Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31), a tyrosine phosphoprotein highly expressed on endothelial cells and leukocytes, is an important component in the regulation of neutrophil transendothelial migration. Engagement of endothelial PECAM-1 activates tyrosine phosphorylation events and evokes prolonged calcium transients, while homophilic engagement of neutrophil PECAM-1 activates leukocyte β-integrins. Although PECAM-1 modulates polymorphoneutrophil transmigration via homophilic PECAM-1–PECAM-1 interaction, the mechanisms underlying endothelial PECAM-1 function are unknown. Proposed mechanisms include (1) formation of a haptotactic gradient that “guides” neutrophils to the cell-cell border, (2) service as a “passive ligand” for neutrophil PECAM-1, ultimately mediating activation of neutrophil β integrins, (3) regulation of endothelial calcium influx, and (4) mediation of SH2 protein association, and/or (5) catenin and non-SH2 protein interaction. Utilizing PECAM-1–null “model” endothelial cells (REN cells), we developed a neutrophil transmigration system to study PECAM-1 mutations that specifically disrupt PECAM-1–dependent signaling and/or PECAM-1 cell localization. We report that interleukin-1β (IL-1β) elicits PECAM-1–dependent transmigration that requires homophilic PECAM–PECAM-1 engagement, but not heterophilic neutrophil PECAM-1 interactions, and is intercellular adhesion molecule-1 dependent. Conversely, whereas IL-8 and leukotriene-B4–mediated transmigration is PECAM-1–independent, PECAM-1 and IL-8–dependent transmigration represent separable and additive components of cytokine-induced transmigration. Surprisingly, neither monolayer PECAM-1–regulated calcium signaling, cell border localization, nor the PECAM-1 cytoplasmic domain was required for monolayer PECAM-1 regulation of neutrophil transmigration. We conclude that monolayer (endothelial cell) PECAM-1 functions as a passive homophilic ligand for neutrophil PECAM-1, which after engagement leads to neutrophil signal transduction, integrin activation, and ultimately transmigration in a stimulus-specific manner.
Introduction
Although the mechanisms regulating initial cell contact and adhesion have been well described,1 the mechanisms underlying endothelial regulation of polymorphoneutrophil (PMN) transmigration are not as well understood. It has been proposed that neutrophil transmigration through endothelial cell (EC) monolayers involves 2 distinct pathways. The first, called type I transendothelial migration (TEM), requires direct neutrophil activation with chemotactic agents such as N-formyl-methionyl-leucinyl-phenylalanine (FMLP), leukotriene-B4 (LTB4), or interleukin-8 (IL-8). The second, type II TEM (“endothelium dependent”), involves endothelial prestimulation by inflammatory mediators, such as IL-1β, that induce endothelial expression of surface proteins and secretion of factors that ultimately activate neutrophils and promote transmigration.2 The mechanisms regulating neutrophil transmigration are thus stimulus- and vascular bed–specific and involve cell adhesion proteins such as intercellular adhesion molecule-1 (ICAM-1), the β-integrins, and others.3-6
One adhesion molecule implicated in neutrophil transmigration is platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31), a 130-kDa immunoglobulin (Ig) superfamily protein that is expressed at high levels at cell-cell borders on endothelial cells,7,8 as well as on leukocytes and platelets.9 PECAM-1 has been implicated as a key regulator of neutrophil transmigration in inflammation,10,11ischemia reperfusion,12,13 and oxidant injury.14 Interestingly, some evidence suggests that PECAM-1 might selectively participate in TEM in the context of cytokine (IL-1β)–mediated (type II) neutrophil TEM but not in chemokine-mediated (type I) TEM.15-18 However, the mechanisms by which endothelial PECAM-1 regulates TEM remain unclear.
There are a number of ways EC PECAM-1 could potentially regulate TEM. Because regulation of PECAM-1–mediated TEM requires homophilic (PECAM-1–PECAM-1) interactions between endothelial cell and neutrophil PECAM-1,15,19,20 it was initially postulated that EC PECAM-1 might function primarily as a cell adhesion molecule, creating a haptotactic gradient that helped to guide neutrophils to endothelial cell-cell borders.15 This hypothesis, which emphasizes the importance of the characteristic cell-cell localization of PECAM-1, has gained further credence by recent evidence suggesting that PECAM-1 functions as the apical component of a step-wise progression of cell adhesion proteins, such as CD99, that regulate monocyte diapedesis following the selectin- and integrin-mediated steps of tethering, rolling, and adhesion.21 However, in both ECs and leukocytes, PECAM-1 has also been found to have a substantial role in activating and modifying signal transduction pathways,9leading to the emergence of at least 4 other mechanistic possibilities.
One of the first signaling functions of PECAM-1 to be recognized was its ability to activate β2 (and β1) integrins on a variety of leukocytes following its engagement.22,23 In neutrophils, this signaling involves PECAM-1 association with phosphatidylinositol 3–(PI3) kinase24 and PECAM-1–dependent activation of the GTPase Rap1 via the PECAM-1 cytoplasmic domain.25 Engagement of PECAM-1 expressed on Jurkat cells has also been found to activate calcium signals,26 a signaling pathway critical to leukocyte TEM.27 Thus, a second mechanism by which endothelial PECAM-1 might regulate transmigration would be as a “passive” homophilic ligand for neutrophil PECAM-1, activating neutrophil signal transduction and integrins in a spatially and temporally appropriate manner.
Neutrophil transmigration has been shown to require increases in intracellular EC calcium levels independent of PMN calcium signaling.27 Interestingly, homophilic PECAM-1 engagement can activate prolonged calcium transients in human umbilical vein endothelial cells (HUVECs) and in an EC-like mesothelioma-derived cell line (REN cells) transfected with human PECAM-1 (RHP cells).28,29 Recently, EC PECAM-1 has also been described as a key regulator of endothelial oxidant-activated calcium signals, a function that may be critical to mediating EC-PMN interactions.30 Thus, PECAM-1–dependent EC calcium channel activation represents a third potential mechanism by which EC PECAM-1 could regulate TEM.
Another potentially important feature of PECAM-1 is that the cytoplasmic domain possesses a tyrosine-containing motif composed of 2 tandem SH2 binding sites (Tyr663/Tyr686) that can be phosphorylated by Src and Csk family kinases.31,32 In ECs, this domain may mediate association with the SH2-containing tyrosine phosphatase SHP2,33,34 while leukocyte and platelet PECAM-1 has been shown to associate with SHP1, PI3K, and other SH2 domain proteins.9,24,35,36 Thus, a fourth mechanism by which EC PECAM could regulate transmigration would be that PECAM-1 engagement or oxidant exposure leads to phosphorylation of the SH2 domain–binding tyrosines on EC PECAM-1 leading to association with SHP2 or other cytoplasmic proteins that in turn influence the transmigration process. Finally, reports of other cytoplasmic proteins, such as β and γ catenin, binding to the cytoplasmic domain of PECAM-137 38 suggest the fifth possibility, that these interactions underlie the regulation of TEM by EC PECAM-1.
Genetic approaches to evaluate these alternatives have proved impractical because of the high levels of constitutive PECAM-1 expression in ECs. We therefore used a previously described PECAM-1–null “endothelial model” cell line, REN, that manifests many phenotypic and signaling characteristics of ECs when stably transfected or virally transduced with human PECAM-1.28-30,39 Utilizing this EC model in a transmigration assay based on standardized in vitro models of leukocyte transmigration,5 40 we have defined the components of PECAM-1–dependent neutrophil transmigration. We also report the effects of specific PECAM-1 mutations known to disrupt monolayer PECAM-1–dependent cationic signaling and/or cell border localization on the regulation of PECAM-1–dependent neutrophil transmigration.
Materials and methods
Antibodies and reagents
Anti–PECAM-1 antibodies used include monoclonal antibody (mAb) 4G6, a murine IgG directed against extracellular loop 6,41 HEC7, a murine mAb against the extracellular loops 1 and 2 (Elias et al,42 kindly provided by Dr William Muller, Weill Medical College of Cornell University, New York, NY), mAb 62, a murine IgG directed against extracellular loop 1,19and Houston, a rabbit polyclonal IgG against the PECAM-1 extracellular domain.8 Other antibodies include murine anti–MHC-1 mAb W6-32 (American Type Culture Collection, Manassas, VA), rabbit anti-β2 microglobulin (Sigma, St Louis, MO), LR6.5 murine IgG directed against the extracellular domain of ICAM-1 (kindly provided by Dr Robert Rothlein, Boehringer Ingelheim, Ridgefield, CT), rabbit anti–IL-8 serum (kindly provided by Dr Robert Streiter, University of California, Los Angeles), nonimmune (NI) rabbit serum, and FITC-conjugated goat antimouse (ICNCappel, Irvine, CA).
Cells and PECAM-1 mutant constructs
REN cells, a human mesothelioma cell line previously isolated in our laboratory,43 were grown in RPMI media (Gibco BRL, Rockville, MD) supplemented with 10% fetal bovine serum (FBS) and 2 mM l-glutamine. The human PECAM-1constructs utilized in this study, RHP, PECAM-ΔCD, PITC(provided by Dr Peter Newman, The Blood Center of Southeastern Wisconsin, Milwaukee), and AAAA (originally referred to as ΔCD+KCYFLAAAA in Sun et al,39 Figure1), were generated through sequence overlap extension, subcloned into the pcDNA-neo vector, and introduced using lipofectin (Gibco BRL) as described.29,39Stable REN cell PECAM-1–transfected cell lines were established through magnetic bead sorting and selection in G418 (0.5 mg/mL; Gibco BRL)–supplemented media. All stably transfected cell lines, but not untransfected REN, uniformly (> 95%) expressed high levels of PECAM-1, demonstrated by fluorescence-activated cell sorting (FACS) and immunoblot, at 2- to 3-fold that of HUVECs. In adenovirus transduction experiments, REN cells were transduced 24 hours prior to experiments with vehicle, Ad.LacZ (control adenovirus encoding theLacZ gene), or Ad.PECAM-1 (adenovirus containing humanPECAM-1) as described.39 Adenovirus was obtained from the University of Pennsylvania Vector Core facility, with transduction performed at the lowest multiplicity of infection (MOI) yielding consistent protein expression. Virus transduction was confirmed by FACS analysis (PECAM-1) or LacZ (βGal) assay (Promega, Madison, WI) on concurrently transduced cell monolayers.
PECAM-1 full-length and mutant constructs.
Schematic representation and calcium signaling and SH2 protein interaction characteristics of the PECAM-1 constructs described in the text. RHP indicates full-length human PECAM-1. The extracellular domain containing 6 Ig-like loops is represented as filled ovals 1-6, the transmembrane domain as a rectangle, and the cytoplasmic domain as a gray rectangle, representing cytoplasmic exons 9-16. PITC is a chimeric construct containing an intact PECAM-1 extracellular domain fused to the nonhomologous ICAM-1 transmembrane and cytoplasmic domains. PECAM-ΔCD is a deletion construct lacking the PECAM-1 cytoplasmic domain. AAAA is a cytoplasmic domain deletion construct, encoding only 9 cytoplasmic amino acid residues, with a mutation in the membrane proximal sequence (KCYFLRKAK→KCYFLAAAA) that disrupts cell border localization. All stably transfected cell lines, but not untransfected REN, uniformly express PECAM-1 (> 95% cells) at levels similar to that of RHP.
PECAM-1 full-length and mutant constructs.
Schematic representation and calcium signaling and SH2 protein interaction characteristics of the PECAM-1 constructs described in the text. RHP indicates full-length human PECAM-1. The extracellular domain containing 6 Ig-like loops is represented as filled ovals 1-6, the transmembrane domain as a rectangle, and the cytoplasmic domain as a gray rectangle, representing cytoplasmic exons 9-16. PITC is a chimeric construct containing an intact PECAM-1 extracellular domain fused to the nonhomologous ICAM-1 transmembrane and cytoplasmic domains. PECAM-ΔCD is a deletion construct lacking the PECAM-1 cytoplasmic domain. AAAA is a cytoplasmic domain deletion construct, encoding only 9 cytoplasmic amino acid residues, with a mutation in the membrane proximal sequence (KCYFLRKAK→KCYFLAAAA) that disrupts cell border localization. All stably transfected cell lines, but not untransfected REN, uniformly express PECAM-1 (> 95% cells) at levels similar to that of RHP.
Neutrophil transmigration assay
Transmigration assays were performed as adapted from previously reported methodologies using ECs and mesothelial cells.5,40 44 REN cells were applied (250 000 cells/well) to fibronectin-treated 3-μm pore, 12-mm diameter Costar transwells (Corning, Cambridge, MA). Confluence was monitored by measurement of transmonolayer resistance utilizing an ohmmeter adapted for 12-mm costar wells (World Precision Instruments, Sarasota, FL). Monolayer resistance was calculated by subtracting monolayer transwell values from the concurrently measured resistance of transwells without cells. Confluence was indicated by development of a maximal plateau of resistance. Correlation of resistance plateaus and confluence was confirmed through immunohistochemical staining of transwell monolayers. Prior to transmigration assays, “luminal” cell monolayers (upper well) were pretreated with cytokines for 24 hours or “abluminal” chemokines and chemoattractants applied (bottom well) at the time of the assay. IL-1β (10 U/mL; Roche, Indianapolis, IN), IL-8 (5 nm; R and D Systems, Minneapolis, MN), LTB4 (100 nm; Sigma), and tumor necrosis factor α (TNFα, 100 U/mL; Roche) were prepared on the day of assay and applied to transwells as indicated.
Human neutrophils were obtained from volunteers following informed consent. PMNs were isolated by Ficoll gradient separation (Robbins Scientific, Sunnyvale, CA) followed by hypotonic red cell lysis. Cell viability was more than 95% by trypan blue dye exclusion following this methodology. Neutrophils were resuspended in RPMI media supplemented with 1% FBS (R1%), counted, and incubated at room temperature in blocking or control antibodies 20 minutes prior to onset of experiments. Monolayers (top well) were concurrently washed gently in phosphate-buffered saline (PBS) then incubated with antibodies in R1% for 20 minutes prior to assays. In some experiments bioactive anti–IL-8 serum45 or NI rabbit serum was added to top (luminal) or bottom (abluminal) wells at 1:200 prior to addition of PMN aliquots.46
Neutrophils (500 000 cells/well) were placed in the upper transwell chambers and transmigration was allowed to take place over 4 hours at 37°C. Cells were harvested from the top and bottom chambers with adherent cells gently washed and added to the respective top and bottom chamber aliquots. Media was aspirated and cells resuspended in 0.1 M K2PO4 (pH 7.0) solution.
Myeloperoxidase (MPO) activity was detected following reaction in 0.083 mg/mL O-diansidine (ICN), Hanks buffered saline (with 0.25% bovine serum albumin [BSA]), and 0.005% hydrogen peroxide. Reactions were terminated after 10 minutes with 0.1% sodium azide and MPO activity measured as optical density at 460 nm.47 A minimum of three 500 000 cell aliquots were measured to yield the maximal (total PMNs) reference standard for each experiment. MPO values were compared with a standard curve performed with each assay ranging from 37 500 to 1.5 million cells. Cell counts and assay conditions were optimized to facilitate data acquisition from the linear portion of the standard curve. Similar to results obtained in monocyte transmigration,48 time-course experiments in IL-1β–stimulated REN and RHP monolayers revealed substantial neutrophil transmigration within 1 hour that reached a maximal plateau by 4 hours (data not shown). As previously described,5this “maximal” plateau time was chosen to ensure that all but the lowest MPO values would fall within the linear portion of the MPO standard curve. Results, whether normalized to negative antibody controls or expressed as the proportion of total PMNs (500 000), represent averaged values from at least 2 separate experiments and at least 3 replicates in each experiment. Data were processed with 2-way Anova with correction for multiple comparisons utilizing Statgraphic Plus software (Manugistics, Rockville, MD).
Adhesion assay
Cells were seeded on 12-well tissue-culture plates (Costar). Confluent monolayers were treated with either IL-1β (10 U/mL) or vehicle for 24 hours; the media were then removed and cells were washed with PBS. Cell monolayers were incubated with control or blocking antibodies in R1% media for 30 minutes and identically treated PMN aliquots were then added. After 30 minutes, nonadhering cells were aspirated and monolayers gently washed with media. Wells were aspirated dry, cells resuspended in 100 μL K2PO4, and MPO assays conducted as described in the previous paragraph. PMN retention in transwell filters was measured by cutting out filters following transmigration (TM) experiments. Filters were then agitated in 100 μL K2PO4 and MPO assays on resultant supernatants conducted as previously described.
Immunofluorescence staining
Cell monolayers were grown to confluence on transwell filters as described in “Neutrophil transmigration assay.” Filters were washed in PBS, fixed with 3% paraformaldehyde for 20 minutes, and then permeabilized with iced 0.5% nonidet P-40 (NP-40) for 1 minute. After washing, fixed monolayers were stained with anti–PECAM-1 mAb 4G6 and counterstained with FITC-conjugated goat anti–mouse IgG as described.39Cells were imaged on a Nikon inverted epifluorescence microscope with a × 40 fluorescence lens and a Nikon digital camera (both from Nikon, Tokyo, Japan).
Results
Phenotypic and functional characteristics of REN and PECAM-1–transfected REN cells
In order to use molecular approaches to directly investigate the mechanisms of PECAM-1–regulated neutrophil transmigration, we utilized a standard transwell chamber transmigration system using the endothelial-like REN cell line. REN cells do not express PECAM-1, but can be stably transfected with high levels of wild-type PECAM-1 that then localize to cell-cell borders, regulates calcium-signaling activity, and manifests tyrosine phosphorylation patterns similar to PECAM-1 in ECs.28-30,39 Like ECs, mesothelial-derived cells, such as REN, also express ICAM-1 and vascular cell adhesion molecule (VCAM), form cobblestone monolayers phenotypically similar to ECs, and can support cytokine-mediated neutrophil transmigration.28,44 49
An important feature of EC monolayers is the differential expression of adhesion molecules such as ICAM-1 and PECAM-1 in response to specific cytokines and chemokines.21 Similar to ECs,50REN and RHP cells manifested marked increases in ICAM-1 expression following exposure to TNFα or IL-1β, but not IL-8 (Table 1). REN cells transfected with wild-type or mutant PECAM (not shown) had no change in PECAM expression following exposure to IL-1β or TNFα (Table 1), again similar to ECs.21
ICAM-1 and PECAM-1 expression in cytokine and chemokine-treated REN and RHP cells
| Cell line (protein) . | No Rx . | TNFα, 24 h . | IL-8, 4 h . | IL-1β, 24 h . |
|---|---|---|---|---|
| REN (ICAM-1) | 1.0 (49.2) | 2.73 (134.7) | 1.0 (49.5) | 2.22 (109.3) |
| RHP (ICAM-1) | 1.0 (68.1) | 2.12 (144.4) | 0.95 (64.9) | 2.45 (168.8) |
| (PECAM-1) | 1.0 (42.3) | 0.91 (38.4) | — | 1.01 (42.7) |
| Cell line (protein) . | No Rx . | TNFα, 24 h . | IL-8, 4 h . | IL-1β, 24 h . |
|---|---|---|---|---|
| REN (ICAM-1) | 1.0 (49.2) | 2.73 (134.7) | 1.0 (49.5) | 2.22 (109.3) |
| RHP (ICAM-1) | 1.0 (68.1) | 2.12 (144.4) | 0.95 (64.9) | 2.45 (168.8) |
| (PECAM-1) | 1.0 (42.3) | 0.91 (38.4) | — | 1.01 (42.7) |
ICAM-1 and PECAM-1 surface expression in cell monolayers treated for 24 hours with IL-1β or TNFα (cytokines) or for 4 hours with IL-8 (chemokine); conditions were identical to transmigration experiments. Representative experiment evaluating protein expression in cells analyzed by FACS. Mean fluorescence intensity values are normalized to baseline ICAM-1 or PECAM-1 expression levels for each cell line (mean fluorescence intensity). Similar results were obtained in at least 3 replicate experiments for each condition. No Rx indicates no treatment; —, not assessed.
Accordingly, REN and RHP cells were plated on transwell filters and confluence was monitored by electrical resistance. Monolayer resistance reached a plateau by days 4 to 6 of approximately 30 to 35 Ωcm2 above transwell resistance, similar to values reported (12 ±13Ωcm2) for endothelial cell monolayers.27 In these confluent RHP monolayers grown on transwell filters (Figure 2A), PECAM-1 demonstrated clear cell border localization, identical to that seen in endothelial cells and RHP cells grown on glass slides.28,39 Importantly, exposure of RHP cells to IL-1β did not alter the cell-cell distribution of PECAM-1, nor was electrical resistance in RHP or REN monolayers affected by cytokine treatment (data not shown), consistent with prior reports in EC monolayers.21,27 51
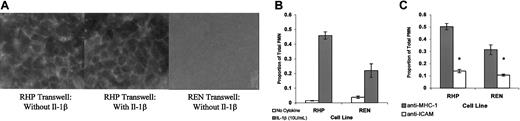
REN and PECAM-1–transfected REN cells are phenotypically similar to ECs and support cytokine-stimulated, ICAM-1–dependent PMN transmigration.
(A) Confluent REN and RHP monolayers plated on transwells stained with anti–PECAM-1 antibody (mAb4G6). RHP monolayers were treated for 24 hours with IL-1β or vehicle exactly as performed in TEM experiments. (B) PMN transmigration through confluent REN and RHP monolayers treated for 24 hours with vehicle or 10 U/mL IL-1β (luminal). (C) PMN transmigration though IL-1β–stimulated REN and RHP monolayers. Monolayers and PMNs were exposed to either isotype-matched anti–MHC-1 or anti–ICAM-1 mAbs (100 μg/mL) 20 minutes prior to and during transmigration. Identical results were obtained using BSA (100 μg/mL) for nonblocking conditions (not shown). Transmigration rates are expressed as the proportion of PMNs migrating through the transwell filter compared with the total number of PMNs added (500 000). In these representative experiments, data represent the mean ± SEM from a minimum of 3 transwells for each condition. (*Significantly different from “unblocked” controls, P < .05.)
REN and PECAM-1–transfected REN cells are phenotypically similar to ECs and support cytokine-stimulated, ICAM-1–dependent PMN transmigration.
(A) Confluent REN and RHP monolayers plated on transwells stained with anti–PECAM-1 antibody (mAb4G6). RHP monolayers were treated for 24 hours with IL-1β or vehicle exactly as performed in TEM experiments. (B) PMN transmigration through confluent REN and RHP monolayers treated for 24 hours with vehicle or 10 U/mL IL-1β (luminal). (C) PMN transmigration though IL-1β–stimulated REN and RHP monolayers. Monolayers and PMNs were exposed to either isotype-matched anti–MHC-1 or anti–ICAM-1 mAbs (100 μg/mL) 20 minutes prior to and during transmigration. Identical results were obtained using BSA (100 μg/mL) for nonblocking conditions (not shown). Transmigration rates are expressed as the proportion of PMNs migrating through the transwell filter compared with the total number of PMNs added (500 000). In these representative experiments, data represent the mean ± SEM from a minimum of 3 transwells for each condition. (*Significantly different from “unblocked” controls, P < .05.)
Similar to ECs, we found that basal (unstimulated) PMN transmigration on REN and RHP monolayers was minimal, but could be markedly enhanced either by creation of a chemotactic gradient (type I transmigration) or by pre-exposure of the EC monolayer to cytokines, such as IL-1β (type II transmigration).2 As shown in Figure 2B, in 4-hour neutrophil (PMN) transmigration experiments utilizing 500 000 PMNs/well, fewer than 5% of total PMNs transmigrated through unstimulated REN and RHP monolayers. However, following pretreatment with IL-1β, REN and RHP monolayers supported a dramatic increase in PMN transmigration (20%-40% of PMNs transmigrating). Transmigration through IL-1β–stimulated RHP monolayers was about double that seen in the IL-1β–stimulated REN monolayers. In addition, when the chemoattractants IL-8 or LTB4 were placed in the lower chamber of the transwell, a marked increase in transmigration also occurred, though at identical levels in both cell types (Figure4).
As seen in Figure 2C, we found that proportionally, more than two thirds of IL-1β–stimulated transmigration in both REN and RHP monolayers was blocked by anti–ICAM-1 antibodies compared with wells treated with the isotype-matched anti–MHC-1–negative control mAb or BSA (not shown). These findings suggest that in both cell lines, similar to most EC beds, the majority of transmigration is ICAM-1 dependent.4 52
Thus, with regard to transmembrane electrical resistance, morphology, and cell and adhesion expression profiles, REN and RHP cells closely resemble EC monolayers. Interestingly, the presence of PECAM-1 appears to contribute an ICAM-1–dependent component to IL-1β–stimulated transmigration, whereas the (small) residual ICAM-1–independent portion of transmigration observed in both REN and RHP monolayers does not appear to be significantly impacted by the presence (or absence) of PECAM-1.
Role of PECAM-1 in IL-1β–mediated type II neutrophil transmigration
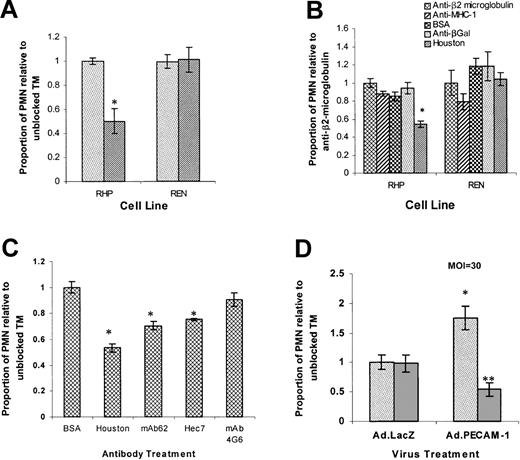
In order to confirm the role of PECAM-1 in neutrophil diapedesis, we first compared the transmigration rate of human neutrophils across IL-1β pretreated REN and RHP monolayers in the setting of a polyclonal anti–PECAM-1–blocking antibody (Houston) or a negative control rabbit antibody directed against the highly expressed cell surface protein, β2 microglobulin. In 4-hour TM experiments, anti-PECAM-1 antibody (Houston) inhibited PMN transmigration by 50% (P < .05%) through RHP monolayers, compared with the proportion of PMNs transmigrating while exposed to anti-β2 microglubulin. Conversely, in REN cells, treatment of monolayers and PMNs with either Houston or anti-β2 microglubulin control yielded no difference in transmigration (Figure 3A). These findings indicate that IL-1β–stimulated transmigration consists of a PECAM-1–dependent (antibody-blockable) and a PECAM-1–independent component (supported by REN or antibody-blocked RHP monolayers) and that heterophilic interactions between neutrophil PECAM-1 and non–PECAM-1 monolayer ligands do not play a role in IL-1β–stimulated PMN transmigration.
IL-1β–stimulated PMN transmigration is composed of PECAM-1–dependent and –independent components.
Cell monolayers were treated for 24 hours with 10 U/mL IL-1β (luminal). Transmigration rates are expressed as the proportion of transmigrating PMNs compared with “unblocked” controls (set at 1.0). Data represent the mean ± SEM from a minimum of 3 transwells for each condition from at least 3 separate experiments unless otherwise indicated. (*Significantly different from “unblocked” controls,P < .05.) (A) Transmigration following exposure of neutrophils and REN or RHP monolayers to anti–PECAM-1 (Houston, ▨) or anti-β2 microglobulin (░) antibodies. (B) Transmigration following exposure of PMN and REN or RHP monolayers to anti-β2 microglobulin (reference set at 1.0), anti–MHC-1, anti-βGal antibodies, BSA, or anti–PECAM-1 (Houston) antibodies (all 100 μg/mL). (C) RHP cells. Luminal BSA (“unblocked” reference control) or anti–PECAM-1 antibodies Houston, mAb62, Hec7, or 4G6 (100 μg/mL) were added as indicated. Data represent the mean ± SEM from a minimum of 3 transwells for each condition from at least 2 separate experiments. (D) Confluent REN monolayers transduced with Ad.PECAM-1 or Ad.LacZ (PECAM null) and subsequently treated for 24 hours with 10 U/mL IL-1β (luminal). PMNs and tranduced REN monolayers were exposed to anti–PECAM-1 (Houston, ▨) or anti–MHC-1 (░) antibodies as indicated. Values are normalized to LacZ transfected (PECAM-1 null), “unblocked” (anti–MHC-1 antibody-treated), negative control. (*Significantly different from Ad.LacZ negative controls,P < .05; **significantly different from “unblocked” Ad.PECAM-1, P < .05.)
IL-1β–stimulated PMN transmigration is composed of PECAM-1–dependent and –independent components.
Cell monolayers were treated for 24 hours with 10 U/mL IL-1β (luminal). Transmigration rates are expressed as the proportion of transmigrating PMNs compared with “unblocked” controls (set at 1.0). Data represent the mean ± SEM from a minimum of 3 transwells for each condition from at least 3 separate experiments unless otherwise indicated. (*Significantly different from “unblocked” controls,P < .05.) (A) Transmigration following exposure of neutrophils and REN or RHP monolayers to anti–PECAM-1 (Houston, ▨) or anti-β2 microglobulin (░) antibodies. (B) Transmigration following exposure of PMN and REN or RHP monolayers to anti-β2 microglobulin (reference set at 1.0), anti–MHC-1, anti-βGal antibodies, BSA, or anti–PECAM-1 (Houston) antibodies (all 100 μg/mL). (C) RHP cells. Luminal BSA (“unblocked” reference control) or anti–PECAM-1 antibodies Houston, mAb62, Hec7, or 4G6 (100 μg/mL) were added as indicated. Data represent the mean ± SEM from a minimum of 3 transwells for each condition from at least 2 separate experiments. (D) Confluent REN monolayers transduced with Ad.PECAM-1 or Ad.LacZ (PECAM null) and subsequently treated for 24 hours with 10 U/mL IL-1β (luminal). PMNs and tranduced REN monolayers were exposed to anti–PECAM-1 (Houston, ▨) or anti–MHC-1 (░) antibodies as indicated. Values are normalized to LacZ transfected (PECAM-1 null), “unblocked” (anti–MHC-1 antibody-treated), negative control. (*Significantly different from Ad.LacZ negative controls,P < .05; **significantly different from “unblocked” Ad.PECAM-1, P < .05.)
In order to utilize previously characterized reagents28,29to further investigate the relationship of EC calcium signaling to transmigration, as well as to facilitate experiments utilizing different antibody isotypes, TM experiments were conducted to directly compare a panel of nonblocking controls with our anti-β2 microglobulin–negative control standard. In IL-1β–stimulated RHP cells, there was no significant difference in TM among control conditions (including BSA alone, an irrelevant rabbit polyclonal anti-βGal antibody, an anti–MHC-1 monoclonal antibody, and the polyclonal anti-β2 microglubulin antibody), while anti–PECAM-1 blocking antibody (Houston) manifested significant blockade compared with all controls (38%-50%). In IL-1β–stimulated REN cells, there was no significant difference in transmigration among any conditions (Figure 3B), confirming our prior findings (Figure 3A). Thus, although we used intact antibodies, there was no evidence of Fc receptor activation of neutrophils by the rabbit polyclonal or mouse monoclonal antibodies. Similarly, we found no difference in MPO content between neutrophils exposed to media, BSA, or any control or anti–PECAM-1 antibodies (data not shown). Having previously demonstrated that neither anti–MHC-1 mAb nor BSA elicits calcium signaling activity in ECs or RHP28 29 and given their equivalence to polyclonal anti-β2 microglobulin as negative controls, we utilized these well-characterized control reagents in our subsequent transmigration experiments.
In order to choose the optimal anti–PECAM-1–blocking reagent, antibody blockade studies were performed comparing the polyclonal antibody (Houston) with 3 monoclonal anti–PECAM-1 antibodies: mAb 62 (directed against Ig-like loop 1), mAb Hec 7 (directed against Ig-like loops 1 and 2), and mAb 4G6 (directed against Ig-like loop 6). Anti–PECAM-1 antibody blockade was dose dependent (with maximal effect observed at 75-100 μg/mL; data not shown) and somewhat epitope specific. As shown in Figure 3C, the polyclonal antibody was most effective, although 2 of the monoclonal antibodies (mAb 62 and Hec7) induced significant (P < .05) blockade of transmigration, confirming prior findings that PECAM-1 homophilic interaction domains are important in mediating PECAM-1–regulated TEM.19Interestingly, mAb 4G6, an antibody known to activate calcium signaling,28 29 did not significantly block TEM. None of these anti–PECAM-1 antibodies blocked PMN transmigration in IL-1β–treated REN cells compared with BSA, anti–MHC-1, or anti-βGal antibody controls (not shown).
In order to assess the contribution of PECAM-1 expression to IL-1β–stimulated transmigration and to rule out artifacts resulting from phenotypic variations between permanently transfected cell lines, we performed TM experiments on IL-1β–stimulated REN monolayers transduced with adenovirus containing human PECAM-1 (Ad.PECAM-1) or a control adenovirus encoding the LacZ gene (Ad.LacZ). This approach resulted in PECAM-1 expression comparable with that of RHP cells in more than 90% to 95% of cells (not shown). Figure 3D shows that in unblocked Ad.PECAM-1–transduced REN cells, we observed an average 75% increase in PMN transmigration compared with unblocked Ad.LacZ (PECAM-1 null) control monolayers (P < .05). This increase was completely blocked in Ad.PECAM-1–transduced REN monolayers treated with anti–PECAM-1 antibodies, whereas anti–PECAM-1 antibody treatment of Ad.LacZ-transduced monolayers did not diminish transmigration. These data confirm the following: (1) PECAM-1–dependent transmigration requires monolayer expression of PECAM-1; (2) neutrophil PECAM-1 heterophilic interactions are not required; and (3) IL-1β–stimulated transmigration consists of both a PECAM-1–dependent component (antibody-blockable) and a PECAM-1–independent component (which is supported by Ad.LacZ-transduced REN or antibody-blocked Ad.PECAM-1–transduced REN monolayers).
Finally, in order to confirm that results attributed to differences in transmigration rates were not simply due to variations in cell adhesion, we performed adhesion assays on cytokine-treated REN cell and REN cell transfectant monolayers. As with ECs, where cytokine-induced TEM is separable from adhesion,53 IL-1β increased PMN binding to REN and RHP monolayers similarly when compared with untreated monolayers. However, this effect was not blocked by addition of anti–PECAM-1 or anti–MHC-1 antibodies (not shown). Additionally, at the end of the 4-hour TEM assays, fewer than 7% of total PMNs were “trapped” in the transwell inserts (or firmly adherent to the luminal or abluminal side) with no significant difference in cell retention found between REN and RHP cells or between antibody conditions (not shown). These results indicate that the differences in PMN transmigration we have interpreted as the “PECAM-1–dependent” component of TEM are not simply an artifact of neutrophil sequestration.
Role of PECAM-1 in IL-8 and LTB4-mediated type I neutrophil transmigration
Our findings clearly identified a PECAM-1–dependent component to IL-1β–mediated type II (cytokine-induced) neutrophil transmigration. To determine if PECAM-1 played a role in type I TEM mediated by CXC chemokines (IL-8) and leukotriene chemoattractants (LTB4), TM assays were conducted on REN and RHP monolayers. IL-8 (5 nm) or LTB4 (100 nm) was added to the abluminal chamber at the time of PMN addition (luminal) and results compared with TM through RHP cell monolayers pretreated for 24 hours with IL-1β. As shown in Figure 4, PMN TM was markedly increased after chemokine addition. Both REN and RHP supported identical transmigration rates of up to 45% to 50% of total PMNs, levels similar to unblocked TM through IL-1β–stimulated RHP monolayers. However, unlike the effect on IL-1β–induced TM through RHP monolayers, addition of anti–PECAM-1 antibody yielded no inhibition of chemokine-induced transmigration through RHP or REN cells. Thus, in contrast to IL-1β–mediated transmigration, chemokine (IL-8) and leukotriene (LTB4)–induced (type I) transmigration is unaffected by the presence of PECAM-1.
PECAM-1–dependent transmigration is chemokine/cytokine specific.
Confluent RHP and REN monolayers treated with 5 nm IL-8 (abluminal) or 100 nm LTB4 (abluminal) at the start of the TM assay or with 10 U/mL IL-1β (luminal) for 24 hours as indicated. Monolayers and neutrophils were treated with luminal anti–PECAM-1 (Houston) or anti–MHC-1 antibodies (100 μg/mL) as indicated. Similar results were obtained using BSA instead of anti–MHC-1 antibody (not shown). Transmigration rates are expressed as the proportion of PMNs migrating through the transwell filter compared with the total number of PMNs added at the start of the experiment. Data represent the mean ± SEM from a minimum of 3 transwells for each condition from 2 separate experiments. (*Significantly different from all other columns,P < .05.)
PECAM-1–dependent transmigration is chemokine/cytokine specific.
Confluent RHP and REN monolayers treated with 5 nm IL-8 (abluminal) or 100 nm LTB4 (abluminal) at the start of the TM assay or with 10 U/mL IL-1β (luminal) for 24 hours as indicated. Monolayers and neutrophils were treated with luminal anti–PECAM-1 (Houston) or anti–MHC-1 antibodies (100 μg/mL) as indicated. Similar results were obtained using BSA instead of anti–MHC-1 antibody (not shown). Transmigration rates are expressed as the proportion of PMNs migrating through the transwell filter compared with the total number of PMNs added at the start of the experiment. Data represent the mean ± SEM from a minimum of 3 transwells for each condition from 2 separate experiments. (*Significantly different from all other columns,P < .05.)
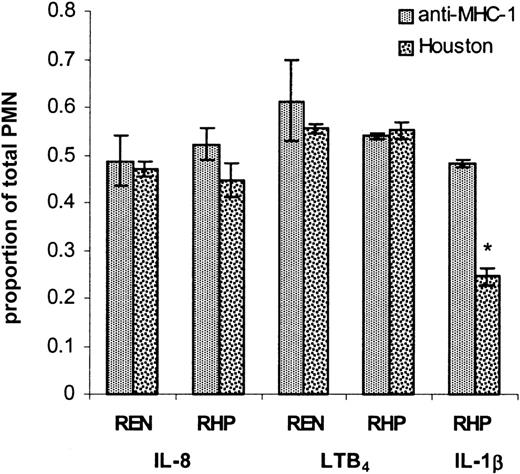
Relationship of IL-8 (type I) transmigration and PECAM-1 in IL-1β–mediated (type II) transmigration
It is well known that IL-8 levels produced by IL-1β–stimulated ECs and mesothelial cells may exceed 1 to 2 nm and underlie at least part of cytokine-induced transmigration.45,46,54 In order to determine the relationship between PECAM-1 and luminal (surface-expressed) or secreted (abluminal) IL-8 in the context of IL-1β–stimulated transmigration, we examined the effects of a bioactive anti–IL-8 serum45 or negative control NI rabbit serum added to either the luminal (top) or abluminal (bottom) chamber in conjunction with (luminal) anti–PECAM-1 (Houston) or anti–MHC-1 antibodies. As shown in Figure 5A, the functional bioactivity of the anti–IL-8 serum was confirmed as abluminal addition of anti–IL-8 serum completely blocked IL-8–stimulated transmigration. As shown in Figure 5B, in IL-1β–treated RHP monolayers, baseline transmigration (50% of total neutrophils added, column 1) was established in wells with control antibodies on the luminal (anti–MHC-1) and abluminal surfaces (NI serum). Similarly, wells treated with luminal anti–MHC-1 antibody manifested no inhibition in PMN transmigration when coincubated with luminal anti–IL-8 serum (67% total PMNs added, column 6) or luminal NI serum (54% total PMNs, column 5). The lack of inhibition in transmigration between columns 5 and 6 suggests that luminal surface expression of IL-8 is not required for IL-1β–mediated transmigration as has been suggested in TNFα-stimulated transmigration.50 Fluorescence cytometry analysis of IL-1β–treated RHP cells similarly revealed no surface IL-8 expression compared with untreated RHP cells (not shown).
IL-1β–stimulated TEM consists of PECAM-1 and IL-8–dependent components that are separable and additive.
(A) Confluent RHP monolayers were treated with 5 nm IL-8 (abluminal) and either 1:200 negative control rabbit serum (NC) or anti–IL-8 serum was added to the abluminal (bottom) chamber at the start of the TM assay. PMNs were allowed to transmigrate for 4 hours. In this representative experiment, data represent the mean ± SEM from a minimum of 3 transwells for each condition. (B) Confluent RHP monolayers were treated for 24 hours with 10 U/mL IL-1β (luminal), washed, and 500 000 PMNs/well were applied to the luminal chamber. Anti–IL-8 serum (1:200) or negative control NI rabbit serum was added to either the luminal (top) or abluminal (bottom) chamber in conjunction with (luminal) anti–PECAM-1 (Houston) or anti–MHC-1 antibodies (100 μg/mL) as indicated (PMN aliquots were treated identically to luminal conditions 20 minutes prior to addition to wells). PMNs were allowed to transmigrate for 4 hours. Transmigration rates are expressed as the proportion of PMNs migrating through the transwell filter compared with the total number of PMNs added at the start of the experiment. Column 1, “unblocked” TM (anti–MHC-1) in the setting of abluminal NI serum; Column 2, “unblocked” TM (anti–MHC-1) in the setting of abluminal anti–IL-8 serum; Column 3, “blocked” TM (Houston) in the setting of abluminal NI serum; Column 4, “blocked” TM (Houston) in the setting of abluminal anti–IL-8 serum; Column 5, “unblocked” TM (anti–MHC-1) in the setting of luminal NI serum; Column 6, “unblocked” TM (anti–MHC-1) in the setting of luminal anti–IL-8 serum. Data represent the mean ± SEM from a minimum of 3 transwells for each condition from 3 separate experiments. (*Columns 2, 3, and 4 are significantly different [P < .05] from column 1. **Column 4 is significantly different from columns 2 and 3 [P < .05].)
IL-1β–stimulated TEM consists of PECAM-1 and IL-8–dependent components that are separable and additive.
(A) Confluent RHP monolayers were treated with 5 nm IL-8 (abluminal) and either 1:200 negative control rabbit serum (NC) or anti–IL-8 serum was added to the abluminal (bottom) chamber at the start of the TM assay. PMNs were allowed to transmigrate for 4 hours. In this representative experiment, data represent the mean ± SEM from a minimum of 3 transwells for each condition. (B) Confluent RHP monolayers were treated for 24 hours with 10 U/mL IL-1β (luminal), washed, and 500 000 PMNs/well were applied to the luminal chamber. Anti–IL-8 serum (1:200) or negative control NI rabbit serum was added to either the luminal (top) or abluminal (bottom) chamber in conjunction with (luminal) anti–PECAM-1 (Houston) or anti–MHC-1 antibodies (100 μg/mL) as indicated (PMN aliquots were treated identically to luminal conditions 20 minutes prior to addition to wells). PMNs were allowed to transmigrate for 4 hours. Transmigration rates are expressed as the proportion of PMNs migrating through the transwell filter compared with the total number of PMNs added at the start of the experiment. Column 1, “unblocked” TM (anti–MHC-1) in the setting of abluminal NI serum; Column 2, “unblocked” TM (anti–MHC-1) in the setting of abluminal anti–IL-8 serum; Column 3, “blocked” TM (Houston) in the setting of abluminal NI serum; Column 4, “blocked” TM (Houston) in the setting of abluminal anti–IL-8 serum; Column 5, “unblocked” TM (anti–MHC-1) in the setting of luminal NI serum; Column 6, “unblocked” TM (anti–MHC-1) in the setting of luminal anti–IL-8 serum. Data represent the mean ± SEM from a minimum of 3 transwells for each condition from 3 separate experiments. (*Columns 2, 3, and 4 are significantly different [P < .05] from column 1. **Column 4 is significantly different from columns 2 and 3 [P < .05].)
In contrast, in IL-1β–stimulated RHP monolayers incubated with anti–MHC-1 antibody and abluminal anti–IL-8 serum (column 2), PMN transmigration was inhibited by 40% compared with abluminal NI control (column 1). These findings are consistent with previous reports that IL-1β treatment of EC monolayers leads to secretion of IL-8 that may play a role as a chemoattractant in PMN transmigration. Utilizing enzyme-linked immunosorbent assay (ELISA) techniques, we confirmed that IL-1β–pretreated REN and RHP monolayers secrete IL-8, whereas untreated monolayers do not (data not shown).
However, in anti–PECAM-1–treated (Houston) monolayers to which NI rabbit serum was added to the abluminal side (column 3), up to a 59% decrease in PMN migration was noted (compared with anti–MHC-1 control in column 1), representing the PECAM-1 blockable component of IL-1β–mediated PMN transmigration. In anti–PECAM-1–treated (Houston) monolayers to which abluminal anti–IL-8 serum was also added (column 4), an 86% decrease in PMN migration was observed (compared with column 1). These findings suggest that IL-8 (type I) and PECAM-1–regulated transmigration are independent and additive components of IL-1β–mediated (type II) PMN transmigration.
Use of PECAM-1 mutant constructs to determine the mechanisms by which PECAM-1 regulates PMN transmigration
Having established an endothelial-like model system in which we could demonstrate a clear PECAM-1–dependent component of IL-1β–mediated transmigration, we used this system to address the question of how monolayer or “endothelial” PECAM-1 regulates PMN transmigration.
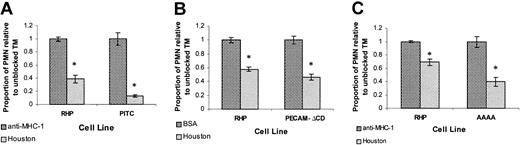
To evaluate the role of PECAM-1–mediated EC signaling, we studied REN cells stably transfected with a series of PECAM-1 mutants. The PITC construct contains the extracellular domain of PECAM-1 fused to the transmembrane and cytoplasmic domains of ICAM-1 (Figure 1). The ICAM-1 cytoplasmic region on this construct lacks the cytoplasmic Tyr663/Tyr686 motif required for tyrosine phosphorylation and disrupts PECAM-1–mediated cationic signaling, but supports homophilic interaction and maintains cell border localization (not shown).29,30 39 We hypothesized that if monolayer PECAM-1–mediated cell signaling (due to either calcium flux or cytoplasmic domain protein-protein interactions) were important in transmigration, the PECAM-1–dependent component of transmigration would be lost. As seen in Figure6A, when transmigration assays on IL-1β–stimulated PITC and RHP monolayers were conducted, treatment with anti–PECAM-1 antibodies (Houston) yielded a more than 80% decrease in transmigration compared with anti–MHC-1 negative control. Similar findings were observed with BSA negative control (not shown).
PECAM-1–dependent transmigration does not require the PECAM-1 cytoplasmic domain or cell border localization.
(A) IL-1β–treated RHP and PITC monolayers exposed to anti–PECAM-1 (Houston) or anti–MHC-1 antibodies. (B) IL-1β–treated RHP and PECAM-ΔCD monolayers exposed to anti–PECAM-1 (Houston) antibodies or BSA (100 μg/mL). Similar results were obtained with anti–MHC-1 antibodies instead of BSA (not shown). (C) IL-1β–treated RHP and AAAA monolayers exposed to anti–PECAM-1 (Houston) antibodies or anti–MHC-1 antibodies. Similar results were obtained with BSA instead of anti–MHC-1 antibodies (not shown). Transmigration rates are expressed as the normalized proportion of migrating PMNs compared with “unblocked” TM (set at 1.0) for each cell line. Data represent the mean ± SEM from a minimum of 3 transwells for each condition from 3 separate experiments. (*Significantly different from “unblocked” negative control, P < .05.)
PECAM-1–dependent transmigration does not require the PECAM-1 cytoplasmic domain or cell border localization.
(A) IL-1β–treated RHP and PITC monolayers exposed to anti–PECAM-1 (Houston) or anti–MHC-1 antibodies. (B) IL-1β–treated RHP and PECAM-ΔCD monolayers exposed to anti–PECAM-1 (Houston) antibodies or BSA (100 μg/mL). Similar results were obtained with anti–MHC-1 antibodies instead of BSA (not shown). (C) IL-1β–treated RHP and AAAA monolayers exposed to anti–PECAM-1 (Houston) antibodies or anti–MHC-1 antibodies. Similar results were obtained with BSA instead of anti–MHC-1 antibodies (not shown). Transmigration rates are expressed as the normalized proportion of migrating PMNs compared with “unblocked” TM (set at 1.0) for each cell line. Data represent the mean ± SEM from a minimum of 3 transwells for each condition from 3 separate experiments. (*Significantly different from “unblocked” negative control, P < .05.)
The preservation of a clear PECAM-1–dependent (antibody blockable) component in the absence of the PECAM-1 cytoplasmic and transmembrane domains (and their potential signaling and protein association functions) suggested that EC PECAM-1 serves primarily as an adhesion protein, either forming a haptotactic gradient (which would require PECAM-1 cell border localization) or functioning as a “passive” ligand for PMN PECAM-1.
To directly address the role of PECAM-1 cell border localization and determine whether a haptotactic gradient is required for PECAM-1–dependent transmigration, we evaluated TM using 2 additional REN cell lines stably transfected with mutant PECAM-1 constructs that disrupt PECAM-1 cell border localization. The first, termed PECAM-ΔCD, lacks the entire cytoplasmic domain and is known to disrupt cell border localization, as well as PECAM-1–dependent calcium and tyrosine signaling events,30,39 but can serve as a ligand for homophilic PECAM-1 binding (not shown). However, because ICAM-1 expression in PECAM-ΔCD was, for some reason, significantly lower than in RHP cells (Table 2), we also utilized a second cytoplasmic deletion construct, termed AAAA, encoding only 9 cytoplasmic amino acid residues with a mutation in the membrane proximal charged stop-transfer sequence (KCYFLRKAK→KCYFLAAAA) that is also known to disrupt cell border localization.39 Notably, ICAM-1 expression before and after IL-1β treatment in REN-AAAA cells was similar to REN, RHP, and PITC cells (Tables 1-2). In TM experiments comparing PECAM-ΔCD and AAAA to RHP cell monolayers, there was an approximately 50% to 60% inhibition of PMN transmigration with anti–PECAM-1 antibodies in both cell lines (Figure 6B-C) compared with nonblocking control, similar to that seen in RHP controls. This indicates that the PECAM-1–dependent component of IL-1β–mediated TEM does not require PECAM-1 cell border localization or signaling and is independent of absolute ICAM-1 expression levels.
ICAM-1 expression in cytokine-treated full-length and mutant PECAM-1–transfected REN cells
| Cell line . | RHP . | PITC . | PECAM-ΔCD . | AAAA . |
|---|---|---|---|---|
| Vehicle | 17.9 (3.34) | 24.0 (5.36) | 2.19 (0.17)* | 23.8 (3.78) |
| IL-1β | 39.7 (11.6) | 35.5 (7.50) | 3.67 (0.93)* | 37.6 (9.10) |
| Fold increase | 2.15 (0.43) | 1.49 (0.17) | 1.66 (0.38) | 1.56 (0.28) |
| Cell line . | RHP . | PITC . | PECAM-ΔCD . | AAAA . |
|---|---|---|---|---|
| Vehicle | 17.9 (3.34) | 24.0 (5.36) | 2.19 (0.17)* | 23.8 (3.78) |
| IL-1β | 39.7 (11.6) | 35.5 (7.50) | 3.67 (0.93)* | 37.6 (9.10) |
| Fold increase | 2.15 (0.43) | 1.49 (0.17) | 1.66 (0.38) | 1.56 (0.28) |
ICAM-1 surface expression in confluent PECAM-1 transfectant cell monolayers treated for 24 hours with IL-1β or vehicle. FACS analysis was conducted on nontrypsinized cells with primary anti–ICAM-1 mAb LR6.5. Mean fluorescence intensity values (± SEM) were obtained from 3 separate experiments. Fold increase (± SEM) is the mean fold increase above untreated ICAM-1 expression (n = 3). There was no significant difference between fold increase values.
PECAM-ΔCD ICAM-1 expression is significantly lower than other cell lines (P < .05).
Discussion
Neutrophil transmigration through cytokine-stimulated endothelial monolayers is a complex process that requires cell adhesion and “adhesion-dependent” intracellular signaling followed by “adhesion-independent” calcium transients and other signal processes in both ECs and PMNs.27,55 The proteins regulating leukocyte TEM include an array of EC and PMN cell adhesion proteins including PECAM-1, ICAM-1 and -2, CD99, junctional adhesion molecule (JAM), integrin-associated protein (IAP), the CD11/18 integrins,4,10,21,51,56 57 and others. It is still not clear, however, how each of these proteins regulates diapedesis, particularly in the context of different transmigration stimuli. The focus of these experiments was to study the role played by “endothelial” PECAM-1 (in contrast to leukocyte PECAM-1) in neutrophil transmigration in response to specific stimuli and to define the role of monolayer PECAM-1 signaling versus ligand functions in this process.
In our model of neutrophil transendothelial migration, REN cells expressing full-length PECAM-1 were phenotypically and functionally similar to endothelial cells, supporting both type II (cytokine [IL-1β]–mediated) and type I (chemokine [IL-8]– and chemoattractant [LTB4]–mediated) transmigration. In cell monolayers treated with IL-1β, PMN transmigration on PECAM-1–expressing REN cells was enhanced compared with untransfected REN cells and was partially blocked by anti–PECAM-1 antibodies against PECAM-1 extracellular loops 1 or 2, while no anti–PECAM-1 antibodies disrupted PMN TM through PECAM-1–“null” REN. These data demonstrate the presence of a PECAM-1–dependent component to cytokine-induced transmigration that requires homophilic interaction between monolayer and PMN PECAM-1 and is independent of heterophilic interactions between neutrophil PECAM-1 and EC non–PECAM-1 ligands.
Consistent with reports that direct-PMN activators mediating type I TEM (such as FMLP and IL-8) may not require PECAM-1,16-18,57 we found that IL-8 and LTB4 elicited equivalent TM rates through REN and RHP monolayers that were unaffected by anti–PECAM-1 antibodies, confirming that regulation by PECAM-1 is stimulus specific. Interestingly, we found that cytokine (IL-1β)–mediated TM is composed of both PECAM-1–dependent and PECAM-1– independent (IL-8–dependent) components that are separable and additive, further refining previous observations that cytokine-mediated TEM involves expression of chemokines such as IL-8.45,46,54 Furthermore, these data suggest a mechanism for the PECAM-dependent and PECAM-independent components of transmigration after prolonged IL-1 exposure. Unlike “pure” type I transmigration,2 in which large amounts of direct neutrophil activators are present and neutrophils do not require a “prestimulated” monolayer (Figure4), transmigration after IL-1 stimulation of the monolayer appears to involve, in part, neutrophil activation caused by secreted IL-8 (at low concentrations), while another component of transmigration requires neutrophil activation through contact-dependent PECAM-1 interactions with the “prestimulated” monolayer.
Having defined the stimuli for and components of PECAM-1–dependent transmigration, we utilized REN cells stably transfected with mutant PECAM-1 isoforms known to selectively disrupt cell border localization, calcium signaling, and protein-protein association to directly address the mechanisms by which PECAM-1 regulates neutrophil transmigration. To our surprise, we found that neither PECAM-1 cell-cell border localization, PECAM-1–mediated calcium signaling, nor the cytoplasmic domain that supports tyrosine phosphorylation and SH2 protein association is absolutely required for PECAM-1–dependent regulation of PMN transmigration. The findings that PECAM-1–dependent leukocyte transmigration requires homophilic PECAM-1–PECAM-1 interaction (Nakada et al19 and Figure 3) and that the extracytoplasmic domain is sufficient to reconstitute PECAM-1–mediated TEM (Figure 6) suggest that the primary function of endothelial cell PECAM-1 is to serve as a “passive” ligand for neutrophil PECAM-1.
Mechanistically, a broad range of stimuli such as crosslinking of neutrophil PECAM-1 or CD98,22,24,25,58 as well as direct PMN activators such as PAF and FMLP,59 have been found to activate leukocyte β-integrins and augment PMN-EC interaction. These stimuli appear to converge on the neutrophil GTPase Rap1 through several pathways, including a calcium-dependent pathway that may involve PI3K and phospholipase C (PLC), as well as a calcium and PLC-independent alternate mechanism activated by FMLP.59 Thus, in type I TEM, it appears that soluble chemokines such as IL-8 or LTB4 can directly activate neutrophil integrins and bypass the need for cytokine prestimulation of ECs and subsequent PECAM-1–induced neutrophil activation. We propose that in IL-1β–mediated (type II) TEM, following EC prestimulation and initial PMN “priming” through activating cell-cell contact, homophilic engagement of “passive” EC PECAM-1 by PMN PECAM-1 may serve as a key neutrophil integrin (CD18) activation step that precedes the steps in diapedesis mediated by distal cell border proteins such as CD99.21 Because PECAM-1 may exist in a monomeric or oligomeric state on ECs mediated, in part, throughcis-extracytoplasmic domain interactions,60 61we have begun an in-depth analysis to assess the role of EC PECAM-1 dimerization or oligomerization in this stage of TEM. One notable limitation of our model system is that it does not lend itself to evaluation of potential cis heterophilic interactions mediated by the EC PECAM-1 extracellular domain, thus making conclusions regarding such potential interactions difficult.
Interestingly, although PECAM-1–dependent transmigration appears to be ICAM-1 dependent (Figure 2C), absolute transmigration rates (not shown) and proportional levels of PECAM-1 involvement were identical in cell lines with large differences in ICAM-1 expression such as PECAM-ΔCD and AAAA (Figure 6 and Table 2). This suggests that there is a threshold level of monolayer ICAM-1 expression (possibly correlating to full occupancy of neutrophil CD18) beyond which TEM does not substantially increase. Similarly, potential neutrophil PECAM-1 heterophilic interactions with EC (monolayer) non–PECAM-1 ligands (including ICAM-1) in the PECAM-1–dependent component of type II transmigration were excluded, as no differences in transmigration through REN (PECAM-1 null) monolayers was observed following incubation of neutrophils with Houston, anti-β2 microglubulin, or any other nonblocking control (Figure 3A-B,D). Finally, in the context of type II transmigration, residual ICAM-1–independent transmigration observed in both REN and RHP monolayers does not appear to require PECAM-1. This component of transmigration may be due to as yet unidentified non–ICAM-1 CD18 ligands as has been postulated,4 62 or perhaps due to residual ICAM activity unblocked by our antibody.
Evaluation of PECAM-1–null mice similarly demonstrates both the subtleties and redundancy of the mechanisms underlying leukocyte TEM. Studies evaluating PMN TEM in IL-1β or thioglycolate-mediated peritonitis demonstrated only a mild defect in PMN migration through the perivascular basement membrane, whereas no TEM defects in other leukocyte subtypes were observed. No effect on overall IL-8, MIP-1α (a CC chemokine), air pouch, IL-1β, or thioglycollate-mediated TEM rates was observed.63 Further evaluation of PECAM-1–null mice revealed that TEM in specific vascular beds (cremasteric muscle venules) involves PECAM-1 in a cytokine-specific (IL-1β not TNFα) and time-dependent manner (4 hours),18 findings consistent with earlier studies suggesting a vascular bed–specific role for PECAM-1.3 Our data are consistent with these findings as we demonstrate that IL-1β–mediated TEM is composed of distinct and separable PECAM-1–dependent and –independent components and that IL-8– and LTB4-mediated TEM is entirely PECAM-1 independent. Similarly, the marked inhibition of transmigration by anti-ICAM antibody in REN cells confirms that PECAM-1 represents only one component of the processes mediating “ICAM-1–dependent” type II transmigration. These findings underscore the functional redundancy allowed by concurrent involvement of alternate PECAM-1–independent pathways and help explain the subtle phenotypes observed in the PECAM-1 knock-out mouse using models of inflammation that likely elicit multiple inflammatory mediators.
Although REN cells appear to be an excellent model for ECs, they do have some differences from ECs that must be considered. For example, REN cells do not express selectins and although they express cadherins at their cell-cell junctions, they have N-cadherin rather than VE-cadherin (data not shown). We do not think that these differences affect our conclusions; however, they could be potentially more important in flow-based systems in which selectins might be more involved. Similarly, contrary to prior findings linking loop 6 epitopes in monocyte TEM through the basement membrane,20 we found no significant blockade of neutrophil transmigration with an anti–loop 6 antibody in this model system. Although this could be due to differences between leukocyte types studied (which, like monocytes and lymphocytes, may have different levels of basal TM or surface protein expression from PMNs64) or due to differences in model systems used, this would not alter our conclusions regarding PECAM-1–dependent transmigration mediated by homophilic PECAM-1 interactions between ECs and PMNs. We are in the process of confirming our observations using ECs derived from PECAM knock-out mice.
In summary, we have used an endothelial model system to study the structural requirements of the PECAM-1 molecule for regulation of cytokine-activated neutrophil transmigration. Contrary to our initial hypothesis, neither monolayer (endothelial) PECAM-1–regulated calcium signaling nor cytoplasmic domain tyrosine phosphorylation events (or protein-protein association) were required for PECAM-dependent TM. Similarly, PECAM-1 cell border localization was not necessary for PECAM-1–dependent TM (Figures 1,6). These findings suggest that monolayer (endothelial) PECAM-1 serves as a passive ligand for neutrophil PECAM-1 that then functions as an active signaling receptor in an ICAM-1–dependent manner. We also confirm that PECAM-1–dependent TM is stimulus specific because chemokine (IL-8)– and chemoattractant (LTB4)–mediated TEM does not require PECAM-1, whereas IL-1β–mediated TM is composed of separable and additive PECAM-1–dependent and type I (PECAM-1–independent) components. Although it remains unclear what the role of PECAM-1 may be in complex disease models where both chemokine and cytokine activation of endothelium might occur, our results explain many of the seemingly conflicting reports regarding PECAM-1 function in leukocyte TEM. In this light, evidence of PECAM-1 regulation of neutrophil function in the context of stimulus12-14 and vascular bed–specific models of inflammation,3,18 as well as roles in models of inflammation characterized by monocyte and lymphocyte activation and transmigration,64 underscores the importance of establishing cell-specific functions for common proteins in the appropriate disease context.
We thank Dr Peter Newman for the PITC mutantPECAM-1 construct, Drs William Muller and Robert Rothlein for the Hec7 and LR6.5 antibodies, and Dr Robert M. Streiter for anti–IL-8 serum. We thank Dr Clayton Buck for his comments and suggestions.
Prepublished online as Blood First Edition Paper, December 5, 2002; DOI 10.1182/blood-2002-08-2396.
Supported by National Institutes of Health grants HL04248 (C.D.O.) and HL49591 (S.M.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Christopher D. O'Brien, c/o Steven M. Albelda, Department of Pulmonary/Critical Care, University of Pennsylvania, 838 BRB II/III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: christoo@mail.med.upenn.edu.


![Fig. 5. IL-1β–stimulated TEM consists of PECAM-1 and IL-8–dependent components that are separable and additive. / (A) Confluent RHP monolayers were treated with 5 nm IL-8 (abluminal) and either 1:200 negative control rabbit serum (NC) or anti–IL-8 serum was added to the abluminal (bottom) chamber at the start of the TM assay. PMNs were allowed to transmigrate for 4 hours. In this representative experiment, data represent the mean ± SEM from a minimum of 3 transwells for each condition. (B) Confluent RHP monolayers were treated for 24 hours with 10 U/mL IL-1β (luminal), washed, and 500 000 PMNs/well were applied to the luminal chamber. Anti–IL-8 serum (1:200) or negative control NI rabbit serum was added to either the luminal (top) or abluminal (bottom) chamber in conjunction with (luminal) anti–PECAM-1 (Houston) or anti–MHC-1 antibodies (100 μg/mL) as indicated (PMN aliquots were treated identically to luminal conditions 20 minutes prior to addition to wells). PMNs were allowed to transmigrate for 4 hours. Transmigration rates are expressed as the proportion of PMNs migrating through the transwell filter compared with the total number of PMNs added at the start of the experiment. Column 1, “unblocked” TM (anti–MHC-1) in the setting of abluminal NI serum; Column 2, “unblocked” TM (anti–MHC-1) in the setting of abluminal anti–IL-8 serum; Column 3, “blocked” TM (Houston) in the setting of abluminal NI serum; Column 4, “blocked” TM (Houston) in the setting of abluminal anti–IL-8 serum; Column 5, “unblocked” TM (anti–MHC-1) in the setting of luminal NI serum; Column 6, “unblocked” TM (anti–MHC-1) in the setting of luminal anti–IL-8 serum. Data represent the mean ± SEM from a minimum of 3 transwells for each condition from 3 separate experiments. (*Columns 2, 3, and 4 are significantly different [P < .05] from column 1. **Column 4 is significantly different from columns 2 and 3 [P < .05].)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-08-2396/3/m_h80734086005.jpeg?Expires=1765888079&Signature=ZyRC4Ub7NP-AHfo7Sra70YHns8ABdrSGBcIc7JwPhZBwkpNUoR-Eh~4X9aJLi7654sXzbgim4ir9lYAw3kgXGC-oDWjmDDe3jznTBqS7E0qIojBXqgxK6ZHWau8nUbepJyf53W0u~pDeMLoVDHGtfdhC31h7oB6jizbd1tYz1WsxdqLWrhY-7yx6RpPTtOOg3P1GygXz0UgHGFuPc1ODwAeW3piMAkwU9AlSPZZb6b5kh45Fgohj91OsdiNGpuzD37HhGjCIeCOENWtlKmGm5RypoXX~Igq~uRYYeLjhgblgo0T87DqSfPcKXGE-EQNuI25N4qTtULvIM72JT2mIXw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal