The importance of αβ versus γδ T-cell subset antigen expression in the classification of peripheral T-cell lymphomas is still unclear. The objective of this study was to investigate the prognostic value of T-cell receptor–δ1 (TCRδ1) expression in primary cutaneous T-cell lymphomas. TCRδ1 cellular expression was assessed in skin biopsy specimens of 104 individuals with cutaneous T-cell lymphoma by immunohistochemistry. Both univariate (Kaplan-Meier) and multivariate (Cox regression) analyses were conducted to determine which variables (T-cell subtype, hemophagocytosis, histologic profile, age, sex, and adenopathy) were significantly associated with survival. Univariate analysis indicated that there was a statistically significant difference in survival between the patients with αβ cutaneous T-cell lymphoma and patients with γδ cutaneous T-cell lymphoma (P < .0001). There was also a statistically significant decrease in survival among patients who had subcutaneous involvement compared with patients who had epidermotropic and/or dermal involvement (P < .0001). Cox model analysis indicated that TCRδ1 expression was the factor that was most closely associated with decreased survival (P < .0001). Among those patients with cutaneous γδ T-cell lymphoma (n = 33), there was a trend for decreased survival for patients who had histologic evidence of subcutaneous fat involvement in comparison with patients who had epidermotropic or dermal patterns of infiltration (P = .067). No other prognostic factors were identified as having a notable association with outcome in this subgroup. TCRδ1 expression in primary cutaneous lymphomas is an independent prognostic factor associated with decreased survival.
Introduction
Peripheral T-cell lymphomas overall represent 10% to 15% of non-Hodgkin lymphoma (NHL) and are a diverse group of lymphoid neoplasms manifesting heterogeneous clinical, histologic, immunophenotypic, cytogenetic, and molecular features.1,2The subclassification of primary cutaneous T-cell lymphomas in the Revised European American Lymphoma (REAL) classification and the World Health Organization (WHO) includes mycosis fungoides/Sézary syndrome (MF/SS), CD30+ T-cell lymphoproliferative disease, and subcutaneous panniculitis–like T-cell lymphoma (SPTCL).3,4 Cutaneous gamma-delta T-cell lymphomas (CGD-TCLs) are not defined as a specific entity in the WHO or REAL classification, nor are they delineated in the classification of primary cutaneous lymphomas proposed by the European Organization for Research and Treatment of Cancer (EORTC).5 However, these tumors appear to have distinctive features.6 Conventional cutaneous T-cell lymphoma (CTCL) typically represents MF/SS and expresses CD4 surface markers. It is a distinct disease from CGD-TCL, which by definition lacks CD4 surface marker expression despite occasional cases of CGD-TCL showing epidermotropism.7
The T-cell receptor consists of either a gamma-delta (γδ) or an alpha-beta (αβ) heterodimer expressed in association with the CD3 complex of proteins on the cell surface.8 The majority of mature T cells express the αβ T-cell receptor. However, 5% of normal T cells express the γδ T-cell receptor.8Gamma-delta T cells have cytotoxic capabilities and can respond to stimuli with lymphokine production and proliferation. Most γδ T cells lack CD4 and CD8 surface markers. However, some γδ T cells in human peripheral blood are CD8+. The exact function of γδ T cells remains unknown.9 Gamma-delta T-cell malignancies are rare and have been found among cases of T-cell lymphoblastic lymphoma/leukemia,10,11 hepatosplenic T-cell lymphoma (HS-TCL),12,13 nasal and extranodal natural killer (NK)/T-cell lymphoma,14 and cutaneous-mucosa–associated T-cell lymphomas including SPTCL.6,14-16 HS-TCLs are nearly always of γδ T-cell origin, as opposed to cutaneous T-cell lymphomas, in which αβ T cells usually predominate.12 Furthermore, γδ HS-TCL and γδ SPTCL express different Vδ subsets of γδ T-cell lymphocytes. Whereas γδ HS-TCL usually belongs to the Vδ1 subset, γδ SPTCLs represent the Vδ2 subset.17 Whereas HS-TCLs are composed of functionally immature cells lacking granzyme B and perforin,12 most other gamma-delta T-cell lymphomas are derived from activated cytotoxic T cells and present preferentially in cutaneous or mucosal sites.18 This clinical distribution corresponds to the distribution of normal gamma-delta T cells.19
CGD-TCLs are not common. In a recent report of 62 cases of cutaneous T-cell lymphoma, only 2 cases were gamma-delta–positive.20 To our knowledge, approximately 40 cases of gamma-delta cutaneous T-cell lymphoma have been reported.6,7,14,16,17,20-30 We recently described the clinical, histologic, and immunohistochemical features of primary cutaneous γδ T-cell lymphoma.6 CGD-TCL shows preferential involvement of the extremities with plaques, tumors, and subcutaneous nodules, some of which ulcerate. CGD-TCL can exhibit diverse histologic patterns, often in the same patient, including epidermotropism and dermal or subcutaneous involvement. CGD-TCLs are Epstein-Barr virus (EBV)–negative clonal T-cell lymphomas that express a mature cytotoxic phenotype with frequent apoptosis.6 Although our previous study and individual case reports suggested that CGD-TCLs have an aggressive clinical course, the significance of T-cell receptor–δ (TCRδ1) expression on the outcome of patients with cutaneous lymphoma has not been studied. In the present study, we evaluated the clinical relevance of TCRδ1 expression in predicting overall survival in individuals affected with primary cutaneous T-cell lymphoma.
Patients and methods
Patient evaluation
Cases with the diagnosis of cutaneous T-cell lymphoma that had frozen skin biopsy specimens available during the period of July 1976 to July 2001 were included in the study, since staining of TCRδ1 is possible only in frozen tissue sections. Clinical evaluation documented primary cutaneous disease without evidence of systemic spread within 1 year of diagnosis. Approval was obtained from the National Cancer Institute (NCI) institutional review board (IRB) for these studies. Informed consent was provided according to the Declaration of Helsinki. Patients seen and treated at the National Institutes of Health (NIH) were enrolled in an IRB-approved protocol. The remaining cases were submitted in consultation for diagnostic evaluation. Because the NIH is a tertiary hospital, the patients enrolled in our study do not represent a truly unselected population. Cases were classified according to the WHO classification.4 T-cell lymphoma subtypes eligible for inclusion in the study were mycosis fungoides; subcutaneous panniculitis–like T-cell lymphoma; and peripheral T-cell lymphoma (PTCL), unspecified. PTCL, unspecified, is a heterogeneous category in the WHO classification, and includes all mature T-cell lymphomas that cannot be assigned to a specific entity. Cases of primary cutaneous CD30+ T-cell lymphoproliferative31disease, including primary cutaneous anaplastic large cell lymphoma, were excluded, as expression of TCRδ1 was not observed in this subset of cases. In addition, primary cutaneous CD30+ T-cell lymphoproliferative disease is a distinct clinicopathologic entity with a known favorable prognosis and distinctive clinical, pathologic, and immunophenotypic features.32 Each patient had a detailed medical history and clinical examination of the skin and lymph nodes. As only 23 of 104 patients had a diagnosis of mycosis fungoides, we did not employ the staging system traditionally used for cutaneous T-cell lymphomas.33 However, we did evaluate the major risk factors identified in this staging scheme in all patients. Thirty-six patients had lymph node biopsies and 68 had examination of the bone marrow. Routine morphologic studies were done on 4-mm hematoxylin-eosin (H&E)–stained sections. We classified skin biopsies by the predominant pattern of lymphomatous involvement as epidermotropic, dermal, or subcutaneous.
The immunophenotypic panel included monoclonal antibodies directed against the lymphocyte-associated antigens: CD3, CD4, CD8, CD5, CD7, CD20, βF1, granzyme B, T-cell intracellular antigen–1 (TIA-1), and perforin. Immunohistochemistry and antigen retrieval were performed as previously described.34Staining for TCR1δ (T Cell Diagnostics, Woburn, MA) was performed in frozen sections. TCRδ1 staining was considered positive when more than 80% of tumor cells showed membrane positivity.
NCI-treated patients with cutaneous T-cell lymphoma were enrolled into successive NCI treatment protocols in which combined-modality therapy (electron beam therapy, chemotherapy, and topical treatment) was evaluated.35 36 Patients with CGD-TCL did not achieve durable complete remissions with any of the available treatment protocols used at NCI. These included conventional CTCL therapies, such as topical steroids (1 individual), psoralen and long wave ultraviolet (PUVA) radiation (7 individuals), interferon-α (IFN-α) (4 individuals), IFN-γ (1 individual), and retinoids; more aggressive treatments including radiation therapy (8 individuals), CHOP (cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone) (6 individuals), polychemotherapy (8 individuals), and bone marrow transplant (1 individual); or investigational therapies including anti-Tac (1 individual) or UCN-01 (7-hydroxy analog of stauropurine, a tyrosine kinase inhibitor) (1 individual). Therefore, the survival of patients in this series represents the natural history of disease altered by the best treatment available during the study period.
Statistical analysis
Survival time was measured in months from time of diagnosis until date of death or last follow-up. Initially, univariate analyses were performed to screen for parameters to evaluate in a more definitive model. The Kaplan-Meier method was used to construct survival curves, and the probabilities of survival between pairs or sets of curves were compared with a log-rank test.37 The following variables were considered for evaluation in the univariate analysis (levels compared are in parentheses): T-cell type (TCRδ+ versus TCRδ−); histologic profile (epidermotropic and/or dermal versus subcutaneous); age (divided into 4 groups based on the quartiles 41, 59, 69 years); sex (male versus female); and adenopathy (yes versus no). Additionally, actuarial analyses were performed with the use of hemophagocytosis (yes versus no); histologic profile (epidermotropic or dermal versus subcutaneous); age (quartiles, 41, 49, 65 years); and sex among those patients classified as γδ (n = 33). The Cox proportional hazards model method was then used to identify the significance of those parameters found to be potentially useful in the univariate analyses, when considered jointly.38 In addition, a likelihood ratio test was performed to determine whether T-cell type was significantly associated with survival after adjustment for other common parameters evaluated.
Results
Clinicopathologic characteristics
The general characteristics of the patients studied are listed in Table 1. Our cohort consisted of 66 men and 38 women with a median age of 59 years. The age range was 13 to 84 years, with only 2 patients under the age of 21. Of 104 cases, 33 expressed TCRδ1 and were βF1 negative. Therefore, they were designated cutaneous γδ T-cell lymphoma (CGD-TCL). All such cases were either CD4−/CD8− or CD8+ and expressed cytotoxic molecules in all cases studied (data not shown). Patients with CGD-TCL had a distinctive clinical presentation with a predominant involvement of the extremities with plaques, tumors, and subcutaneous nodules, some of which ulcerated (Figure1). There were 56% (41 of 71) of patients with primary cutaneous αβ T-cell lymphomas who presented with tumors, as compared with 73% (24 of 33) of patients with CGD-TCL.
Clinical characteristics of patients with primary cutaneous T-cell lymphomas
| . | All TCL . | αβ (n = 71) . | γδ (n = 33) . |
|---|---|---|---|
| Age, years | |||
| Median | 59 | 62 | 49 |
| Range | (13-84) | (17-84) | (13-82) |
| Sex | |||
| Male | 66 | 47 | 19 |
| Female | 38 | 24 | 14 |
| Adenopathy (6 not done) | |||
| Positive | 26 | 22 | 4 |
| Negative | 72 | 44 | 28 |
| Histologic profile | |||
| Epidermotropic or dermal | 71 | 59 | 12 |
| Subcutaneous | 33 | 12 | 21 |
| Provisional subtype in WHO classification | |||
| Mycosis fungoides | 23 | 19 | 4 |
| SPTCL | 23 | 9 | 14 |
| PTCL, unspecified | 58 | 43 | 15 |
| Bone marrow (36 not done) | |||
| Positive | 5 | 5 | 0 |
| Negative | 63 | 45 | 18 |
| Node biopsy (68 not done) | |||
| Positive | 5 | 4 | 1 |
| Negative | 31 | 21 | 10 |
| . | All TCL . | αβ (n = 71) . | γδ (n = 33) . |
|---|---|---|---|
| Age, years | |||
| Median | 59 | 62 | 49 |
| Range | (13-84) | (17-84) | (13-82) |
| Sex | |||
| Male | 66 | 47 | 19 |
| Female | 38 | 24 | 14 |
| Adenopathy (6 not done) | |||
| Positive | 26 | 22 | 4 |
| Negative | 72 | 44 | 28 |
| Histologic profile | |||
| Epidermotropic or dermal | 71 | 59 | 12 |
| Subcutaneous | 33 | 12 | 21 |
| Provisional subtype in WHO classification | |||
| Mycosis fungoides | 23 | 19 | 4 |
| SPTCL | 23 | 9 | 14 |
| PTCL, unspecified | 58 | 43 | 15 |
| Bone marrow (36 not done) | |||
| Positive | 5 | 5 | 0 |
| Negative | 63 | 45 | 18 |
| Node biopsy (68 not done) | |||
| Positive | 5 | 4 | 1 |
| Negative | 31 | 21 | 10 |
Dermatologic and histologic features of cutaneous gamma-delta T-cell lymphoma.
(A) Characteristic ulcerated tumors covered with hemorrhagic crust. (B) Multiple pink to plum–colored tumors in an upper extremity. (C) Low magnification shows an example of the coexistent epidermotropic, dermal, and subcutaneous patterns of involvement in a single biopsy. Original magnification, × 25. There is psoriasiform epidermal hyperplasia and a bandlike lymphocytic infiltrate in the papillary dermis with epidermotropism. The infiltrate extends into the reticular dermis with a perivascular pattern. In addition, there is infiltration of subcutaneous tissue reminiscent of lobular panniculitis. (D) The dermal infiltrate is positive for TCRδ1 by immunohistochemistry (avidin-biotin complex [ABC] immunoperoxidase; methyl green counter stain). Original magnification, × 100. Adapted from Toro et al,6 and reproduced with permission from Jaffe et al.4
Dermatologic and histologic features of cutaneous gamma-delta T-cell lymphoma.
(A) Characteristic ulcerated tumors covered with hemorrhagic crust. (B) Multiple pink to plum–colored tumors in an upper extremity. (C) Low magnification shows an example of the coexistent epidermotropic, dermal, and subcutaneous patterns of involvement in a single biopsy. Original magnification, × 25. There is psoriasiform epidermal hyperplasia and a bandlike lymphocytic infiltrate in the papillary dermis with epidermotropism. The infiltrate extends into the reticular dermis with a perivascular pattern. In addition, there is infiltration of subcutaneous tissue reminiscent of lobular panniculitis. (D) The dermal infiltrate is positive for TCRδ1 by immunohistochemistry (avidin-biotin complex [ABC] immunoperoxidase; methyl green counter stain). Original magnification, × 100. Adapted from Toro et al,6 and reproduced with permission from Jaffe et al.4
Histologic patterns of involvement were evaluated in all 104 patients. Dominant epidermotropic or dermal involvement was present in 71 cases. Of this group, 23 were classified within the spectrum of mycosis fungoides, and 48 were classified as PTCL, unspecified. In addition, 33 cases had subcutaneous involvement. Of these cases, 10 cases were PTCL, unspecified, and 23 cases were classified as SPTCL: 9 were αβ and 14 were γδ. The designation of SPTCL was based on a predominant pattern of subcutaneous infiltration, rimming of fat spaces by cytotoxic neoplastic T-cell lymphocytes, and prominent apoptosis as previously described.15
Univariate analysis
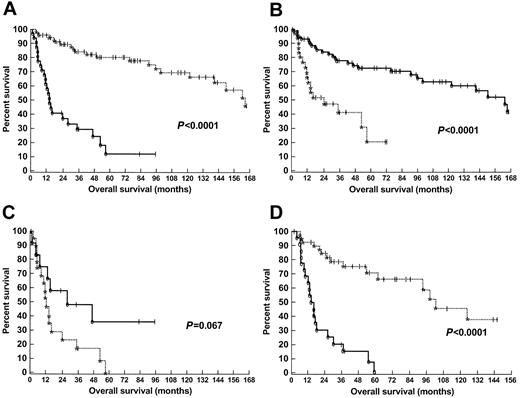
The results of tests for significance from the univariate analysis are summarized in Table 2. There was a very large and statistically significant difference in survival between the patients with cutaneous αβ T-cell lymphoma and individuals affected with cutaneous γδ T-cell lymphoma (P < .0001) (Figure 2A). In addition, there was a statistically significant decrease in survival in individuals who had subcutaneous involvement in comparison with patients who had epidermotropic and/or dermal involvement (P < .0001) (Figure 2B). However, this was not consistent in magnitude between the patients with cutaneous αβ T-cell lymphoma and patients with cutaneous γδ T-cell lymphoma (P = .73 within αβ, and P = .067 within γδ). Thus, an interaction between these 2 parameters was worthy of investigation in the Cox models (“Cox proportional hazards model analysis”).
Univariate analysis
| Variable . | P, log-rank, 2-tailed . |
|---|---|
| Univariate analysis of primary cutaneous T-cell lymphomas | |
| T-cell type (αβ versus γδ) | < .0001 |
| Histologic profile, epidermotropic or dermal versus subcutaneous | < .0001 |
| Age, among 4 groups | .93 |
| Sex | .047 |
| Adenopathy, normal versus positive | .66 |
| Bone marrow, normal versus positive | .05 |
| Univariate analysis of cutaneous γδ T-cell lymphomas | |
| Histologic profile, epidermotropic or dermal versus subcutaneous | .067 |
| Age, among 4 groups | .14 |
| Sex | .77 |
| Adenopathy, normal versus positive | .17 |
| Hemophagocytic syndrome, negative versus positive | .21 |
| Variable . | P, log-rank, 2-tailed . |
|---|---|
| Univariate analysis of primary cutaneous T-cell lymphomas | |
| T-cell type (αβ versus γδ) | < .0001 |
| Histologic profile, epidermotropic or dermal versus subcutaneous | < .0001 |
| Age, among 4 groups | .93 |
| Sex | .047 |
| Adenopathy, normal versus positive | .66 |
| Bone marrow, normal versus positive | .05 |
| Univariate analysis of cutaneous γδ T-cell lymphomas | |
| Histologic profile, epidermotropic or dermal versus subcutaneous | .067 |
| Age, among 4 groups | .14 |
| Sex | .77 |
| Adenopathy, normal versus positive | .17 |
| Hemophagocytic syndrome, negative versus positive | .21 |
Kaplan and Meier plots of patients with cutaneous T-cell lymphoma.
(A) Survival of individuals with cutaneous T-cell lymphoma according to T-cell–receptor immunophenotype. A comparison was made between patients with alpha-beta (dotted line) and gamma-delta (solid line) cutaneous T-cell lymphomas. Significance was determined by the log-rank test. (B) Survival of individuals with cutaneous T-cell lymphoma according to histologic profile. A comparison of survival was made between patients with subcutaneous involvement (dotted line), and epidermotropic and/or dermal involvement (solid line). Significance was determined by the log-rank test. (C) Survival of individuals with cutaneous gamma-delta T-cell lymphoma according to histologic profile. A comparison of survival was made between patients with subcutaneous involvement (dotted line), and epidermotropic and/or dermal involvement (solid line). Significance was determined by the log-rank test. (D) Survival of patients presenting with cutaneous tumors according to T-cell–receptor immunophenotype. A comparison was made between patients with alpha-beta (dotted line) and gamma-delta (solid line) cutaneous T-cell lymphomas. Significance was determined by the log-rank test.
Kaplan and Meier plots of patients with cutaneous T-cell lymphoma.
(A) Survival of individuals with cutaneous T-cell lymphoma according to T-cell–receptor immunophenotype. A comparison was made between patients with alpha-beta (dotted line) and gamma-delta (solid line) cutaneous T-cell lymphomas. Significance was determined by the log-rank test. (B) Survival of individuals with cutaneous T-cell lymphoma according to histologic profile. A comparison of survival was made between patients with subcutaneous involvement (dotted line), and epidermotropic and/or dermal involvement (solid line). Significance was determined by the log-rank test. (C) Survival of individuals with cutaneous gamma-delta T-cell lymphoma according to histologic profile. A comparison of survival was made between patients with subcutaneous involvement (dotted line), and epidermotropic and/or dermal involvement (solid line). Significance was determined by the log-rank test. (D) Survival of patients presenting with cutaneous tumors according to T-cell–receptor immunophenotype. A comparison was made between patients with alpha-beta (dotted line) and gamma-delta (solid line) cutaneous T-cell lymphomas. Significance was determined by the log-rank test.
There was a marginally statistically significant difference in survival between individuals with and without bone marrow involvement, although data were only available on 65% of all patients (P = .05). There was no statistical difference in survival between individuals with and without adenopathy (P = .66). There was a marginally statistically significant difference in sex when data from all patients were analyzed (P = .047). However, age was not associated with decreased survival (P = .93).
To determine if the poorer prognosis of CGD-TCL was related to the higher incidence of either clinical tumors or subcutaneous involvement in these patients, we examined the prognostic significance of immunophenotype (αβ versus γδ) within these subsets. In the subset of patients presenting with clinical tumors, γδ immunophenotype still predicted for poor prognosis (P < .0001) (Figure 2D). Moreover, individuals with subcutaneous involvement and a γδ immunophenotype had a poorer survival than individuals with subcutaneous involvement and an αβ immunophenotype (P = .0003).
We also examined the statistical significance of a variety of parameters within the subset of patients with cutaneous γδ T-cell lymphoma. As shown in Table 2, subcutaneous involvement (P = .067) was the only parameter that showed nearly statistical significance within this subset of patients (Figure 2C). The development of a hemophagocytic syndrome did not reach statistical significance (P = .21).
Cox proportional hazards model analysis
The results from the Cox regression analysis are summarized in Table 3. On the basis of the results from the univariate analyses, the following variables were considered for inclusion in the Cox regression analysis: T-cell type (αβ versus γδ), histologic profile (epidermotropism and/or dermal versus subcutaneous involvement), and sex (male versus female). No other parameters were included because for all other univariatesP > .15. Because multiple factors did not emerge with respect to being potentially statistically significant in the patients with a γδ phenotype, no Cox model was created for that subset.
Multivariate analysis of primary cutaneous T-cell lymphomas
| Model and variables . | P3-150 . | Hazard ratio (95% CI) . |
|---|---|---|
| Model 1 | ||
| Histologic profile | < .0001 | 0.25 (0.13-0.48) |
| Model 2 | ||
| Histologic profile | .11 | 0.55 (0.26-1.14) |
| T-cell type | < .0001 | 0.16 (0.08-0.35) |
| Model 3 | ||
| T-cell type | < .0001 | 0.13 (0.07-0.25) |
| Model and variables . | P3-150 . | Hazard ratio (95% CI) . |
|---|---|---|
| Model 1 | ||
| Histologic profile | < .0001 | 0.25 (0.13-0.48) |
| Model 2 | ||
| Histologic profile | .11 | 0.55 (0.26-1.14) |
| T-cell type | < .0001 | 0.16 (0.08-0.35) |
| Model 3 | ||
| T-cell type | < .0001 | 0.13 (0.07-0.25) |
CI indicates confidence interval.
By χ2 test.
A Cox regression analysis, initially excluding T-cell type to establish the joint importance of all other factors under consideration, showed that histologic profile (epidermotropic and/or dermal versus subcutaneous involvement) was significantly associated with survival (model 1). Once T-cell subtype was added to the model (model 2), a likelihood ratio test showed that T-cell subtype was significantly associated with survival (P < .0001) after adjustment for histologic profile. However, histologic profile was no longer statistically significantly associated with survival, which was also confirmed by a likelihood ratio test (P = .11). When the histologic profile was removed from the 2-factor model, model 2, leaving only T-cell type (model 3), T-cell type showed a strong association with survival.
T-cell type and histologic profile interactions were also tested, with both T-cell type and histologic profile effects in the model, since there appeared to be a differential effect in the univariate analyses. However, there was no statistically significant interaction between T-cell type and histologic profile (likelihood ratio,P = .22).
A likelihood ratio test was also performed to test whether sex was associated with survival after adjustment for T-cell type. In addition, T-cell type by sex interaction was evaluated after inclusion of both T-cell type and sex effects in the model. However, neither sex (likelihood ratio, P = .26) nor T-cell type by sex interaction (likelihood ratio, P = .13) was significantly associated with survival.
Discussion
While the emphasis of the WHO classification is on the definition of disease entities, peripheral or mature T-cell lymphomas remain poorly understood. Lineage is the starting point in the subclassification of most lymphoid malignancies, yet the literature on primary cutaneous γδ T-cell lymphoma has been limited, because of the rarity of this lymphoma and the inability to study large numbers of patients. Most small series and case reports have indicated an aggressive clinical course. However, no other study has evaluated the impact of a γδ immunophenotype on the clinical outcome of primary cutaneous T-cell lymphoma in patients with diverse patterns of involvement. This study was designed to evaluate whether TCRδ expression was of prognostic significance in primary cutaneous T-cell lymphomas. Immunohistochemic studies for TCRδ1 were used to provide direct evidence of γδ T-cell derivation. In addition, all TCRδ1+ cases tested were βF1−, consistent with a γδ T-cell derivation (data not shown).
We found that there was a statistically significant decrease in survival among individuals affected with γδ cutaneous T-cell lymphoma in comparison with individuals with αβ cutaneous T-cell lymphoma (Figure 2A). The median survival for individuals with γδ T-cell lymphoma was 15 months, whereas the median survival of individuals with αβ T-cell lymphoma was 166 months. Cox proportional hazard model analysis confirmed that TCRδ1 expression was strongly associated with decreased survival.
We then investigated whether there was a difference in survival related to the depth of cutaneous involvement for all patients. We found a statistically significant decrease in survival in individuals who had subcutaneous involvement in comparison with individuals who had epidermotropic or dermal involvement (Figure 2B). We further examined the significance of these histologic parameters within only the γδ T-cell group. Subcutaneous involvement in CGD-TCL was associated with a trend for decreased survival in comparison with individuals who had epidermotropic or dermal involvement (Figure 2C). Those with subcutaneous involvement had a median survival of 13 months as compared with those with epidermotropic or dermal involvement, who had a more favorable median survival of 29 months. We also sought to identify other clinical and pathologic features of prognostic significance in patients with CGD-TCL. However, age, sex, adenopathy, and hemophagocytic syndrome did not have a significant association with survival.
Clinical tumors and subcutaneous involvement were more commonly seen in patients with CGD-TCL than in patients with αβ T-cell tumors (Table1). However, these factors alone cannot account for the poor survival of patients with γδ T-cell disease. In the subset of patients presenting with clinical tumors, γδ immunophenotype still predicted for poor prognosis (P < .0001) (Figure 2D). Moreover, individuals with subcutaneous involvement and a γδ immunophenotype had poorer survival than individuals with subcutaneous involvement and an αβ immunophenotype (P = .0003).
In the present study, we have demonstrated that TCRδ1 expression can be useful to predict the clinical outcome of patients with primary cutaneous T-cell lymphomas. Clinically, patients had aggressive disease and were resistant to multiagent chemotherapy and/or radiation. Within 5 years of diagnosis, 66% (22 of 33) patients were dead of their disease and 7 patients had progressive disease. It is of interest that none of the patients with CGD-TCL had bone marrow involvement; only 1 had lymph node involvement; and only 4 had adenopathy. However, biopsy evaluation of these sites was limited; only 36 patients had lymph node biopsies, and 68 had bone marrow biopsies. Similarly to MF/SS, CGD-TCL may spare the bone marrow even in advanced and leukemic stages. This pattern of dissemination is consistent with the tendency of CGD-TCL to preferentially involve mucocutaneous sites and may also be a reflection of the migratory features of “cutaneous T cells.” However, because of the poor clinical outcome, early diagnosis is indicated in patients with CGD-TCL.
Prior studies have indicated that mucosal/cutaneous γδ T-cell lymphoma represents a proliferation of functionally mature T-cell lymphocytes that express TIA-1 and release the cytotoxic proteins granzyme B and perforin, capable of inducing cellular apoptosis.6,14 This distribution of disease reflects the localization of normal γδ T cells, which are believed to play a role in host mucosal and epithelial immune responses.8,19Mucosal and cutaneous γδ T-cell lymphomas differ from hepatosplenic γδ T-cell lymphomas, which are derived from functionally immature γδ T cells, positive for TIA-1, but negative for granzyme B and perforin.12,18,39 It is possible that the activated cytotoxic phenotype contributes to the aggressive clinical behavior, as suggested in prior studies.7 For example, cutaneous epidermotropic CD-8+ cytotoxic T-cell lymphoma is characterized by generalized patches, plaques, papulonodules, and tumors, spread to unusual sites but not to the lymph nodes, and an aggressive course (median survival, 32 months). Histologically, it shows a bandlike infiltrate of epidermotropic T cells and necrosis. The neoplastic cells express CD3, CD8, CD7, CD45RA, βF1, and TIA-1 markers, whereas CD2 and CD5 were frequently absent.40However, not all cutaneous lymphomas of cytotoxic phenotype are aggressive. For example, cutaneous anaplastic large cell lymphoma (ALCL) has an indolent clinical course but expresses a cytotoxic phenotype.34
All patients in this study presented with disease initially confined to the skin. A characteristic clinical feature of patients with CGD-TCL was the presence of necrotic tumors or nodules, affecting primarily the extremities. This clinical feature should raise a suspicion for the diagnosis of CGD-TCL. We had found this characteristic quite helpful in identifying cases during physical examination. We identified 3 histologic patterns of involvement in the skin: epidermotropic, dermal, and subcutaneous. However, usually more than one histologic pattern was present in the same patient in different biopsy specimens or even in some cases within a single biopsy specimen (Figure 1). Epidermal infiltration from mild epidermotropism to pagetoid reticulosis–like has been previously reported in some cases of CGD-TCL.6 7 We observed only mild to moderate epidermotropism in our cases.
Subcutaneous involvement has been reported in γδ T-cell lymphoma.24 Moreover, in several recent studies, approximately 25% of cases of SPTCL were identified as being of γδ T-cell derivation on the basis of the expression of TCRδ1 or inferential evidence based on absent staining for βF1 in conjunction with a double-negative cytotoxic T-cell phenotype.15,16,41CGD-TCL with subcutaneous involvement shares many clinical and histologic features with SPTCL of αβ derivation, including expression of cytotoxic molecules such as TIA-1 and perforin and the presence of an atypical lymphocytic infiltrate with rimming of fat spaces within the subcutaneous tissue. However, in the published cases, certain differences with classical SPTCL of αβ origin have been noted. For example, the cases of γδ SPTCL have been noted to exhibit dermal involvement, in addition to the classic subcutaneous panniculitis–like infiltrate, as well as aggressive clinical behavior.15,16 41 As in previous reports, we found that cases of CGD-TCL with panniculitic features often manifested dermal as well as subcutaneous infiltrates, and variations in the histologic pattern were seen. However, not all cases with subcutaneous involvement in our series fulfilled the criteria for SPTCL. Of 33 cases with subcutaneous involvement, only 23 were diagnosed as SPTCL: 14 of γδ phenotype and 9 of αβ phenotype.
Arnulf et al14 made the observation that mucocutaneous γδ T-cell lymphomas could resemble SPTCL, nasal NK/T-cell lymphoma, or even enteropathy-associated T-cell lymphoma. Our study expands the diversity of histologic patterns described in the skin in γδ T-cell lymphomas and, more importantly, shows that irrespective of histologic pattern, γδ immunophenotype has important prognostic implications.
The distinctive clinical presentation in conjunction with a spectrum of histologic patterns and the fact that TCRδ1 expression is an independent prognostic factor associated with decreased survival suggest that CGD-TCL has distinctive features. Further studies are needed to determine if mucocutaneous γδ T-cell lymphoma should be designated a separate disease entity in future classification schemes.39 Analysis of T-cell subtype expression (αβ versus γδ) may be clinically indicated in the evaluation of patients with primary cutaneous T-cell lymphomas.
Prepublished online as Blood First Edition Paper, January 9, 2003; DOI 10.1182/blood-2002-05-1597.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jorge R. Toro, Genetic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, 6120 Executive Blvd, Executive Plaza S, Rm 7012, Rockville, MD 20892-7231; e-mail: torojo@exchange.nih.gov.

![Fig. 1. Dermatologic and histologic features of cutaneous gamma-delta T-cell lymphoma. / (A) Characteristic ulcerated tumors covered with hemorrhagic crust. (B) Multiple pink to plum–colored tumors in an upper extremity. (C) Low magnification shows an example of the coexistent epidermotropic, dermal, and subcutaneous patterns of involvement in a single biopsy. Original magnification, × 25. There is psoriasiform epidermal hyperplasia and a bandlike lymphocytic infiltrate in the papillary dermis with epidermotropism. The infiltrate extends into the reticular dermis with a perivascular pattern. In addition, there is infiltration of subcutaneous tissue reminiscent of lobular panniculitis. (D) The dermal infiltrate is positive for TCRδ1 by immunohistochemistry (avidin-biotin complex [ABC] immunoperoxidase; methyl green counter stain). Original magnification, × 100. Adapted from Toro et al,6 and reproduced with permission from Jaffe et al.4](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/9/10.1182_blood-2002-05-1597/4/m_h80934249001.jpeg?Expires=1769095882&Signature=IS6FAXhms2G0CkaQ8Zs54Ci07zCgRP-AgTjnxrot~jLH36imDr6NiACQuUsMegU4nouqIbD4Z86gn1GCue-KXaUKRENVYBPDFJpO4gBdb7emxE30QjuJzXRqDy2a9lHsR8qD7BXUEjyI3pXRv~JvYi4driVWLlgeSjDZI58i1MwHuDZXV-OHMscWbFP5qttVN1NfD0trHWb24fsm-5-sU07HhuP2nHYlBOiWawv8l924rHn7eUS466iiDcWxNxLakLajzvy3Fa4HZz0CHHWxgZpXgJgYk5oMI3qJPud6Li-Jk3PqaBAzWkGAdQyhMSKmNVfVHFXPpED~PmoOQSDDAg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal