemia specimen. The lower limit of detection of this approach was determined to be 0.5%.
A bone marrow specimen was defined to be positive if a distinct cluster of blast-sized cells present at a level equal to or exceeding 0.5% of the total number of nucleated cells analyzed for that specimen manifested aberrant cell surface antigen expression as defined by one or more of the following characteristics: (1) expression of nonmyeloid antigens, (2) asynchronous expression of myeloid-associated antigens, (3) over- or underexpression of myeloid-associated antigens, or (4) absence of expression of myeloid-associated antigens.12 Aberrant blasts identified by intensity of antigen expression required the population to be at least 0.5 decades disparate from the relative intensity of antigens expressed on corresponding normal cells.21 Samples lacking cells specifically meeting the above criteria were considered negative. List-mode data from each specimen were independently evaluated by 2 investigators. Reported flow cytometric results differed between the 2 investigators for 10 of 549 (2%) specimens, and the disputes were arbitrated by reanalyzing the list-mode data jointly. In each of these instances, consensus was achieved. Throughout this flow cytometric analysis phase, both investigators were blinded to any corresponding pathologic or clinical data about the enrolled patients. Once a consensus value had been recorded for all specimens, the data set was submitted to the study statistician to determine whether any relationship between flow cytometric data and observed clinical outcomes existed.
Statistical analysis
We initially performed a focused evaluation of 178 of 252 patients in whom at least one marrow specimen was available for analysis before delivery of intensification chemotherapy or allogeneic transplantation to evaluate the prognostic significance of detectable occult leukemia early in treatment (Figure1). Because at least one marrow specimen for flow cytometric evaluation was available from 74 additional children with AML in whom no sample had been submitted before commencement of intensification therapy, separate statistical analyses were also performed for the entire 252-patient cohort. All analyses included flow cytometric data obtained from marrow specimens obtained 14 or more days after the initial initiation of chemotherapy. Flow cytometric data obtained coincident with or after the time that a relapse was morphologically defined were excluded. If more than one specimen was available for analysis, the case was considered positive for occult leukemia if greater than or equal to 0.5% aberrant cells were detected by the flow cytometric assay in any specimen obtained prior to any morphologic relapse.
Patients lost to follow-up were censored at their last known point of study, with a cutoff date of June 27, 2001, and July 8, 2000, for CCG-2961 and CCG-2941 patients, respectively. Because the flow cytometric analyses were performed at several times after remission induction for each patient, and because the number of submitted samples varied between patients, time-varying Cox regression models were used to determine the prognostic significance of the flow cytometric measurement on relapse-free survival (RFS), the time from achieving remission at the end of the first course of induction therapy to relapse or death due to progressive disease, censoring death due to other causes. Similarly, this method was used to determine the prognostic significance of the flow cytometric measurement on overall survival (OS), the time from achieving remission at the end of the first course of induction therapy to death from any cause. Time-varying multivariate analyses were used to relate the risk of relapse after remission induction and the risk of death to the identification of occult leukemia. The analyses controlled for sample time intervals, age, sex, race, white blood cell (WBC) count at diagnosis, presence of splenomegaly or hepatomegaly cytogenetics, and presence of more than 15% blasts in the marrow after the first course of induction. Cytogenetic risk groups were defined as reported by the Medical Research Council.1
Kaplan-Meier analyses were performed from the end of consolidation therapy on a subset of 145 patients in which flow status did not vary after consolidation therapy.22 Thus, time-varying analyses are not required. Differences in RFS and OS were tested for significance using the log-rank statistic.23 ReportedP values are 2-sided.
Other patient characteristics including age, sex, race, WBC count at diagnosis, French-American-British (FAB) classification type, presence or absence of organomegaly, and cytogenetics were compared between those patients with and without occult leukemia identified by flow cytometric analysis. The significance of observed differences in proportions was tested using the Chi-squared test and Fisher exact test when data were sparse. For continuous data, the Mann-Whitney test was used to compare the medians of distributions.24
Results
Relationship between detection of occult leukemia by flow cytometry before delivery of intensification therapy and clinical outcome
Because strategies that modify subsequent therapeutic intensity based on relapse risk require early identification of high-risk patients in remission, we initially limited our analysis to 178 (71%) of 252 patients with responsive disease in whom at least one marrow specimen was available for analysis before delivery of intensification therapy. Statistical comparison of the demographic and clinical characteristics between these 178 children enrolled in CCG B-942 and 451 other children with similarly responsive disease enrolled in CCG-2961 treatment protocol but who were not enrolled in CCG B-942 revealed no significant difference with regard to age, sex, FAB morphologic classification, cytogenetic classification, WBC count at diagnosis, presence of either splenomegaly or hepatomegaly, relapse rate, overall survival, or event-free survival (P > .05). A significant difference was identified for one ethnic group. Hispanics comprised 11% and 19% of the B-942 study group and the remaining CCG-2961 study patients in remission, respectively (P = .014). In all other respects, the cohort studied for the presence of occult leukemia before delivery of intensification therapy was demographically and clinically representative of the larger AML treatment group.
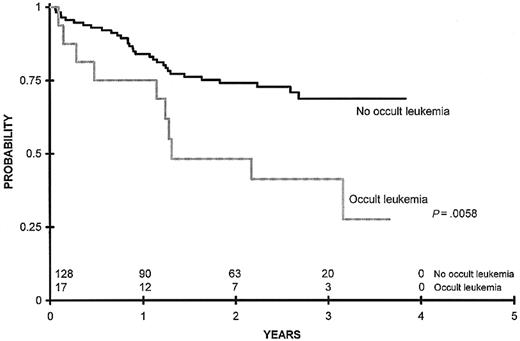
Among these 178 patients with responsive disease, 23 (13%) had greater than or equal to 0.5% aberrant blasts detectable by the flow cytometric assay before any morphologic relapse. The relative risk of relapse after remission induction was estimated in a univariate model to be 3.3 times (95% confidence interval [CI] = 1.7-6.6) higher during time intervals when occult leukemia was detected by flow cytometric assay (Table 3;P = .001). Further restriction of the cohort to 145 patients alive and without recurrent leukemia at the end of consolidation therapy yielded 17 (11.7%) patients with occult leukemia detected by flow cytometry at a single time point. Because of the increased incidence of relapse, death occurred more frequently among patients harboring occult leukemia before delivery of intensification therapy. For this cohort, overall survival (censored at the time of allogeneic bone marrow transplantation) 3 years after the end of consolidation was 41% ± 26% versus 69% ± 10% for patients with and without occult leukemia, respectively (Figure2; P = .0058).
Time-dependent univariate and multivariate Cox models demonstrating relative risk of leukemic relapse or death according to various risk factors
| Characteristic . | Samples obtained before intensification therapy . | Samples obtained before and after intensification therapy . | ||||||
|---|---|---|---|---|---|---|---|---|
| RR for RFS . | P . | RR for OS . | P . | RR for RFS . | P . | RR for OS . | P . | |
| Univariate model | ||||||||
| Leukemia detectable by flow cytometry | 3.33 | .001 | 3.00 | .001 | 4.18 | < .0001 | 3.45 | < .0001 |
| Multivariate model | ||||||||
| Leukemia detectable by flow cytometry | 3.57 | .0004 | 2.75 | .007 | 4.07 | < .0001 | 3.11 | < .0001 |
| Age, 0-2 y or 10-21 y | 1.68 | .096 | 1.96 | .036 | 1.55 | .072 | 2.26 | .002 |
| Sex, female | 0.88 | .616 | 0.94 | .819 | 0.89 | .600 | 0.97 | .882 |
| Race, black | 2.24 | .041 | 1.36 | .467 | 2.01 | .035 | 1.53 | .192 |
| Spleen, slightly or greatly enlarged | 1.26 | .445 | 0.68 | .243 | 1.20 | .480 | 0.96 | .865 |
| Liver, slightly or greatly enlarged | 1.19 | .613 | 1.89 | .056 | 0.89 | .683 | 1.34 | .279 |
| WBC count ≥ 100 | 1.23 | .618 | 1.71 | .139 | 1.79 | .061 | 1.59 | .126 |
| > 15% marrow blasts by morphology3-150 | 3-151 | 3-151 | 2.58 | .091 | 1.64 | .290 | 2.15 | .058 |
| Characteristic . | Samples obtained before intensification therapy . | Samples obtained before and after intensification therapy . | ||||||
|---|---|---|---|---|---|---|---|---|
| RR for RFS . | P . | RR for OS . | P . | RR for RFS . | P . | RR for OS . | P . | |
| Univariate model | ||||||||
| Leukemia detectable by flow cytometry | 3.33 | .001 | 3.00 | .001 | 4.18 | < .0001 | 3.45 | < .0001 |
| Multivariate model | ||||||||
| Leukemia detectable by flow cytometry | 3.57 | .0004 | 2.75 | .007 | 4.07 | < .0001 | 3.11 | < .0001 |
| Age, 0-2 y or 10-21 y | 1.68 | .096 | 1.96 | .036 | 1.55 | .072 | 2.26 | .002 |
| Sex, female | 0.88 | .616 | 0.94 | .819 | 0.89 | .600 | 0.97 | .882 |
| Race, black | 2.24 | .041 | 1.36 | .467 | 2.01 | .035 | 1.53 | .192 |
| Spleen, slightly or greatly enlarged | 1.26 | .445 | 0.68 | .243 | 1.20 | .480 | 0.96 | .865 |
| Liver, slightly or greatly enlarged | 1.19 | .613 | 1.89 | .056 | 0.89 | .683 | 1.34 | .279 |
| WBC count ≥ 100 | 1.23 | .618 | 1.71 | .139 | 1.79 | .061 | 1.59 | .126 |
| > 15% marrow blasts by morphology3-150 | 3-151 | 3-151 | 2.58 | .091 | 1.64 | .290 | 2.15 | .058 |
RR indicates relative risk.
After first cycle of induction.
Marrow blasts by morphology had 0 variance when added to the RFS multivariate model. This multivariate model does not include bone marrow blasts.
Survival comparing outcomes between patients with any occult leukemia detectable by flow cytometry at the end of consolidation therapy censoring for allogeneic transplant.
Overall survival 3 years after the end of consolidation was 41% ± 26% versus 69% ± 10% for patients with and without occult leukemia, respectively (P = .0058). n = 128 patients.
Survival comparing outcomes between patients with any occult leukemia detectable by flow cytometry at the end of consolidation therapy censoring for allogeneic transplant.
Overall survival 3 years after the end of consolidation was 41% ± 26% versus 69% ± 10% for patients with and without occult leukemia, respectively (P = .0058). n = 128 patients.
Influence of intensification therapy and other clinical characteristics on the early detection of occult leukemia before treatment intensification
Because the type of intensification therapy received likely influenced patient outcome in this study, subanalyses were performed to ascertain the potential differential effects of chemotherapy versus matched related donor allogeneic transplantation. When the 115 patients who actually received chemotherapy intensification were analyzed separately from the remaining patients who received bone marrow transplants or were withdrawn from protocol therapy, the relative risk of relapse was estimated to be 3.0 (95% CI = 1.3-7.0,P = .013) and the relative risk of death was estimated to be 3.9 (95% CI = 1.7-9.0, P = .001) during time intervals when occult leukemia was detected by flow cytometric assay. Analyses restricted to patients receiving allogeneic bone marrow transplantation were not performed because of small sample sizes.
A similar subanalysis was performed to determine the relative effect of “good risk” cytogenetics on the ability of the flow cytometric assay to predict poor outcome. Removal of 41 patients from the 178 patient data set who were known to have leukemias characterized by the core binding factor chromosomal abnormalities t(8;21) and inv(16) did not significantly change the findings. In this subanalysis, the relative risk of relapse was estimated to be 2.9 (95% CI = 1.4-5.8,P = .003) during time intervals when occult leukemia was detected by flow cytometric assay. The lower risk of relapse for patients lacking detectable occult leukemia was thus not solely due to an overrepresentation of patients bearing core binding factor abnormalities in this subgroup.
Time-varying multivariate Cox regression was used to relate the risk of relapse after remission induction to the flow cytometric measurement in the 178 patients in whom samples were evaluable prior to delivery of intensification therapy (Table 3). This multivariate analysis controlled for allogeneic marrow transplantation, variable intervals between sample submission, age, sex, race, WBC count at diagnosis, presence of splenomegaly or hepatomegaly, and morphologic presence of more than 15% blasts in the marrow after the first course of induction. Morphologic presence of more than 15% blasts in the marrow after the first course of induction showed no variance in univariate or multivariate models for RFS, but these data were included in the OS models. During time intervals when occult leukemia was detected by the flow cytometric assay, the relative risk of relapse was estimated to be 3.6 (95% CI = 1.8-7.2, P = .0004) and the relative risk of death was estimated to be 2.7 (95% CI = 1.3-5.7,P = .007). In these analyses, detection of residual leukemia by flow cytometry before delivery of intensification therapy was the factor most predictive of death and relapse, with a relative risk exceeding that contributed by elevated WBC count at diagnosis, a poor initial morphologic response to induction therapy, and other poor risk features (Table 3).
Relationship between detection of occult leukemia by flow cytometry before and after delivery of intensification therapy and clinical outcome
Our study was designed to monitor for occult leukemia both before and after the delivery of intensification therapy. At least one marrow specimen for flow cytometric evaluation was available from 74 additional children with AML in whom no sample had been submitted before commencement of intensification therapy. Combining data from these 74 patients with the 178 patients described above comprised 252 patients with treatment-responsive disease. Of these 252 patients, 41 (16%) had evidence of occult leukemia detectable by the flow cytometric assay at a level of greater than or equal to 0.5% prior to any morphologic relapse. During time intervals when occult leukemia was detected by the flow cytometric assay, the relative risk of relapse was estimated in a univariate analysis to be 4.2 (95% CI = 2.6-6.8,P < .0001). Because therapeutic intensification with allogeneic transplantation in first remission has been associated with a reduced risk of subsequent relapse,25 the data were reanalyzed censoring patients who received an allogeneic transplant at the time of transplantation. In this analysis, the relative risk of relapse was estimated to be 5.3 (95% CI = 3.2-8.6,P < .0001).
Among patients who relapsed in either cohort, the median time to relapse was significantly shorter among those who harbored detectable leukemia during remission (168.5 days versus 293 days,P = .008). Patients harboring occult leukemia detectable by flow cytometry during morphologic remission were also more likely to die than those lacking detectable leukemia. During time intervals when occult leukemia was detected by the flow cytometric assay, the relative risk of death was estimated to be 3.4 (95% CI = 2.2- 5.4,P < .0001).
A multivariate analysis controlling for variable intervals between sample submission, age, sex, race, WBC count at diagnosis, presence of splenomegaly or hepatomegaly, and presence of more than 15% blasts in the marrow after the first course of induction, was performed for this cohort of 252 patients (Table 3). During time intervals when occult leukemia was detected by the flow cytometric assay, the relative risk of relapse was estimated to be 4.1 (95% CI = 2.4-7.0,P < .0001) and the relative risk of death was estimated to be 3.1 (95% CI = 1.9-5.1, P < .0001). Detection of occult leukemia during remission by the flow cytometric assay was the most potent predictor of both relapse and death in the multivariate analysis, relatively independent of other prognostic factors. Although the presence of more than 15% blasts in the marrow after the first course of induction was predictive of relapse in the univariate model, this factor did not reach statistical significance in the multivariate analysis. A separate regression analysis that included allogeneic bone marrow transplantation as a time-dependent covariate demonstrated similar associations—the relative risk of relapse was 4.8 (95% CI = 2.8-8.4, P < .0001) and relative risk of death remained 3.1 (95% CI = 1.9-5.1, P < .0001) during time intervals when occult leukemia was detectable by flow cytometry.
Relationship between serial monitoring for occult leukemia by flow cytometry and clinical outcome
Among 41 patients responding to therapy in whom occult leukemia was detected by flow cytometry, 32 had 2 or more marrow specimens submitted after induction therapy that were evaluable by this technique for residual disease while the patients were receiving ongoing treatment. Per the AML treatment protocols, these marrows were submitted at diagnosis and upon completion of induction, consolidation, and intensification courses of therapy. In 13 of these 32 children, no disease was detectable in at least one early marrow specimen obtained but the flow cytometric assay detected leukemic blast cells later during the course of treatment. Leukemic relapse occurred in 11 (85%) of these 13 patients in whom disease levels were rising. Thus, a strategy that includes monitoring for residual disease at several time points during treatment might improve the positive predictive value of the flow cytometric assay. Conversely, in 12 of these patients, leukemia was detected by flow cytometry in an early specimen that was not detectable by the flow cytometric assay in at least one subsequent marrow sample. Despite the appearance that leukemic burdens were declining over the course of therapy, leukemia recurred in 7 of these 12 (58%) children. Although these results suggest that the flow cytometric assay is less likely to accurately distinguish those patients who will remain in long-term remission, serial monitoring does appear to successfully identify patients who will likely relapse.
Relationship of demographic, biologic, cytogenetic, and clinical characteristics on the detection of occult leukemia
Statistical comparison of the demographic and clinical characteristics between patients with and without leukemia detectable by flow cytometry revealed no significant difference with regard to age, sex, WBC count at diagnosis, or presence of splenomegaly or hepatomegaly (Table 1). Patients harboring occult leukemia were more likely to be of black race, but this association was of marginal statistical significance (P = .06). Cytogenetic results were available from 152 (60%) of the 252 study patients. Because patients with APL were enrolled in a separate treatment protocol, only one patient with the t(15;17) translocation was represented in the CCG B-942 study group. Among 152 patients in whom cytogenetic data were available, 34 (22%) had leukemia characterized by t(8;21). As might be anticipated from studies that have shown the favorable prognostic value of this cytogenetic abnormality,26 only one of these patients had occult leukemia detectable by flow cytometry during remission. Patients with occult leukemia during morphologic remission were somewhat more likely to have leukemias characterized by normal cytogenetics (P = .057). No other statistically significant differences between patients with and without occult leukemia were detected among the remaining cytogenetic groups, but small numbers of patients in each group might preclude identification of a real difference were it to exist.
A multivariate analysis controlling for variable intervals between sample submission, age, sex, race, WBC count at diagnosis, presence of splenomegaly or hepatomegaly, and presence of more than 15% blasts in the marrow after the first course of induction, was performed in the smaller subset of patients in whom cytogenetic data were available. During time intervals when occult leukemia was detected by the flow cytometric assay, the relative risk of relapse was estimated to be 4.3 (95% CI = 2.1-8.9, P < .0001) and the relative risk of death was estimated to be 2.0 (95% CI = 1-4.2,P = .05). In comparison, patients whose leukemia expressed standard or poor risk cytogenetics were 1.8 times more likely to relapse (P = .093) and 2.5 times more likely to die (P = .016) than those expressing good risk cytogenetics in the multivariate model. Thus, with inclusion of cytogenetics in the multivariate model, detection of occult leukemia during remission by the flow cytometric assay remained the most potent predictor of relapse.
Discussion
Despite aggressive induction chemotherapy for AML, recurrent leukemia is common but unpredictable using current laboratory assays. Multidimensional flow cytometry, a quantitative assay that enables the detection of occult leukemia cells during complete morphologic remission, was evaluated for its ability to predict outcome in 252 children and adolescents treated for AML in 2 nearly identical prospective, cooperative group trials. In this study, one-sixth of patients responding to induction therapy appeared to harbor detectable levels of occult leukemia. These patients with residual disease were 4 times more likely to relapse, and 3 times more likely to die than their counterparts who lacked evidence of occult leukemia. These prognostic relationships were also present among the subset of 178 children in whom marrow specimens were evaluated for residual disease before intensification therapy was delivered, thus providing rationale for tailoring intensification therapy based on a patient's unique risk of recurrent disease.
Historically, delivery of induction chemotherapy alone resulted in only brief periods of remission among adult patients with AML, suggesting that residual leukemic blast cells that were not fully eradicated by initial treatment were responsible for recurrent disease.27 For this reason, investigators have coupled induction with consolidation and subsequent intensification regimens with the intent of eliminating residual disease.28-30 In current treatment strategies, further aggressive therapy was justifiably applied to the entire study cohort because an individual patient's risk of relapse is impossible to ascertain. Recently, the Medical Research Council of the United Kingdom and others characterized highly predictive parameters that should enable future clinical trials to apply therapeutic risk group stratification.1 However, even with this new prognostic index, fully half of patients with AML remain in a standard risk group, of whom 50% experience relapse after treatment with standard chemotherapeutic regimens. Alternative means of identifying these patients are vital because pre-emptive delivery of intensified therapy might prevent relapse in this high-risk cohort.
Early response to treatment is of critical prognostic value in the treatment of acute leukemias. Data from our blinded, prospective study suggest that multidimensional flow cytometry analysis of marrow specimens can quantify leukemic burden in such responding children early during therapy and identify those who will likely relapse. In multivariate analyses, immunophenotypic evidence of occult leukemia before delivery of intensification therapy was associated with a higher relative risk of relapse than all other known risk factors evaluated. In conjunction with other established prognostic factors in use, detection of occult leukemia early during the course of treatment might allow intensification strategies to be tailored to a patient's relapse risk. Employing risk group stratification might enable patients with a lower risk of relapse to avoid exposure to unnecessary therapy. Conversely, strategies to intensify therapy might benefit those at a higher risk of relapse.
After the delivery of near myeloablative chemotherapy, it is notoriously difficult to distinguish normal recovering marrow elements from residual leukemia by morphologic evaluation of marrow aspirates. Some patients who formally have more than 5% marrow blasts after induction therapy ultimately achieve complete remission without the delivery of additional chemotherapy, whereas others manifest increasing levels of leukemia. It has thus been suggested that multidimensional flow cytometry can be used to accurately differentiate between these 2 clinical scenarios.21 In the 252-patient multivariate analysis, the relative risk for relapse among responding patients with leukemia detected by flow cytometry was 4.1 versus 1.6 for those who had more than 15% morphologically identified blasts after induction therapy. Thus, flow cytometric evidence of leukemia appeared to be more predictive of relapse than morphologic assessment in children responding to induction chemotherapy. These findings suggest that flow cytometric evaluation of marrow specimens obtained after delivery of induction chemotherapy might provide a more accurate assessment of remission status than that offered by conventional morphologic techniques.
Unlike PCR-based assays for recurring fusion genes that are present in a minority of patients with AML or flow cytometric assays that require patient-specific antibody cocktails, we evaluated a flow cytometric assay employing a standardized panel of antibody combinations in every patient with AML. Our approach sought to identify leukemic blast cells based on cell surface antigen expression that differed from normal hematopoiesis. Although some marrow specimens were analyzed more than 72 hours from the time of marrow aspiration, resulting in poor sample quality, the assay appeared to be sufficiently robust to detect cells with abnormal cell surface antigen expression with a general lower limit of detection of 5 × 10−3. Employment of a standard 3-color antibody panel regardless of the unique immunophenotype that a leukemic specimen displayed at diagnosis simplified specimen handling and enabled rapid processing in a clinically oriented flow cytometry laboratory. Moreover, changes in leukemic immunophenotype during the course of therapy can also be detected with this approach.
Using patient-specific cocktails of antibodies enabling assay sensitivity in the range of 1 × 10−4, San Miguel et al defined 4 risk categories for subsequent relapse among the 70% to 80% of adult patients with AML bearing sufficiently aberrant phenotypic abnormalities amenable to their detection strategy.15Relapse occurred in 85% of patients harboring levels of residual leukemia greater than 1% in the first marrow aspirate obtained during morphologic remission. In their report, 81 (64%) of 126 patients in remission had evidence of more than 0.1% cells expressing a leukemia-associated phenotype. Similarly, we identified detectable leukemia in 54% of pediatric patients with AML who achieved remission in our retrospective study.18 Both of these studies evaluated patients who had less than 5% blasts by marrow morphology for residual disease. While our prospective study of 252 patients is not directly comparable to these studies because some patients who were responding to chemotherapy did not necessarily have less than 5% morphologically identified blasts, only 13% of pediatric patients had evidence of more than or equal to 0.5% cells expressing an aberrant immunophenotype before delivery of intensification therapy. Quite likely, improved assay sensitivity would identify more patients with occult leukemia at this time point. But because the delivered intensity of AML induction using idarubicin31 was higher in the CCG-2961 AML treatment study than that experienced by patients in the previous study of minimal residual disease,18 it is also possible that the depth and quality of remissions has improved as well.
Although some responding patients lacking detectable leukemia during morphologic remission also eventually experienced relapse, the median time to relapse was 124 days later than that observed among those with detectable occult leukemia. The inability of the flow cytometric assay to predict relapse in these patients was probably due to the fact that leukemic cells were present at a level below the sensitivity of the standard 3-color antibody panel flow cytometric technique that we employed. Sample degradation due to processing delay may have reduced the ability of the assay to detect rare leukemic cells as well. Because approximately 40% of children who enter remission and who are treated with chemotherapy intensification subsequently relapse, employment of the flow cytometric assay helped predict a third of the total number of relapses that occurred. In order to increase the positive predictive value of the assay, several modifications to our current strategy are being evaluated as a means of increasing sensitivity while maintaining specificity for leukemic cells. Reducing the time interval between sample collection and analysis, increasing the number of collected data events, and addition of a fourth antibody to each tube may further improve the sensitivity of the assay.
In this prospective analysis of 252 children and adolescents treated for AML, detection of leukemic cells during remission imparted an approximately 4-fold greater risk of subsequent relapse and 3-fold greater risk of death than those without detectable leukemia. The Children's Oncology Group is evaluating the feasibility of a prospective, randomized study to determine whether intensification of therapy for patients with occult leukemia during remission will result in fewer relapses and improved overall survival.
We are indebted to Samantha Burns for technical services and Fred Appelbaum for critical review of the manuscript. The AML Reference Laboratory of the Children's Cancer Group provided diagnostic leukemia specimens for this study. A list of participating investigators appears in the .
The Children's Cancer Group institutions and their respective principal investigators who enrolled patients in this study are: Raymond Hutchinson, C.S. Mott Children's Hospital, Ann Arbor, MI; Roshni Kulkarni, Michigan State University, East Lansing, MI; David R Freyer, DeVos Children's Hospital, Grand Rapids, MI; Hassan Yaish, Henry Ford Hospital, Detroit, MI; Leonard A Mattano Jr, Kalamazoo Center for Medical Studies, Kalamazoo, MI; Jerry Z Finklestein, Harbor/University of California at Los Angeles (UCLA) and Miller Children's Hospital, Long Beach, CA; Vonda L Crouse, Valley Children's Hospital, Fresno, CA; Carole G Hurvitz, Cedars-Sinai Medical Center, Los Angeles, CA; Paul Baranko, Phoenix Children's Hospital, Phoenix, AZ; Katherine K Matthay, University of California at San Francisco (UCSF) School of Medicine, San Francisco, CA; James Feusner, Children's Hospital of Oakland, Oakland, CA; Stephen A Feig, UCLA School of Medicine, Los Angeles, CA; Jennifer Pearce, Albany Medical Center, Albany, NY; Ronald S Oseas, Sunrise Children's Hospital, Sunrise Hospital and Medical Center, Las Vegas, NV; Paul S Gaynon, University of Wisconsin - Children's Hospital Madison, Madison, WI; Torrey L Mitchell, University of Illinois - Rockford, Rockford, IL; James Miser, Ronald Chard, J Russell Geyer, Children's Hospital and Regional Medical Center, Seattle, WA; Daniel Niebrugge, Mary Bridge Hospital, Tacoma, WA; Philip Herzog, Group Health Cooperative of Puget Sound, Seattle, WA; Frank A Reynolds, Deaconess Medical Center, Spokane, WA; Lorrie F Odom, The Children's Hospital, Denver, CO; Susan Shurin, Rainbow Babies and Children's Hospital, Cleveland, OH; Mustafa Barudi, Western Reserve Care System - Tod Children's Hospital, Youngstown, OH; Gerald S Gilchrist, Mayo Clinic and Foundation, Rochester, MN; Stephen C Elliott, Raymond Blank Children's Hospital, Des Moines, IA; Nathan Kobrinsky, MeritCare Hospital, Fargo, ND; Marwan D Hanna, Dakota Midwest Cancer Institute, Sioux Falls, SD; Ten Suan Goh, Allan Blair Cancer Centre, Regina, SK; Gregory H Reaman, Children's National Medical Center, Washington, DC; John F Kelleher, Baystate Medical Center, Springfield, MA; Rebecca L Byrd, Children's Hospital-Kings Daughters, Norfolk, VA; Joseph E Gootenberg, Georgetown University Medical Center, Washington, DC; Ruth E Luddy, Sinai Hospital of Baltimore, Baltimore, MD; Randy Hock, St Vincent Hospital and Medical Center, Indianapolis, IN; Dorothy R Barnard, Isaac Walton Killam (IWK) Grace Health Centre, Halifax, NS; Herbert A Cooper, Joseph Wiley, Stuart H Gold, University of North Carolina at Chapel Hill, Chapel Hill, NC; Paul S Gaynon, Children's Hospital Los Angeles, Los Angeles, CA; Willye B Powell, Southern California Permanente Medical Group, Downey, CA; Antranik Bedros, Loma Linda University Medical Center, Loma Linda, CA; Judith Sato, City of Hope National Medical Center, Duarte, CA; Felicity Hodder, Santa Barbara Cottage Children's Hospital, Santa Barbara, CA; Lawrence Ettinger, Richard Drachtman, University of Medicine and Dentistry of New Jersey, New Brunswick, NJ; Peri Kamalakar, Newark Beth Israel Medical Center, Newark, NJ; Frederick B Ruymann, Children's Hospital of Columbus, Columbus, OH; Emmett Broxson, Children's Medical Center Dayton, Dayton, OH; Maxine Hetherington, The Children's Mercy Hospital, Kansas City, MO; Sergio Piomelli, Michael Weiner, Leonard H Wexler, Columbia Presbyterian College of Physicians and Surgeons, New York, NY; Ronald Kline, Atlantic Health System, Florham Park, NJ; Peter F Coccia, University of Nebraska Medical Center, Omaha, NE; A Kim Ritchey, Children's Hospital of Pittsburgh, Pittsburgh, PA; John Lukens, James Whitlock, Vanderbilt Children's Hospital, Nashville, TN; Robert Ettinger, Ronnie Neuberg, South Carolina Cancer Center, Columbia, SC; Ray Pais, East Tennessee Children's Hospital, Knoxville, TN; F Leonard Johnson, James Nachman, University of Chicago Medical Center, Chicago, IL; John Hyo Kwon, Lutheran General Children's Medical Center, Park Ridge, IL; Thomas Loew, Gregory Brandt, Southern Illinois University School of Medicine, Springfield, IL; Robert Neerhout, Lawrence Wolff, H Stacy Nicholson, Doernbecher Children's Hospital - Oregon Health Sciences University, Portland, OR; Kenneth Lazarus, Southwest Texas Methodist Hospital, Dallas, TX; John Iacuone, Children's Hematology/Oncology Team at Covenant Children's, Lubbock, TX; Trib Vats, Texas Tech University Health Sciences Center - Amarillo, Amarillo, TX; William Wood, Joseph Neglia, University of Minnesota Cancer Center, Minneapolis, MN; H James Nickerson, Marshfield Clinic, Mashfield, WI; Marura O'Leary, Children's Health Care - Minneapolis, Minneapolis, MN; John Priest, Joanne M Hilden, Children's Hospitals and Clinics - St Paul, St Paul, MN; Rochelle Yanofsky, CancerCare Manitoba, Winnipeg, MB; Rudolph Roskos, Munkund Dole, J Roloff, Quain and Ramstad Clinic, Bismark, ND; Michael Willoughby, David Baker, Princess Margaret Hospital for Children, Perth, Western Australia; Anna Meadows, Beverly Lange, Children's Hospital of Philadelphia, Philadelphia, PA; Rita Meedk, Christiana Care Health Services/A.I. duPont, Wilmington, DE; Narayan Shah, Geisinger Medical Center, Danville, PA; Aaron Rausen, New York University Medical Center, New York, NY; Eva Radel, Montefiore Medical Center, Bronx, NY; Arnold Altman, University of Connecticut Health Center, Farmington, CT; Mark Weinblatt, North Shore University Hospital - Cornell University Medical Center, Manhasset, NY; Mary Ann Bonilla, Saint Barnabas Medical Center, Livingston, NJ; Mitchell Cairo, Violet Shen, Children's Hospital of Orange County, Orange, CA; Philip Breitfeld, Indiana University - Riley Children's Hospital, Indianapolis, IN; Richard O'Brien, William Carroll, Primary Children's Medical Center, Salt Lake City, UT; Emma Harwood, Mountain States Tumor Institute, Boise, ID; Christopher Fryer, Paul C Rogers, British Columbia Children's Hospital, Vancouver, BC; Robert Wells, Children's Hospital Medical Center, Cincinnati, OH; Elizabeth Kurczynski, John Bergsagel, P Charlton Davis, Children's Healthcare of Atlanta at Scottish, Atlanta, GA; Alexander Koufos, Children's Hospital Medical Center, Akron, OH; John Neely, Pennsylvania State Children's Hospital, Hershey Medical Center, Hershey, PA; Salvatore Bertolone, Kosair Children's Hospital, Louisville, KY; and Ihsan Al-Khalil, Memorial Medical Center/Backus Children's, Savannah, GA.
Prepublished online as Blood First Edition Paper, December 27, 2002; DOI 10.1182/blood-2002-10-3064.
Children's Cancer Group institutions and their respective principal investigators who enrolled patients in this study are listed in the “.”
Supported by grants CA13539 and CA10382. I.D.B. is supported by an FM Kirby/American Cancer Society Clinical Research Professorship.
M.R.L. is employed by Hematologics, whose service was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Eric L. Sievers, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N D5-280, Seattle, WA 98109; e-mail: esievers@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal