Mechanisms underlying fetal hemoglobin (HbF) reactivation in stress erythropoiesis have not been fully elucidated. We suggested that a key role is played by kit ligand (KL). Because glucocorticoids (GCs) mediate stress erythropoiesis, we explored their capacity to potentiate the stimulatory effect of KL on HbF reactivation, as evaluated in unilineage erythropoietic culture of purified adult progenitors (erythroid burst-forming units [BFU-Es]). The GC derivative dexamethasone (Dex) was tested in minibulk cultures at graded dosages within the therapeutical range (10−6 to 10−9M). Dex did not exert significant effects alone, but synergistically it potentiated the action of KL in a dose-dependent fashion. Specifically, Dex induced delayed erythroid maturation coupled with a 2-log increased number of generated erythroblasts and enhanced HbF synthesis up to 85% F cells and 55% γ-globin content at terminal maturation (ie, in more than 80%-90% mature erythroblasts). Equivalent results were obtained in unicellular erythroid cultures of sibling BFU-Es treated with KL alone or combined with graded amounts of Dex. These results indicate that the stimulatory effect of KL + Dex is related to the modulation of γ-globin expression rather than to recruitment of BFU-Es with elevated HbF synthetic potential. At the molecular level, Id2 expression is totally suppressed in control erythroid culture but is sustained in KL + Dex culture. Hypothetically, Id2 may mediate the expansion of early erythroid cells, which correlates with HbF reactivation. These studies indicate that GCs play an important role in HbF reactivation. Because Dex acts at dosages used in immunologic disease therapy, KL + Dex administration may be considered to develop preclinical models for β-hemoglobinopathy treatment.
Introduction
Human hemoglobin (Hb) is heterogeneous at all stages of development, from embryonic through adult life. The fetal period is characterized by the synthesis of fetal Hb (HbF, α2 γ2), which is associated with a small amount of adult Hb (HbA, α2 β2) (5%-10% of total Hb). The perinatal phase involves a gradual and reciprocal switch from HbF to HbA.1 In adult life, the residual HbF (less than 1%) is restricted to F cells, which represent less than 5% of red blood cells (RBCs).2,3 In diverse conditions associated with accelerated bone marrow regeneration (stress erythropoiesis), HbF synthesis may be reactivated up to 10% to 20%.4,5 HbF reactivation provides an interesting model of partial reverse of the HbF→HbA perinatal switch. More important, therapeutic administration of agents stimulating HbF production may be beneficial in patients with β-globin disorders, such as sickle cell anemia or β-thalassemia. In sickle cell anemia, HbF interferes with sickling by inhibiting the polymerization of HbA in vitro and in vivo.6,7 In β-thalassemia a significant increase in HbF, replacing the defective HbA, may diminish the precipitation of unpaired α-globin chains.8
Cell cultures and animal models have allowed the identification of several agents reactivating HbF synthesis,9 including chemical inducers and cytokines. The clinical use of hydroxyurea, though partially successful in the treatment of sickle cell anemia,10,11 has been hindered by myelotoxicity, potential long-term carcinogenicity, and only moderate therapeutic effects in most patients.12 Histone deacetylase inhibitors, particularly butyrate analogs, increase HbF levels in primary cultures of human erythroid cells.13 Their clinical use, however, is rendered difficult by their rapid degradation. In sickle cell anemia, the intravenous administration of arginine butyrate enhances HbF synthesis, but the effect gradually declines on prolonged infusion. Pulse regimens may offset this decline but may result in a suboptimal amount of the drug and a lower stimulatory effect.14
In addition to chemical inducers, HbF synthesis may be stimulated by hematopoietic growth factors (HGFs).15-18 Particularly, kit ligand (KL) markedly stimulated HbF production in sickle cell anemia17 and healthy hematopoietic progenitor cell (HPC) cultures.18 In vitro, the stimulating effect of KL is potentiated by histone deacetylase inhibitors; however, these chemical inducers, particularly when used alone, exert an inhibitory effect on erythropoiesis.19 In vivo KL stimulates HbF synthesis and potentiates the effects of hydroxyurea.20 Here again, the combined administration of KL and hydroxyurea in the clinical setting is limited by the toxic effects of the latter agent.
To overcome these limitations, we have explored whether the stimulating effect of KL may be potentiated by other therapeutic agents widely used in the clinical setting. Among the hormones that stimulate erythropoiesis, glucocorticoids (GCs) are of particular interest, as indicated by in vitro and in vivo studies.21-23 We have therefore investigated the effects of the GC derivative dexamethasone (Dex) on HbF reactivation in erythroid bursts generated by purified adult erythroid burst-forming units (BFU-Es) in minibulk and single-cell unilineage erythroid cultures. The results indicate that Dex alone does not affect HbF reactivation and erythroid cell proliferation, whereas combined treatment with Dex and KL exerts a synergistic stimulatory effect on both parameters.
Materials and methods
Recombinant human HGFs and chemical inducers
Interleukin-3 (IL-3), granulocyte macrophage–colony-stimulating factor (GM-CSF), KL, and erythropoietin (EPO) were supplied by Behring (Behringwerke AG, Marburg, Germany), Sandoz (Basel, Switzerland), Peprotech (London, England), and Amgen (Thousand Oaks, CA), respectively. Dex in a water-soluble form was provided by Sigma Chemical (St Louis, MO).
HPC purification
Adult peripheral blood (PB) was obtained from healthy adult male donors after informed consent. Blood (450 mL) was collected in preservative-free, citrate/phosphate/dextrose/adenine (CPDA-1) anticoagulant. A buffy coat was obtained by centrifugation (Beckman J6M/E, 1400 rpm per 20 minutes at room temperature; Beckman Instruments, Fullerton, CA). Low-density cells (less than 1.077 g/mL) were isolated using a Ficoll gradient as previously described.24
The CD34+ HPCs were then purified by using the MiniMACS CD34 isolation system (Miltenyi, Bergisch Gladbach, Germany) following the manufacturer's instructions. Purified cells were more than 90% CD34+, as evaluated by fluorescence-activated cell sorting (FACS) analysis.
HPC unilineage erythropoietic culture
Mini-bulk culture.
Purified HPCs were grown in fetal calf serum (FCS)–free liquid culture (5 × 104 cells/mL/well) in a fully humidified atmosphere of 5% CO2/5% O2/90% N2 and were induced to unilineage erythropoietic differentiation by an erythroid-specific HGF cocktail (EPO and low-dose IL-3/GM-CSF), as reported.25 26 The HGF cocktail was supplemented or not supplemented with KL (100 ng/mL), Dex (10−6 to 10−9 M), or both.
Unicellular sibling BFU-E culture.
Unicellular cultures (0.5 cell/well) were performed in flat-bottomed 96-microwell plates in 0.1 mL FCS-free medium25-27supplemented with 5% FCS. To selectively stimulate erythroid differentiation, the cultures were supplemented with the HGF cocktail (EPO and IL-3/GM-CSF) indicated in the previous paragraph. At days 3 to 4, the 4 cell clones were identified. The 4 sibling cells picked up by a micromanipulator were seeded in 4 different wells,19 each containing 0.1 mL of the same erythroid medium supplemented with plateau levels of KL (100 ng/mL), alone or combined with graded amounts of Dex (10−7-10−8-10−9 M). Clones were analyzed from day 14 through days 28 to 30.
Morphology analysis.
Cells were harvested from day 14 through day 28, smeared on glass slides by cytospin centrifugation, and stained with standard May-Grünwald-Giemsa. For single sibling colony analysis, polylysine-coated glass slides were used.
HbF assays: F-cell number and γ-chain content
HbF assays were carried out at terminal erythroid maturation (ie, at days 14-16 for control ± Dex cultures and at days 20-22 or 28-30 for KL or KL + Dex cultures, respectively). In all culture conditions, the percentage of late erythroblasts (polychromatophilic 2 and orthochromatic) was more than 80% to 90%.
F cells
The percentage of mature erythroblasts containing HbF was evaluated by indirect fluorescence as previously described.3 Briefly, erythroid cells were cytocentrifuged on a glass slide, fixed for 5 minutes in acetone-methanol (9:1, vol/vol), washed 3 times with phosphate-buffered saline (PBS) and once with PBS containing 2 mg/mL bovine serum albumin (BSA), and incubated for 40 minutes at 37°C with anti-human HbF monoclonal antibody (mAb; Caltag Laboratories, Burlingame, CA). Slides were washed twice with PBS and once with PBS/BSA, incubated for 30 minutes at room temperature with fluorescein isothiocyanate (FITC)–conjugated F(ab′)2antimouse immunoglobulin G (IgG; Dakopatts, Copenhagen, Denmark), and extensively washed in PBS. Slides were then mounted in PBS/glycerol (50:50) and observed under an Axiophot Zeiss microscope (Zeiss, Jena, Germany) equipped for fluorescence. As a negative control, cells were incubated with healthy mouse IgG instead of anti-HbF and were processed as indicated above in this paragraph.
In some experiments, HbF containing erythroblasts were also evaluated by flow cytometry. Briefly, erythroid cells were fixed and permeabilized with the Cytofix/Cytoperm kit (PharMingen, San Diego, CA), washed in PBS containing 0.1% Triton X-100, incubated with anti-HbF mAb, and then washed with anti-mouse IgG antibody, as indicated above. The percentage of fluorescent cells and the intensity of fluorescence (mean fluorescence intensity [MFI]) were evaluated using a FACScan (Becton Dickinson, San Diego, CA).
γ-chain content
High-performance liquid chromatography (HPLC) separation of globin chains was performed according to previously published methods.28 Briefly, cell lysates from bulk culture, or single sibling erythroid colonies, or both were separated on chromatographic columns (Merck LiChrospher 100 CH8/2, 5 μm; E. Merck, Darmstadt, Germany) using as eluents a linear gradient of acetonitrile/methanol/0.155 M sodium chloride (eluent A; pH 2.7, 68:4:28 vol/vol/vol) and acetonitrile/methanol/0.077 M sodium chloride (eluent B; pH 2.7, 26:33:41 vol/vol/vol). Gradient was from 20% to 60% eluent A in 60 minutes at a flow rate of 0.8 mL/min. The optimal absorbance of the different globin was evaluated at 214 nm because absorbance coefficients of the different chains are identical at this wavelength.28
Id2, Tal1, and GATA1 expression
Id2, Tal1, and GATA1 expression was investigated at the protein level by Western blotting on erythroid minibulk cultures. Briefly, 2.5 × 105 cells were obtained at different days of erythroid culture and were lysed in 20 μL boiling sodium dodecyl sulfate (SDS) sample buffer. Samples were boiled for 10 minutes and then centrifuged at 10 000 rpm for 10 minutes at 4°C to remove debris. Whole cell lysates were resolved by SDS–polyacrylamide gel electrophoresis. The same lysate amount was added to all lanes. Tal1, Id2, and GATA1 expression were detected using an anti-Tal1 rabbit serum 1080,29 an anti-Id2 rabbit polyclonal antibody sc-489, and an anti- GATA1 rat monoclonal antibody sc-266 (Santa Cruz Biotechnology, CA), respectively. Anti-β actin CP01 (Oncogene, Boston, MA) was used as loading control.
Results
In the first series of experiments we analyzed the effect of Dex in minibulk erythroid culture. This synthetic glucocorticoid was added in a dose-response fashion (10−6 to 10−9 M) to unilineage erythroid culture supplemented or not with plateau levels of KL (100 ng/mL).
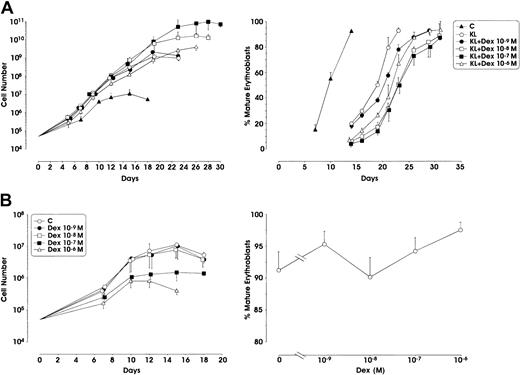
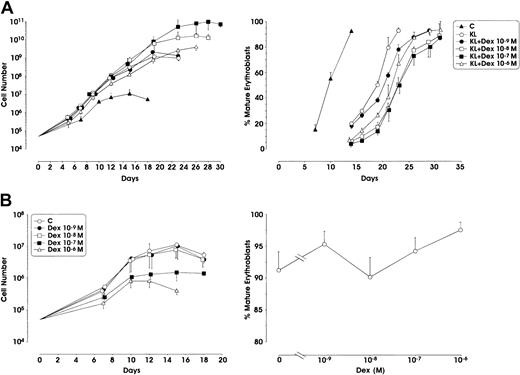
When used in combination with KL, Dex at the 10−7 M concentration remarkably stimulated erythroid cell proliferation with a 2 log rise of the cell number output, compared with KL alone (from 109 to 1011 cells starting from 5 × 104 CD34+cells) (Figure1A). Interestingly, Dex at 10−8 M also enhanced cell proliferation, whereas at lower (10−9 M) or higher (10−6 M) doses it did not significantly stimulate erythroid cell growth. In KL + Dex culture conditions, terminal erythroid maturation (defined by the presence of more than 80% to 90% polychromatophilic 2 and orthochromatic erythroblasts) was delayed up to 28 to 30 days of culture (1 or 2 weeks later than KL-treated or control erythroid cultures, respectively) (Figure 1A). On the contrary, when supplemented alone, Dex did not stimulate cell proliferation compared with control culture (Figure 1B).
Effect of Dex on erythroid cell growth and maturation.
(A) Growth curve from minibulk HPC erythroid cultures supplemented or not supplemented with KL (100 ng/mL) and graded amounts of Dex (10−9 to 10−6 M). C indicates control. Total cell number (left) and percentage of mature erythroblasts (polychromatophilic 2 + orthochromatic erythroblasts) during erythroid differentiation (right) are shown. Mean ± SEM values from 5 separate experiments. Variance analysis was performed to evaluate the difference between KL and each Dex dose.P < .001 when comparing KL with Dex 10−7 or 10−8 M. The difference between KL and Dex 10−6 or 10−9 M is not significant. (B) Left panel, total cell number from minibulk erythroid cultures supplemented only with Dex; right panel, erythroid maturation, as evaluated in terms of mature erythroblasts on days 14 to 16 of culture. C indicates control. Mean ± SEM values from 4 separate experiments.P < .01 and P < .001 when comparing C with Dex 10−7 and 10−8 M, respectively.
Effect of Dex on erythroid cell growth and maturation.
(A) Growth curve from minibulk HPC erythroid cultures supplemented or not supplemented with KL (100 ng/mL) and graded amounts of Dex (10−9 to 10−6 M). C indicates control. Total cell number (left) and percentage of mature erythroblasts (polychromatophilic 2 + orthochromatic erythroblasts) during erythroid differentiation (right) are shown. Mean ± SEM values from 5 separate experiments. Variance analysis was performed to evaluate the difference between KL and each Dex dose.P < .001 when comparing KL with Dex 10−7 or 10−8 M. The difference between KL and Dex 10−6 or 10−9 M is not significant. (B) Left panel, total cell number from minibulk erythroid cultures supplemented only with Dex; right panel, erythroid maturation, as evaluated in terms of mature erythroblasts on days 14 to 16 of culture. C indicates control. Mean ± SEM values from 4 separate experiments.P < .01 and P < .001 when comparing C with Dex 10−7 and 10−8 M, respectively.
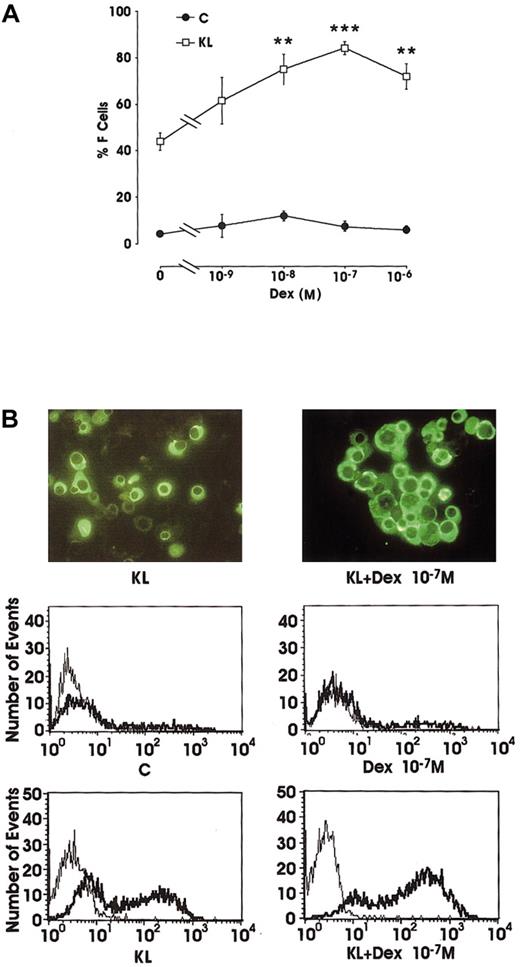
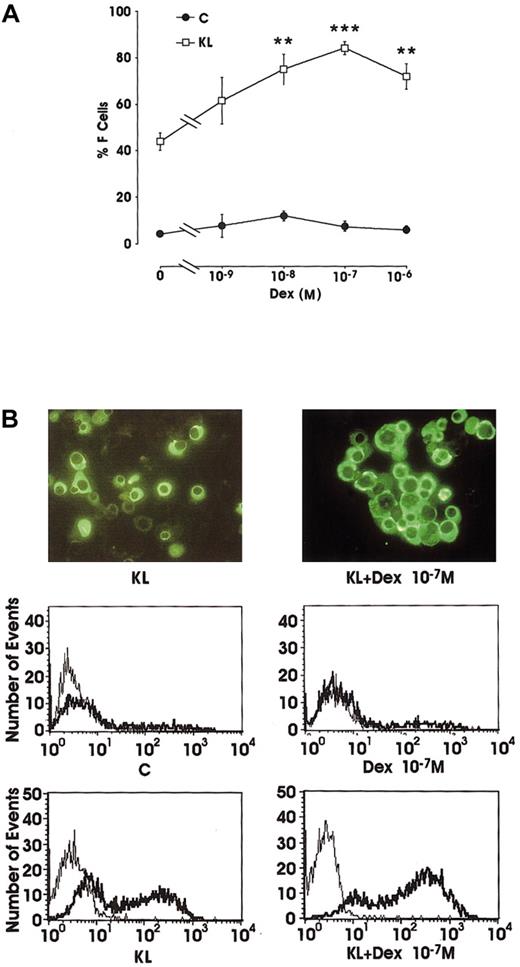
Evaluation of F-cell frequency by immunofluorescence (IF) in minibulk erythroid cultures showed that Dex alone slightly reactivated the production of F erythroblasts (from 4% in control erythroid cultures to 12% at 10−8 M Dex). However, in combination with KL, Dex induced a striking increase of F cells (up to 84% at 10−7 M) compared with KL alone (42%) (Figure2A). In these different culture conditions, F cells were also analyzed by flow cytometry (Figure 2B). The data confirmed the rise of F-cell frequency in KL ± Dex cultures and showed a parallel increase in the intensity of fluorescence staining.
F-cell analysis.
(A) Percentage of F cells in minibulk unilineage erythroid cultures supplemented or not supplemented with KL ± graded amounts of Dex (10−9 to 10−6 M). C indicates control cultures. Mean ± SEM values from 5 separate experiments. **P < .01 and ***P < .001 when compared with KL alone. The difference between KL and KL + Dex 10−9 M and between C and all Dex dosages is not significant. (B) F-cell evaluation by indirect immunofluorescence labeling (top; original magnification × 400) or by flow cytometry (middle and bottom; thin lines indicate negative controls, and thick lines, anti-HbF Ab–treated cells). Representative results are shown.
F-cell analysis.
(A) Percentage of F cells in minibulk unilineage erythroid cultures supplemented or not supplemented with KL ± graded amounts of Dex (10−9 to 10−6 M). C indicates control cultures. Mean ± SEM values from 5 separate experiments. **P < .01 and ***P < .001 when compared with KL alone. The difference between KL and KL + Dex 10−9 M and between C and all Dex dosages is not significant. (B) F-cell evaluation by indirect immunofluorescence labeling (top; original magnification × 400) or by flow cytometry (middle and bottom; thin lines indicate negative controls, and thick lines, anti-HbF Ab–treated cells). Representative results are shown.
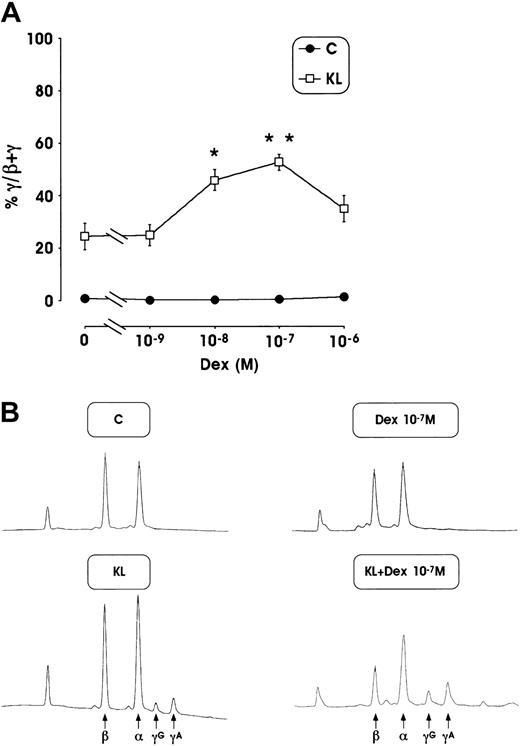
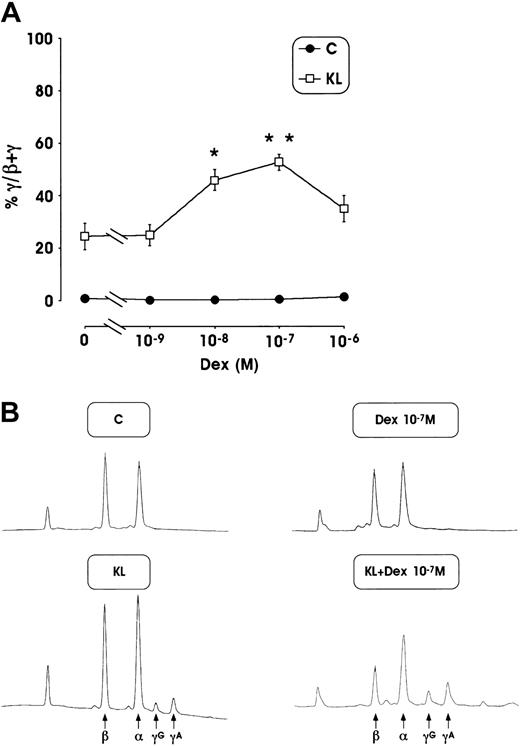
This striking HbF reactivation was confirmed by analysis of γ-chain content using an HPLC assay. The mean values of γ-globin content from 3 separate experiments, performed in minibulk erythroid cultures supplemented with KL + Dex, show a strong increase of γ-globin chains (up to 53% at 10−7 M Dex) compared with control culture supplemented with Dex or KL alone (less than 1% or 24.5%, respectively) (Figure 3A). Here again, a marked enhancement of γ-globin content (45%) was also observed in erythroid cultures grown in the presence of KL and lower Dex dosages (10−8 M). Representative globin-chain HPLC scans from mature erythroblasts differentiated in unilineage erythroid culture in the presence or absence of KL ± Dex at 10−7 M (Figure 3B) show a clear increase of γA and γG chain peaks.
γ-chain content.
(A) Percentage of γ-globin chain content evaluated by the HPLC system in mature erythroblasts obtained from minibulk HPC erythroid cultures supplemented or not with KL ± graded amount of Dex (10−9 to 10−6 M). Mean ± SEM values from 5 separate experiments. *P < .05 and **P < .01 when compared with KL alone. (B) Globin chain HPLC scans from mature erythroblasts obtained in minibulk erythroid cultures supplemented or not with KL ± Dex (representative results). C indicates control culture.
γ-chain content.
(A) Percentage of γ-globin chain content evaluated by the HPLC system in mature erythroblasts obtained from minibulk HPC erythroid cultures supplemented or not with KL ± graded amount of Dex (10−9 to 10−6 M). Mean ± SEM values from 5 separate experiments. *P < .05 and **P < .01 when compared with KL alone. (B) Globin chain HPLC scans from mature erythroblasts obtained in minibulk erythroid cultures supplemented or not with KL ± Dex (representative results). C indicates control culture.
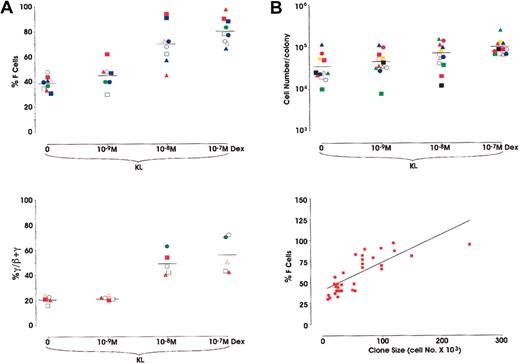
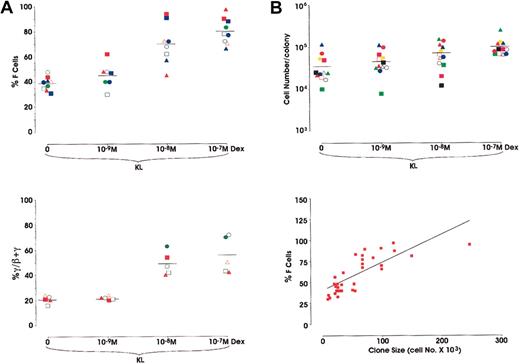
In a second series of experiments we analyzed the effects of Dex, alone or in combination with KL, in sibling BFU-E colonies. Briefly, the purified HPCs were grown in unicellular unilineage erythroid liquid cultures. After 2 cell divisions, the 4 sibling BFU-Es were subdivided by micromanipulation and individually reseeded in secondary erythroid cultures supplemented with KL ± Dex from 10−7 to 10−9 M. Results confirm the data obtained in minibulk cultures (Figure 4). With respect to F-cell frequency in 9 different sibling clones, Dex induced a comparable dose-related HbF reactivation (38.8%-80% F cells in KL and KL + Dex 10−7 M, respectively) (Figure 4A, top). Moreover, in 5 other sibling clones, analysis of γ-globin content showed a parallel increase of HbF synthesis (20.8%-55.4% γ-chains in KL vs KL + Dex 10−7 M, respectively) (Figure 4A, bottom). This HbF reactivation was paralleled by an increase in colony size (3.5 × 104 to 1 × 105; mean values from 12 different sibling clones grown in KL or KL + Dex 10−7 M, respectively) (Figure 4B, top). Obviously, because erythroid maturation was delayed in KL + Dex-treated cultures, we analyzed erythroid clones from sibling BFU-Es composed of approximately 80% to 90% mature erythroblasts. Percentages of F cells and cell number per colony values were strictly correlated (P < .001) (Figure 4B, bottom). Finally, to investigate the molecular mechanisms underlying HbF reactivation induced by KL + Dex, we evaluated the expression of Id2, Tal1, and GATA1.
HbF reactivation in sibling BFU-E colonies.
(A) Top: Percentage of F cells evaluated in single sibling BFU-E colonies grown in unilineage erythroid cultures supplemented with KL ± Dex at 3 different concentrations (10−9, 10−8, or 10−7 M). Results from 9 experiments, each including 4 sibling colonies each represented by the same symbol (mean values are also indicated). P < .01 when comparing the 10−8 M and 10−7 M Dex groups with the control group. Bottom: Percentage of γ-globin chains evaluated by HPLC in the unicellular sibling BFU-E cultures. Results from 5 experiments, each including 4 sibling colonies.P < .01 when comparing the 10−8 M and 10−7 M Dex groups with the control group. (B) Top: Single values of cell number/colony from 12 different clones, each including 4 sibling BFU-E colonies, grown as described above (mean values are also indicated). Bottom: Direct correlation between F-cell frequency (percentage values) and cell number × 103 in 36 sibling colonies (P < .001).
HbF reactivation in sibling BFU-E colonies.
(A) Top: Percentage of F cells evaluated in single sibling BFU-E colonies grown in unilineage erythroid cultures supplemented with KL ± Dex at 3 different concentrations (10−9, 10−8, or 10−7 M). Results from 9 experiments, each including 4 sibling colonies each represented by the same symbol (mean values are also indicated). P < .01 when comparing the 10−8 M and 10−7 M Dex groups with the control group. Bottom: Percentage of γ-globin chains evaluated by HPLC in the unicellular sibling BFU-E cultures. Results from 5 experiments, each including 4 sibling colonies.P < .01 when comparing the 10−8 M and 10−7 M Dex groups with the control group. (B) Top: Single values of cell number/colony from 12 different clones, each including 4 sibling BFU-E colonies, grown as described above (mean values are also indicated). Bottom: Direct correlation between F-cell frequency (percentage values) and cell number × 103 in 36 sibling colonies (P < .001).
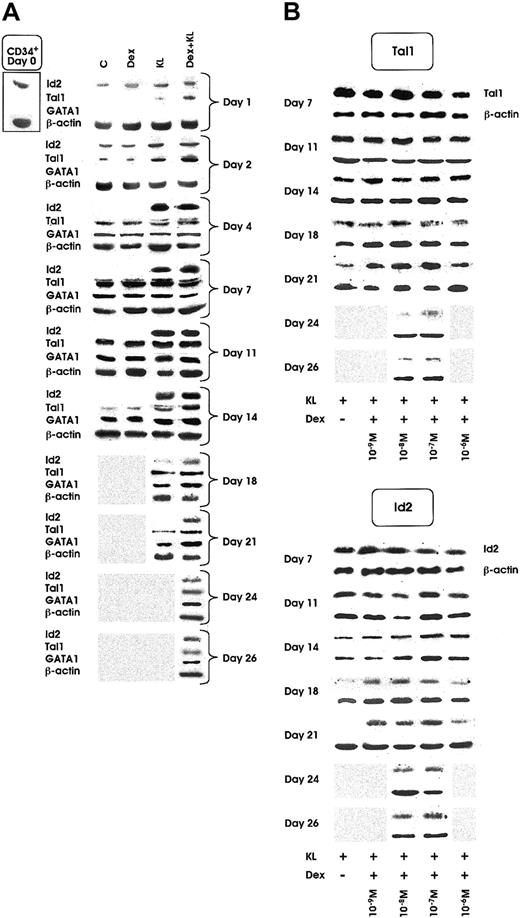
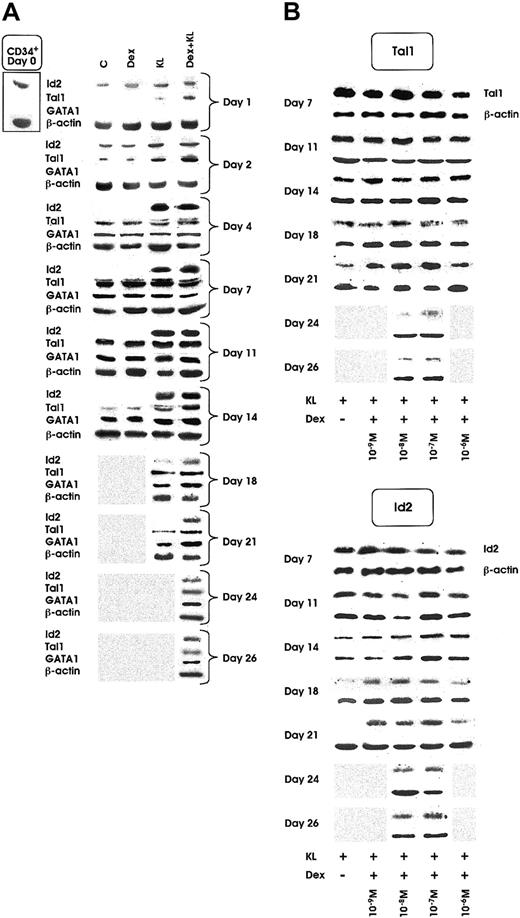
Id2 protein was clearly detected in quiescent HPCs and in all culture conditions during the first 2 days of culture. Starting from day 2, the expression decreased in control (C) and Dex cultures and slightly increased in KL and KL + Dex cultures. From day 4 of culture to terminal maturation, Id2 protein was virtually undetectable in control erythroid cultures supplemented or not supplemented with Dex. In contrast, it was expressed at sustained levels up to day 18 and day 26 in KL and KL + Dex cultures, respectively (Figure5A). Specifically, Id2 expression remained detectable to day 26 only in KL cultures supplemented with Dex ranging from 10−7 to 10−8 M (Figure 5B, right panel).
Id2, Tal1, and GATA1 protein expression as evaluated by Western blot.
(A) Id2, Tal1, and GATA1 expression from day 0 to day 14, 21, or 26 in minibulk HPC erythroid cultures supplemented or not with Dex at optimal dosage (10−7 M) KL or KL + Dex, respectively. C indicates control cultures. β-Actin controls are also included. Shaded area indicates sample not available (ie, the culture had already reached terminal maturation and had therefore been terminated). (B) Tal1 (top panel) and Id2 (bottom panel) expression in minibulk HPC erythroid cultures supplemented with KL and different doses of Dex (from 10−6 to 10−9 M) from day 7 to complete maturation (day 21 for KL ± Dex 10−6 M or 10−9 M and day 26 for KL + Dex 10−7 or 10−8 M, respectively). β-Actin controls are also included. Shaded area indicates sample not available.
Id2, Tal1, and GATA1 protein expression as evaluated by Western blot.
(A) Id2, Tal1, and GATA1 expression from day 0 to day 14, 21, or 26 in minibulk HPC erythroid cultures supplemented or not with Dex at optimal dosage (10−7 M) KL or KL + Dex, respectively. C indicates control cultures. β-Actin controls are also included. Shaded area indicates sample not available (ie, the culture had already reached terminal maturation and had therefore been terminated). (B) Tal1 (top panel) and Id2 (bottom panel) expression in minibulk HPC erythroid cultures supplemented with KL and different doses of Dex (from 10−6 to 10−9 M) from day 7 to complete maturation (day 21 for KL ± Dex 10−6 M or 10−9 M and day 26 for KL + Dex 10−7 or 10−8 M, respectively). β-Actin controls are also included. Shaded area indicates sample not available.
As shown in Figure 5A, Tal1 protein was virtually undetectable in quiescent HPCs. In control cultures, supplemented or not with Dex 10−7 M, Tal1 protein expression was detectable from day 2 to day 14, whereas in KL and KL + Dex cultures a marked increase of Tal1 was observed from day 1 to day 21 and day 26, respectively (Figure 5A). Like Id2, Tal1 protein was detected until day 26 only in KL cultures supplemented with optimal Dex concentrations (10−7 to 10−8 M) (Figure 5B, left panel).
GATA1 expression, virtually undetectable in quiescent HPCs and until day 2 of culture, was induced at day 4 in all erythroid cultures, supplemented or not with KL and Dex (Figure 5A). In control ± Dex wells, GATA1 protein was detectable to day 14, whereas in KL and KL + Dex cultures, GATA1 was extended to day 21 and day 26, respectively (Figure 5A).
Discussion
Stress erythropoiesis is triggered by tissue hypoxia, which may be induced by blood loss, anemia, or oxygen deprivation. It has been established that GCs are important mediators of erythropoietic stress. GCs regulate a variety of physiological responses and developmental processes by binding to their cognate nuclear receptor GCR.30,31 In vitro, the GCR cooperates with the activated EPO receptor (EPOR) and Kit to induce long-term proliferation of erythroid progenitors while delaying their differentiation.22 In vivo, GCR is also required for the rapid expansion of murine erythroid progenitors under stress conditions such as erythrolysis or hypoxia.23
In postnatal conditions associated with erythropoietic stress, HbF synthesis is reactivated up to 10% to 20% relative γ-globin content.4,5 It has been suggested that the increased EPO levels observed in these conditions may mediate HbF reactivation.32 In clinical trials, however, large dosages of recombinant EPO induced only a modest and variable increase of HbF.33 We previously reported that KL reactivates HbF synthesis and enhances erythroid proliferation in single-cell erythropoietic culture.18,19 In the present study we have evaluated the effect of GCs on human HbF reactivation in minibulk and unicellular erythroid cultures of purified adult HPCs. We observed that the combined addition of Dex and KL triggered a marked increase of HbF synthesis compared with the erythroid cultures grown with KL alone. This increase was not linked to defective erythroblast maturation because HbF content was consistently monitored in 80% to 90% of mature erythroblasts. Similarly, Dex exerted a stimulatory action on erythroid cell proliferation only when combined with KL. Experiments on unicellular culture of 4 sibling BFU-Es indicate that the synergistic stimulatory effect of the Dex + KL combination is mediated through a direct dose-related effect of Dex on HbF synthesis, but they rule out a recruitment of BFU-Es with elevated HbF synthesis potential.19
Here again, we observed a direct correlation between the enhanced erythroid proliferation and the reactivation of HbF, as previously reported18,19 and confirmed by others.34 We suggest that in adult stress erythropoiesis, HbF synthesis may be reactivated by enhanced proliferation of early erythroid cells, triggered by KL and GCs. Hypothetically, a decline of KL activity may similarly mediate perinatal Hb switching. Indeed, erythroid cell proliferation is elevated in fetal life, gradually declines in the perinatal period, and bottoms in steady-state adult life.35 KL activity is more elevated in fetal/perinatal life than in postnatal life, including the erythropoietic response to KL.36 37 Ongoing studies aim to verify this model of Hb switching.
The effects of GCs on erythropoiesis have been investigated in cultures stimulated by EPO only or by EPO and KL. In erythroid cultures of unpurified fetal liver HPCs stimulated with EPO alone, Dex exerts a dose-dependent inhibitory effect.38 This report is in line with our observations on purified adult HPCs in control erythroid cultures that show a dose-related inhibitory effect at 10−7 to 10−6 M Dex. On the other hand, studies carried out on bone marrow HPCs indicate that Dex synergizes with KL in sustaining erythroid cell proliferation.22 The latter finding is confirmed here. Altogether, it is apparent that Dex may exert either stimulatory or inhibitory effects on erythroid cell proliferation, mainly depending on the applied GC dosage and the presence or absence of KL.
To shed light on the molecular mechanisms underlying the stimulatory effects of KL + Dex on erythroid cell proliferation and HbF synthesis, we have analyzed the expression of Id2, Tal1, and GATA1 transcription factors (TFs) at the protein level in erythroid cultures. Circumstantial evidence suggests that Id2 and Tal1 may stimulate HbF production. Specifically, Id2 enhances γ-globin expression in erythroleukemic cell lines,39 and Tal1, in cooperation with other TFs, interacts with a conserved E-box in the β-globin control region enhancer to stimulate γ-globin gene transcription.40 Furthermore, GATA1 plays a key role in erythroid cell survival and proliferation.41 42
We observed that Id2 protein, though expressed in CD34+cells, is gradually suppressed in control ± Dex cultures at days 1 to 2, down to undetectable levels from day 4. In sharp contrast, Id2 expression is sustained in the KL culture, particularly if supplemented with Dex, suggesting that Id2 may significantly contribute to stimulate erythroid cell proliferation and to delay erythroblast maturation. Because enhanced erythroid proliferation strictly correlates with and possibly causes HbF reactivation,18,19 34 it is hypothesized that the persistent Id2 expression induced by the addition of KL ± Dex may stimulate early erythroid proliferation and hence HbF synthesis.
Our observations are in line with other reports on the proliferative and antidifferentiative Id2 effects in diverse hematopoietic cell types.43 Specifically, Id2-deficient mice show defective natural killer (NK) cell differentiation and lymph node formation.44,45 Furthermore, Id2 exerts a negative role in erythroid29 and B-lymphoid46 differentiation. In both lineages Id2 is expressed in early precursors but not in mature cells.29,47 Our observations on erythroid cell differentiation here and in our earlier report29 are apparently at variance with studies by Zhang et al,47which report Id2 expression in maturing erythroid precursors. This discrepancy may be reconciled in view of the sharp differences in cell culture conditions (unpurified vs purified hemopoietic progenitor cells, serum-rich vs serum-free medium).
Tal1 and GATA1, though undetectable in HPCs, are induced in erythroid culture. Specifically, Tal1 is detected in KL ± Dex cultures26,29 starting from day 1; interestingly, the induction in control ± Dex wells starts on day 2 and is altogether less pronounced in the initial culture period. In all groups, GATA1 is induced from day 4 onward. In all culture conditions, the expression of both TFs is sustained and declines in terminal erythroid cells. Therefore, the expression is more prolonged with KL ± Dex addition (as reported by Miller et al48 for Tal1), which induces a more extensive erythroid proliferation period. The initial enhancement of Tal1 expression induced by KL ± Dex may contribute to stimulate early erythroid proliferation. Indeed, Tal1 potentiates antiapoptotic mechanisms in early erythropoiesis (R. De Maria et al, manuscript submitted). On the other hand, Tal1 enhances c-kit expression and thereby maintains the sensitivity of erythroid cells to KL.49 Along these lines, the KL-induced prolongation of GATA1 expression may contribute to potentiate erythroblast proliferation. To clarify the molecular mechanisms underlying HbF reactivation, studies on transduction of Id2, Tal1, and GATA1 genes are in progress and will be presented in a separate report.
It is noteworthy that the peak effect of Dex on erythroid cell proliferation and HbF reactivation was observed at 10−7 M concentration, whereas a marked stimulatory effect on both parameters was monitored at 10−8 M. Interestingly, these concentrations are reached in plasma following the administration of Dex for therapeutic purposes.50 These observations pave the way to preclinical studies aiming to assess HbF reactivation induced by KL and low-dose Dex in suitable animal models,20 including the evaluation of side effects related to prolonged treatment with KL and Dex. In parallel, studies51 based on erythroid culture of progenitors from patients with β-thalassemia and sickle cell anemia may contribute to evaluations of the therapeutic potential of HbF reactivation induced by KL and low-dose Dex in β-hemoglobinopathies.
We thank M. Blasi and M. Fontana for editorial assistance and A. Zito for the graphics.
Prepublished online as Blood First Edition Paper, November 7, 2002; DOI 10.1182/blood-2002-05-1477.
Supported in part by an intramural grant from the Istituto Superiore di Sanitá, Rome, Italy (M.G.).
M.G. and U.T. contributed equally to this manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Cesare Peschle, Kimmel Cancer Center, Room 609, Thomas Jefferson University, 233 South 10th St, Philadelphia, PA 19107-5541; e-mail:cesare.peschle@mail.tju.edu.