Osteoclasts are derived from hematopoietic precursor cells belonging to the monocyte/macrophage lineage. Osteoclast development has been reported to be regulated by several molecules such as macrophage colony-stimulating factor (M-CSF), receptor activator of nuclear factor (NF)-κB ligand (RANKL), and a decoy receptor of RANKL, osteoprotegerin (OPG). Recently, it was demonstrated that the Notch signaling pathway regulates myeloid differentiation and antagonizes cell fate determination, however, the effect of Notch signaling on the osteoclast lineage has not been reported. In this study, we examined the effect of signaling via Notch receptors on the differentiation into osteoclasts by using cells from the bone marrow, spleen, and peritoneal cavity, and a cloned macrophagelike cell line. Osteoclastogenesis was inhibited by an immobilized Notch ligand, Delta-1. The dish-adherent bone marrow cells precultured with M-CSF expressed both Mac-1 and M-CSF receptors, c-Fms; osteoclastogenesis of these cells was efficiently inhibited. The immobilized Delta-1 also down-regulated the surface c-Fms expression, while the c-Fms gene expression was not changed. Genes for Notch receptors and Notch ligands are expressed in not only hematopoietic cells but also stromal cells that support osteoclast development. Constitutively active Notch1-transfected stromal cells showed increased expression of RANKL and OPG genes, and strong inhibition of M-CSF gene expression, resulting in reduction of their ability to support osteoclast development. Taken together, these findings indicate that Notch signaling affects both osteoclast precursors and stromal cells and thereby negatively regulates osteoclastogenesis.
Introduction
The control of cell fate by Notch signaling was first described for Drosophila melanogaster neural/epidermal precursors as a mechanism involving lateral inhibition.1,2At present, 4 Notch receptors, Notch-1, -2, -3, and -4, and their ligands, Delta-1, -3, and -4, and Jagged-1 and -2 have been identified in mammals.3-11 Notch ligands bind to Notch receptors through their extracellular domain, trigger proteolytic processing, and release the Notch intracellular domain (NIC) from the cell membrane. Cleaved NIC interacts with the DNA-binding transcription factor CBF1/RBP-Jκ, and the complex is translocated into the nucleus.12,13 This complex binds to the Hairy enhancer of split (Hes1) promoter and stimulates the transcription of the Hes1 gene.14,15Overexpressed NIC acts as a constitutively active form of Notch receptor.16
Recently, several studies have reported the roles of Notch signaling in hematopoietic cell development.17-20 NIC transgenic mice have defective B-cell development.21 Notch signaling influences the decision of differentiation into αβ versus γδT cells,22 as well as that of differentiation into CD4 versus CD8 T cells.23 Delta-1 blocks differentiation into the B-cell lineage, while it promotes the emergence of T/natural killer (NK) cell precursors.24 In myeloid cell lineages, an immobilized form of the extracellular domain of Delta-1 induces monocytes to undergo apoptosis when treated with macrophage colony-stimulating factor (M-CSF)25 and inhibits the differentiation of monocytes into mature macrophages when treated with granulocyte/macrophage (GM)–CSF, but permits their differentiation into dendritic cells in the presence of GM-CSF and interleukin-4 (IL-4).26
Osteoclasts are also included in the myeloid lineage and are derived from hematopoietic precursor cells shared with the macrophage and dendritic cell lineages.27,28 Osteoclast differentiation is a multistep process that eventually leads to expression of tartrate-resistant acid phosphatase (TRAP), multinucleation, and bone-resorbing activity.29-33 Osteoclastogenesis is dependent on stromal cells that support hematopoiesis,34-36 and it has been demonstrated that the critical molecules produced by stromal cells are M-CSF37and receptor activator of nuclear factor (NF)-κB ligand (RANKL).38 39 However, the effects of Notch signaling on osteoclastogenesis have not been reported yet.
In this study, we used culture systems for osteoclast development to assess the role of Notch signaling in hematopoiesis. Stromal cells produce not only M-CSF and RANKL,38,40 but also several other molecules that affect hematopoiesis, including Notch ligands. When a truncated form of the Notch ligand Delta-1 was immobilized on culture dishes, the Hes1 transcription in bone marrow (BM) cells cultured in these dishes in the presence of M-CSF was activated. We also found that Notch signaling reduced the expression of an M-CSF receptor, c-Fms, in M-CSF–treated adherent BM cells and inhibited their osteoclastogenesis. Furthermore, the activated Notch-1 on stromal cells reduced M-CSF (Csfm) gene expression and enhanced RANKL (Tnfsf11) and osteoprotegerin (OPG)/osteoclastogenesis inhibitory factor (OCIF; Tnfrsf11b) gene41 42expression, resulting in reduction of the ability to support osteoclastogenesis. Thus, this study showed that Notch signaling regulates osteoclast development directed to osteoclast precursors and through stromal cells.
Materials and methods
Cell culture
Murine femoral BM cells from C57BL/6 mice (Japan Clea, Tokyo, Japan) were cultured with 50 ng/mL recombinant human (rh) M-CSF (a gift from Drs K. Yamanishi and M. Takahashi, Otsuka Pharmaceutical). These cells were maintained in alpha-minimum essential medium (α-MEM; Gibco-BRL, Grand Island, NY) containing 10% fetal calf serum (FCS; Tace Scientific, Melbourne, Australia). A macrophagelike cell line, C7-TY, was maintained in α-MEM supplemented with 10% FCS and 50 ng/mL rhM-CSF.43
A BM-derived stromal cell line, ST2, was maintained in RPMI 1640 (Gibco-BRL) supplemented with 5% FCS and 50 μM 2-mercaptoethanol.44 ST2NIC cells were maintained in the same medium, except that it was supplemented with 1 μg/mL tetracycline (Tc; Sigma, St Louis, MO).
Induction of differentiation into osteoclasts and dendritic cells
Twenty-four-well tissue-culture plates (Corning Costar, Corning, NY) and 100-mm dishes (Corning Costar) were coated with a FLAG-fused human Delta-1 (Delta-1–FL), which lacked the transmembrane and cytoplasmic domains or a FLAG fusion protein ofEscherichia coli bacterial alkaline phosphatase (BAP-FL; Sigma) at the concentration of 10 μg/mL in phosphate-buffered saline (PBS) for one day at 4°C. After the solutions were removed, the cells were plated in the 24-well plates. For the induction of osteoclasts, these cells were cultured in [α-MEM containing 10% FCS, 50 ng/mL rhM-CSF, and 25 ng/mL rhRANKL (Pepro Tech EC, London, England) for 6 days.38 For induction of dendritic cells, cells were plated in 6-well plates coated with Delta-1–FL or BAP-FL and cultured with α-MEM containing 10% FCS, 100 U/mL GM-CSF (a gift from Dr T. Sudo, Toray Industries), and 50 ng/mL rh IL-4 (Pepro Tech EC) for 6 days.45 46 Spent medium was replaced with fresh medium every third day.
TRAP staining
Cultured cells were fixed with 10% formalin (Wako Pure Chemical Industries, Osaka, Japan) in PBS for 10 minutes, and subsequently with ethanol-acetone (50:50 vol/vol; Wako) for 1 minute at room temperature, and incubated in acetate buffer (pH 5.0; Sigma) containing naphthol AS-MX phosphate (Sigma) as a substrate and fast red violet Luria-Bertani (LB) salt (Sigma) as a stain in the presence of 50 mM sodium tartrate (Wako).47 TRAP+cells with more than 2 nuclei were scored as TRAP+-multinucleated cells (MNCs).
Frequency analysis of osteoclast precursors
One day before the assay, the ST2 monolayer was allowed to form in a 96-well plate (Corning Costar). Before seeding the cells from BM cells and M-CSF–cultured BM cells, the medium was removed from the well and 20 dilutions of freshly prepared bone marrow cells and 3 dilutions of M-CSF–cultured cells diluted in 200 μL of medium containing dexamethasone (DEX) and 1α, 25-(OH)2D3 were added. Each cell dilution series was performed in a total of 96 wells. The medium was changed every other day by aspirating the medium and adding 200 μL of fresh medium. At 6 days after the initiation of the cultures, the cells in the well were stained. Wells containing TRAP+ cells were judged as osteoclast positive. We selected the appropriate number of TRAP+ cell–positive wells from each experimental group and calculated 1/frequency of the presence of osteoclast precursors in the fractions using the following formula: 1/frequency = N/{ln[T/(T−P)]}, where N is the number of cells added per well, T is the number of wells per group (96 wells), and P is the number of positive wells per group.48
Polymerase chain reaction (PCR) analysis
Total RNA was prepared using ISOGEN (Nippon Gene, Toyama, Japan). First-strand cDNA synthesis was performed by using ReverTraAce (Toyobo, Osaka, Japan) and primed with oligo(dT)12-18primer (Gibco-BRL) in a 20-μL reaction mixture. The first-strand cDNA mixture (1 μL) was subjected to PCR with Taqpolymerase (Toyobo) in a 25-μL volume. The PCR conditions were as follows: 94°C (3 minutes), 60°C (2 minutes), and 72°C (3 minutes) for the primary cycle; 94°C (45 seconds), 60°C (1 minute), and 72°C (1.5 minutes) for the following 36 cycles. The extension time in the last cycle was 3 minutes. The following primers were used:Delta1 (GenBank accession number X80903), 5′-CTGAGGTGTAAGATGGAAGCG-3′/5′-CAACTGTCCATAGTGCAATGG-3′;Jagged1 (AF 171092), 5′-ATTCGATCTACATAGCCTGTGAG-3′/5′-CTATACGATGTATTCCATCCGGT-3′;Jagged2 (AF038572), 5′-TGTCAGCCACGGAGCAGTCATT-3′/5′-TCTCACGTTCTTTCCTGCGCTT-3′;Notch1 (Z11886), 5′-TGTGACAGCCAGTGCAACTC-3′/5′-TGGCACTCTGGAAGCACTGC-3′;Notch2 (D32210), 5′-ACATCATCACAGACTTGGTC-3′/5′-CATTATTGACAGCAGCTGCC-3′;Notch3 (X74760), 5′-ACACTGGGAGTTCTCTGT-3′/5′-GTCTGCTGGCATGGGATA-3′; Notch4 (U43691), 5′-TGCCTGCACAATGGTACCTG-3′/5′-TCTGGCTTCAGTGCCTTAAG-3′;Hes1(BC018375), 5′-GCCAGTGTCAACACGACACCGG-3′/5′-TCACCTCGTTCATGCACTCG-3′;Tnfrsf11a (RANK; AF019046), 5′-CCAGGGGACAACGGAATCAG-3′/5′-GGCCGGTCCGTGTACTCATC-3′; c-Fms(X68932), 5′-GGACTATGCTAACCTGCCAA-3′/5′-GAGAAAGAGAACTAGGGGTG-3′; glyceraldehyde-3-phosphate (Gapd;M32599), 5′-CACCACCATGGAGAAGGCCGGG-3′/5′-GTGTAGCCCAAGATGCCCTTCA-3′; hypoxanthine phosphoribosyltransferase (Hprt;J00423), 5′-GTAATGATCAGTCAACGGGGGAC-3′/ 5′-CCAGCAAGCTTGCAACCTTAACCA-3′.
Flow cytometry
The following antibodies (Abs) were used for staining: M1/70 (anti–Mac-1; Immunotech, Marseille, France); AFS98 (anti–c-Fms),49 anti-CD11c (PharMingen, San Diego, CA); and anti–I-Ab (PharMingen). Stained cells were analyzed using a flow cytometer (EPICS XL; Coulter, Palo Alto, CA).
Tc-regulated system
ST2 cells were first transfected with a construct that expressed the Tc-controlled transactivator-internal ribosomal entry site (IRES)-hph gene under the control of the CAG promoter, and cultured in the presence of 25 μg/mL hygromycin B (Sigma). Resistant cells were further cotransfected with a construct that expressed a constitutively active form of the Notch-1; this construct contained an IRES element followed by the gene for green fluorescent protein (GFP) under the control of the human cytomegalovirus-1 (CMV*-1) promoter (Tc-responsive promoter) and a PGK-neor construct. The cells were then selected in the presence of 200 μg/mL G418 (Gibco-BRL), 25 μg/mL hygromycin B, and 1 μg/mL Tc.50 51 Transfections were performed with LipofectAMINE 2000 Reagent (Gibco-BRL).
Northern blot analysis
ST2NIC cells were cultured for 4 days as described in “Cell culture.” Total RNA was prepared using ISOGEN. Total RNA (25 μg) was electrophoresed in a 1% agarose formaldehyde gel, transferred to a Hybond-N+ membrane (Amersham Bioscience, Piscataway, NJ), and probed with [32P]-labeled M-CSF (also known as Csfm), RANKL (Tnfsf11), OCIF (Tnfrsf11b) cDNA, and Gapd DNA fragments. TheGapd probe was a DNA fragment amplified by PCR with the following primers : 5′-CACCACCATGGAGAAGGCCGGG-3′/5′-GTGTAGCCCAAGATGCCCTTCA-3′.
Results
Immobilized Delta-1–FL inhibits osteoclast development
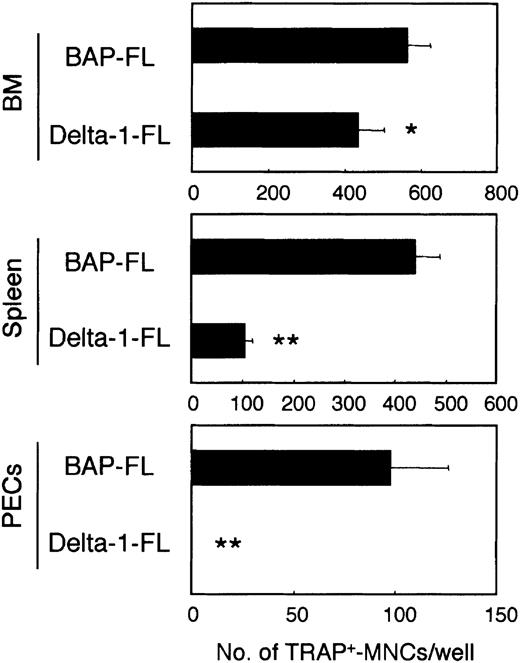
To assess the effects of Notch ligands on osteoclast differentiation, we cultured freshly prepared cells from the BM, spleen, and peritoneal cavity with M-CSF and RANKL in dishes coated with BAP-FL as a control, or Delta-1–FL. Osteoclast precursors are present in various tissues, including BM, spleen, and the peritoneal cavity. In an in vitro study, we have found that these cells can differentiate into multinucleated TRAP+ osteoclasts that have bone-resorbing activity (data not shown).
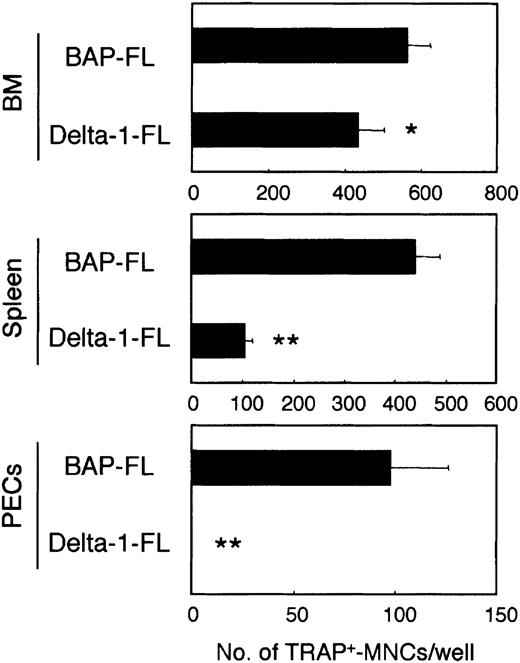
The osteoclast formation was significantly inhibited in the cultures in the dishes coated with Delta-1–FL. The degree of inhibition of the formation of multinucleated osteoclasts was different for osteoclast precursors from different tissues (the rates of reduction were as follows: BM, 23.3%; spleen, 75.9%; and peritoneal cavity, 100.0%; Figure 1).
Immobilized Delta-1–FL inhibits osteoclast development.
Freshly prepared BM cells, spleen cells, and cells from the peritoneal cavity (PECs) were cultured on dishes coated with Delta-1–FL or BAP-FL in the presence of RANKL (25 ng/mL) and M-CSF (50 ng/mL). After 6 days of culturing, the number of TRAP+-MNCs was counted. Bars indicate means ± SDs of triplicate cultures. *P < .05 and **P < .005 indicate significantly different from the corresponding cultures in dishes coated with BAP-FL.
Immobilized Delta-1–FL inhibits osteoclast development.
Freshly prepared BM cells, spleen cells, and cells from the peritoneal cavity (PECs) were cultured on dishes coated with Delta-1–FL or BAP-FL in the presence of RANKL (25 ng/mL) and M-CSF (50 ng/mL). After 6 days of culturing, the number of TRAP+-MNCs was counted. Bars indicate means ± SDs of triplicate cultures. *P < .05 and **P < .005 indicate significantly different from the corresponding cultures in dishes coated with BAP-FL.
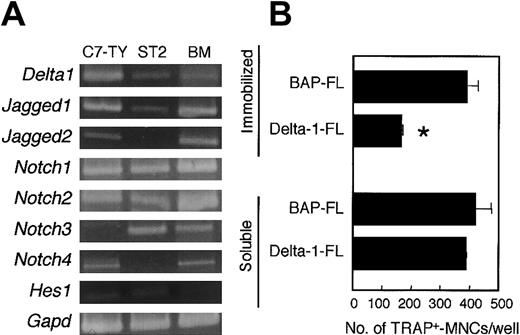
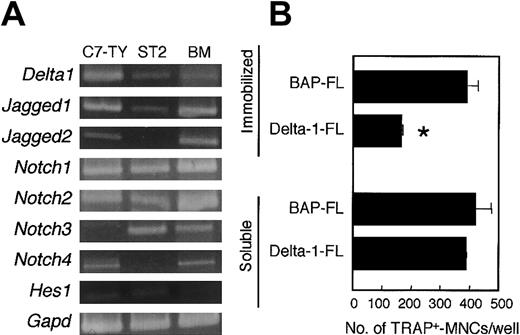
Cells from BM, spleen, and peritoneal cavity also contained a variety of cell lineages other than osteoclast precursors. Reverse transcriptase (RT)-PCR analysis showed that there were cells expressing Notch receptors Notch1, Notch2, Notch3, andNotch4 in BM. A BM-derived stromal cell line, ST2, also expressedNotch1, Notch2, and Notch3 (Figure2A). Thus, it is not clear whether the inhibition of osteoclastogenesis by an immobilized Delta-1–FL (as shown in Figure 1) results from a direct effect on osteoclast precursors or from a secondary effect through stromal cells or other hematopoietic cell lineages.
C7-TY, ST2, and BM cells express genes for components of Notch signaling, and immobilized Delta-1–FL directly inhibits osteoclastogenesis of C7-TY cells.
(A) RT-PCR analyses of genes for components of Notch signaling were performed by using cDNAs from C7-TY, ST2, and BM cells. (B) C7-TY cells cultured with RANKL (25 ng/mL) on dishes coated with Delta-1–FL or BAP-FL, or in the presence of soluble Delta-1–FL (1 μg/mL) or BAP-FL (1 μg/mL). After 6 days of culturing, the number of TRAP+-MNCs was counted. Bars indicate means ± SDs of triplicate cultures. *Indicates significantly different from the corresponding cultures in dishes coated with BAP-FL (P < .005).
C7-TY, ST2, and BM cells express genes for components of Notch signaling, and immobilized Delta-1–FL directly inhibits osteoclastogenesis of C7-TY cells.
(A) RT-PCR analyses of genes for components of Notch signaling were performed by using cDNAs from C7-TY, ST2, and BM cells. (B) C7-TY cells cultured with RANKL (25 ng/mL) on dishes coated with Delta-1–FL or BAP-FL, or in the presence of soluble Delta-1–FL (1 μg/mL) or BAP-FL (1 μg/mL). After 6 days of culturing, the number of TRAP+-MNCs was counted. Bars indicate means ± SDs of triplicate cultures. *Indicates significantly different from the corresponding cultures in dishes coated with BAP-FL (P < .005).
C7-TY cells, a clonal macrophagelike cell line43 that has the potential to differentiate into osteoclasts even when treated with RANKL only, expressed Notch receptors Notch1,Notch2, and Notch4, but not Notch3 (Figure2A). We assessed the effect of immobilized Delta-1–FL on C7-TY cells. Osteoclastogenesis of C7-TY cells was also inhibited by immobilized Delta-1–FL (Figure 2B). Thus, the inhibition of osteoclast formation by Notch signaling appears to be due at least in part to direct effects on osteoclast precursors.
In contrast, the soluble form of Delta-1–FL did not affect the osteoclastogenesis of BM cells, spleen cells, PECs (data not shown), or C7-TY cells (Figure 2B). Neither the soluble nor the immobilized form of BAP-FL affected osteoclastogenesis, so we used immobilized BAP-FL as a negative control, unless otherwise indicated.
Immobilized Delta-1–FL induces Hes1 gene expression
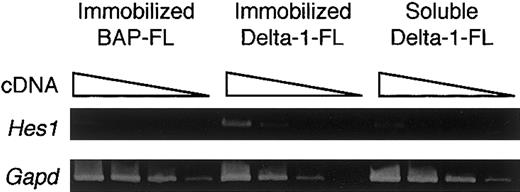
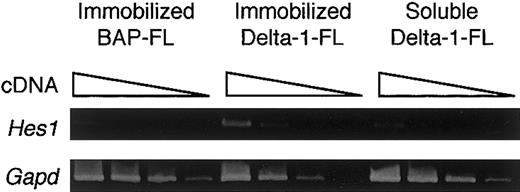
To determine whether the immobilized Delta-1–FL functions as an agonist or antagonist of Notch signaling, we investigated the gene expression of a transcription factor, Hes1, whose expression is regulated by Notch signaling. RT-PCR analysis showed that BM cells cultured with M-CSF for 4 days on immobilized Delta-1–FL showed increased expression of the Hes1 gene compared with those on immobilized BAP-FL (Figure 3). Addition of soluble Delta-1–FL did not affect Hes1 expression (Figure 3). These data indicate that immobilized Delta-1–FL functions as an agonistic ligand, but soluble Delta-1–FL does not affectHes1 gene expression.
Induction of Hes1 gene expression by immobilized Delta-1–FL.
Total RNAs were prepared from dish-adherent BM cells cultured in the presence of immobilized Delta-1–FL or BAP-FL, or soluble Delta-1–FL, plus M-CSF (50 ng/mL) for 4 days. Serial dilutions (1:1, 1:9, 1:81, 1:729) of cDNAs were subjected to PCR amplification specific for theHes1 and Gapd genes.
Induction of Hes1 gene expression by immobilized Delta-1–FL.
Total RNAs were prepared from dish-adherent BM cells cultured in the presence of immobilized Delta-1–FL or BAP-FL, or soluble Delta-1–FL, plus M-CSF (50 ng/mL) for 4 days. Serial dilutions (1:1, 1:9, 1:81, 1:729) of cDNAs were subjected to PCR amplification specific for theHes1 and Gapd genes.
Osteoclast precursors in the BM are enriched by culturing with M-CSF
We cultured freshly prepared BM cells with 50 ng/mL M-CSF to enrich the cells that have the potential to differentiate into mature osteoclasts. On day 2, BM cells treated with M-CSF showed macrophagelike morphology. Although not all BM cells treated with M-CSF (designated BMMCs) attached to culture dishes, the dish-adherent cells increased and nonadherent cells decreased during the culture period (Figure 6C).
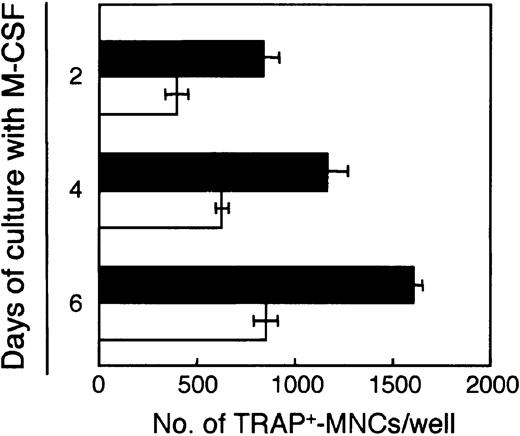
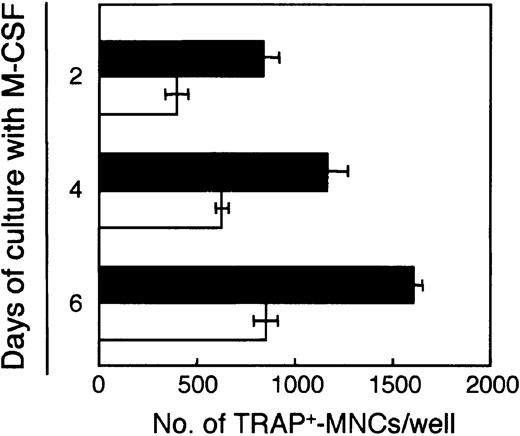
We assessed the potential of these BMMCs to differentiate into osteoclasts. After 2, 4, or 6 days of culturing with M-CSF, 103 each adherent and nonadherent cells were inoculated into 24-well plates, and induced to differentiate into osteoclasts with M-CSF and RANKL for 6 days. Generation of osteoclasts increased as the length of preculturing with M-CSF increased (Figure4). Freshly prepared BM cells contained 1 osteoclast precursor per 63.4 cells, whereas more than 1 of 3 cells in both adherent (1/2.7) and nonadherent (1/2.3) cells cultured with M-CSF for 4 days were capable of differentiating into osteoclasts. These data suggest that M-CSF enriched the cells that have the potential to differentiate into osteoclasts.
Osteoclast precursors in the BM cells are enriched by culturing with M-CSF.
Freshly prepared BM cells were cultured with M-CSF (50 ng/mL) for 2, 4, or 6 days, and 103 adherent or nonadherent cells were inoculated into each well of 24-well plates and treated with M-CSF (50 ng/mL) and RANKL (25 ng/mL). After a further 6 days of culturing, the number of TRAP+-MNCs was counted. Bars indicate means ± SD of triplicate cultures. The results obtained with adherent (▪) and nonadherent (■) cells were significantly different on each day of culturing (adherent versus nonadherent cells on days 2, 4, and 6,P < .01; day 2 versus 4 and 6, day 4 versus 6 for both adherent and nonadherent cells, P < .01).
Osteoclast precursors in the BM cells are enriched by culturing with M-CSF.
Freshly prepared BM cells were cultured with M-CSF (50 ng/mL) for 2, 4, or 6 days, and 103 adherent or nonadherent cells were inoculated into each well of 24-well plates and treated with M-CSF (50 ng/mL) and RANKL (25 ng/mL). After a further 6 days of culturing, the number of TRAP+-MNCs was counted. Bars indicate means ± SD of triplicate cultures. The results obtained with adherent (▪) and nonadherent (■) cells were significantly different on each day of culturing (adherent versus nonadherent cells on days 2, 4, and 6,P < .01; day 2 versus 4 and 6, day 4 versus 6 for both adherent and nonadherent cells, P < .01).
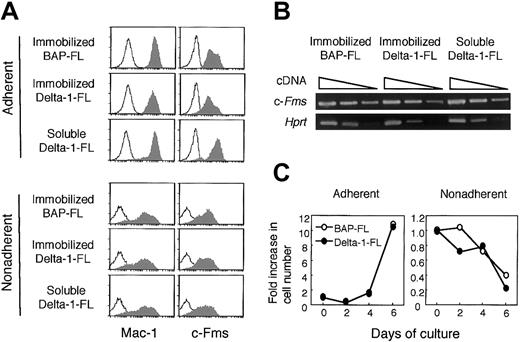
Flow cytometric analysis revealed that almost all of the adherent cells cultured with M-CSF for 4 days expressed myeloid cell lineage markers Mac-1 and c-Fms on their surface (Mac-1+, 98.4%; c-Fms+, 93.9%; Mac-1+ and c-Fms+, 93.7%). The majority of nonadherent cells also expressed Mac-1 (Mac-1+, 77.8%; c-Fms+, 52.7%; Mac-1+ and c-Fms+, 50.5%). M-CSF preferentially induced Mac-1 and c-Fms double-positive cells from BM cells (Mac-1+, 60.5%; c-Fms+, 1.8%; Mac-1+ and c-Fms+, 1.8%). Although there were also Mac-1 and/or c-Fms–expressing cells in the nonadherent cell fraction, the fluorescence intensities were heterogeneous and less bright than those of adherent cells (Figure 6A).
M-CSF stimulation induces the expression of Notch receptors and their ligands on BMMCs
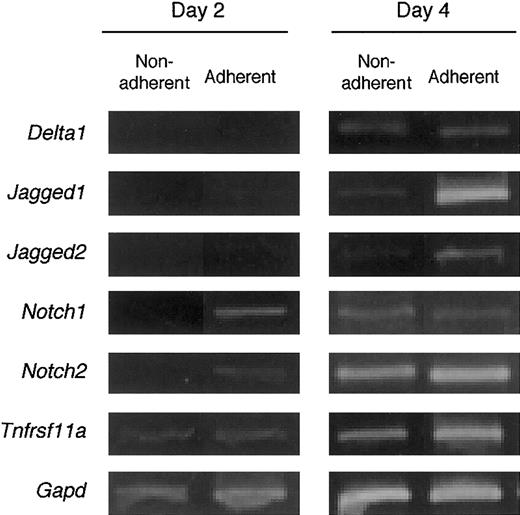
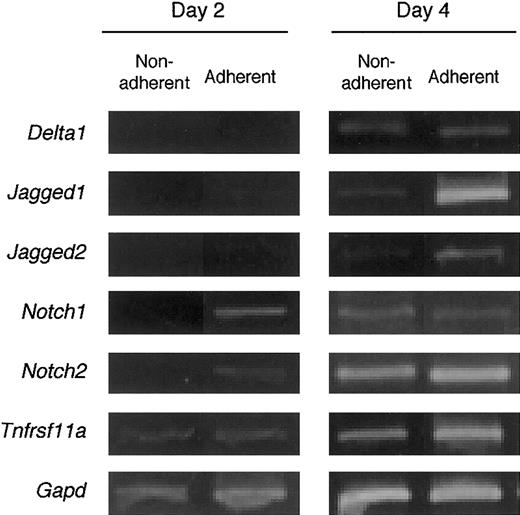
The expression of Notch receptors and their ligands on BMMCs was analyzed. After 2 days of culturing with M-CSF, the dish-adherent cells expressed Notch1 and Notch2 mRNAs, but nonadherent cells did not. On day 4, significant expression ofNotch1 and Notch2 was detected in both adherent and nonadherent cells. M-CSF stimulation induced the expression of Notch ligands Delta1, Jagged1, andJagged2 on day 4 of culturing. These data show that BMMCs expressed Notch receptors and ligands, and that this expression was delayed on nonadherent cells compared with that on adherent cells. Expression of RANK (Tnfrsf11a), a receptor for RANKL, was detected in adherent and nonadherent cells on day 2, and the expression was enhanced on day 4 (Figure 5).
BM cells cultured with M-CSF express genes for components of Notch signaling and RANK.
Freshly prepared BM cells were cultured with M-CSF (50 ng/mL). On days 2 and 4 of culture, dish-adherent and -nonadherent cells were harvested and the gene expression for Notch receptors and their ligands, and RANK (Tnfrsf11a) was examined by RT-PCR analyses. The expression of these genes was enhanced by culturing with M-CSF.
BM cells cultured with M-CSF express genes for components of Notch signaling and RANK.
Freshly prepared BM cells were cultured with M-CSF (50 ng/mL). On days 2 and 4 of culture, dish-adherent and -nonadherent cells were harvested and the gene expression for Notch receptors and their ligands, and RANK (Tnfrsf11a) was examined by RT-PCR analyses. The expression of these genes was enhanced by culturing with M-CSF.
Immobilized Delta-1–FL reduces c-Fms expression on BMMCs
Because immobilized Delta-1–FL inhibited osteoclastogenesis, we assessed whether the immobilized Delta-1–FL might negatively affect the commitment of BM cells to differentiate into osteoclast precursors, or the proliferation of osteoclast precursors. We cultured BM cells with M-CSF for 4 days with soluble Delta-1–FL, or on BAP-FL– or Delta-1–FL–coated dishes, and analyzed harvested cells for the expression of myeloid cell markers by flow cytometry. Almost all of the adherent cells expressed c-Fms; however, the fluorescence intensity of the cell surface differed depending on the treatment. Compared with the cells cultured on BAP-FL–coated dishes, the cells cultured on Delta-1–FL–coated dishes showed reduced c-Fms expression. In contrast, the addition of soluble Delta-1–FL slightly enhanced the c-Fms expression (Figure 6A). Because soluble Delta-1–FL did not affect Hes1 expression, it is not clear how soluble Delta-1–FL enhanced c-Fms expression (Figure 3).
Immobilized and soluble Delta-1–FL reciprocally regulate c-Fms expression on adherent cells and immobilized Delta-1–FL, but do not affect the cell growth of BM cells.
(A) BM cells were cultured with M-CSF (50 ng/mL) for 4 days in dishes coated with Delta-1–FL, or BAP-FL, or in the presence of soluble Delta-1–FL (1 μg/mL) and then analyzed by flow cytometry. The shaded histograms represent staining with anti–Mac-1 and anti–c-Fms antibodies and the open histograms represent staining with a control antibody. (B) Total RNAs were prepared from dish-adherent BM cells cultured in the presence of immobilized Delta-1–FL or BAP-FL, or soluble Delta-1–FL, plus M-CSF (50 ng/mL) for 4 days. Serial dilutions (1:1, 1:9, 1:81) of cDNAs were subjected to PCR amplification specific for the c-Fms and Hprt genes. (C) Freshly prepared BM cells were cultured with M-CSF (50 ng/mL) on Delta-1–FL– or BAP-FL–coated dishes. After 2, 4, and 6 days of culturing, the numbers of dish-adherent and -nonadherent cells were counted.
Immobilized and soluble Delta-1–FL reciprocally regulate c-Fms expression on adherent cells and immobilized Delta-1–FL, but do not affect the cell growth of BM cells.
(A) BM cells were cultured with M-CSF (50 ng/mL) for 4 days in dishes coated with Delta-1–FL, or BAP-FL, or in the presence of soluble Delta-1–FL (1 μg/mL) and then analyzed by flow cytometry. The shaded histograms represent staining with anti–Mac-1 and anti–c-Fms antibodies and the open histograms represent staining with a control antibody. (B) Total RNAs were prepared from dish-adherent BM cells cultured in the presence of immobilized Delta-1–FL or BAP-FL, or soluble Delta-1–FL, plus M-CSF (50 ng/mL) for 4 days. Serial dilutions (1:1, 1:9, 1:81) of cDNAs were subjected to PCR amplification specific for the c-Fms and Hprt genes. (C) Freshly prepared BM cells were cultured with M-CSF (50 ng/mL) on Delta-1–FL– or BAP-FL–coated dishes. After 2, 4, and 6 days of culturing, the numbers of dish-adherent and -nonadherent cells were counted.
We performed RT-PCR analysis for the c-Fms gene to examine whether the reduction of c-Fms on the cell surface by immobilized Delta-1–FL was due to transcriptional regulation. The results showed that the level of the c-Fms transcript was not affected by immobilized or soluble Delta-1–FL (Figure 6B). This suggests that the reduction of c-Fms on the cell surface by Notch signaling was due to posttranscriptional regulation.
We also assessed the effect of immobilized Delta-1–FL on M-CSF–dependent cell growth when BM cells were cultured with M-CSF for 2, 4, and 6 days on the BAP-FL, or Delta-1–FL–coated dishes, and found that the numbers of recovered adherent and nonadherent cells from the 2 types of dishes were not significantly different (Figure 6C).
Immobilized Delta-1–FL inhibits the differentiation of BMMCs to osteoclasts
As shown in Figure 1, immobilized Delta-1–FL inhibited osteoclast formation of spleen cells and PECs more efficiently than that of BM cells. Therefore, we assessed whether this difference might have resulted from the presence of a variety of osteoclast precursors or variation of their developmental stages. We precultured freshly prepared BM cells with 50 ng/mL M-CSF for 4 days, and subsequently induced these BMMCs to differentiate into osteoclasts by treatment with RANKL and M-CSF for 6 days. Dish-adherent cells precultured with M-CSF efficiently differentiated into TRAP+ osteoclasts. When the induction of these adherent cells to osteoclasts was performed in the presence of immobilized Delta-1–FL, the formation of osteoclasts was strongly inhibited (Figure 7A,C). In contrast, the osteoclastogenesis of nonadherent cells precultured with M-CSF was not inhibited by immobilized Delta-1–FL (Figure 7A,C). Addition of soluble Delta-1-FL to cultures did not affect osteoclast formation from either the adherent or nonadherent cells (data not shown).
Effects of immobilized Delta-1–FL on the differentiation into osteoclasts and dendritic cells of BM cells precultured with M-CSF.
BM cells cultured with M-CSF for 4 days were harvested, and adherent and nonadherent cells were further cultured with M-CSF (50 ng/mL) and RANKL (25 ng/mL) in wells coated with (A) Delta-1–FL or BAP-FL (2 μg), or (C) various doses of Delta-1–FL (2, 1, 0.5, 0.05, and 0 μg). (B) After 6 days, TRAP staining was performed. Adherent and nonadherent cells were cultured with GM-CSF (100 U/mL) and IL-4 (25 ng/mL) in the wells coated with Delta-1–FL or BAP-FL (2 μg) for 6 days, and the harvested cells were stained with anti-I-Aband anti-CD11c antibodies, and analyzed by flow cytometry. The numbers of I-Ab-high and CD11c+ cells were calculated as follows: recovered cell number/well × % positive cells/100. Bars indicate means ± SD of triplicate cultures. *Indicates significantly different from the corresponding cultures in dishes coated with BAP-FL (P < .01).
Effects of immobilized Delta-1–FL on the differentiation into osteoclasts and dendritic cells of BM cells precultured with M-CSF.
BM cells cultured with M-CSF for 4 days were harvested, and adherent and nonadherent cells were further cultured with M-CSF (50 ng/mL) and RANKL (25 ng/mL) in wells coated with (A) Delta-1–FL or BAP-FL (2 μg), or (C) various doses of Delta-1–FL (2, 1, 0.5, 0.05, and 0 μg). (B) After 6 days, TRAP staining was performed. Adherent and nonadherent cells were cultured with GM-CSF (100 U/mL) and IL-4 (25 ng/mL) in the wells coated with Delta-1–FL or BAP-FL (2 μg) for 6 days, and the harvested cells were stained with anti-I-Aband anti-CD11c antibodies, and analyzed by flow cytometry. The numbers of I-Ab-high and CD11c+ cells were calculated as follows: recovered cell number/well × % positive cells/100. Bars indicate means ± SD of triplicate cultures. *Indicates significantly different from the corresponding cultures in dishes coated with BAP-FL (P < .01).
Nonadherent cells, but not adherent cells, from BMMCs maintain the potential to differentiate into the dendritic cell lineage
To clarify the difference between the differentiation potentials of the adherent and nonadherent cells, we induced these cells to differentiate into the dendritic cell lineage. Osteoclasts and dendritic cells have been reported to have common precursors.27 28 After BM cells were cultured with M-CSF for 4 days, the adherent and nonadherent cells were recultured with GM-CSF and IL-4 in Delta-1–FL–coated wells for 6 days. I-Ab-high CD11c+ cells, whose phenotype corresponded to the dendritic cell lineage, were efficiently induced from nonadherent cells, but not from adherent cells (Figure 7B). Immobilized Delta-1–FL did not affect the induction of I-Ab-high CD11c+ cells (Figure 7B). Thus, nonadherent cells maintained the potential to differentiate into not only osteoclasts but also dendritic cells, whereas adherent cells lost the potential to differentiate into dendritic cells.
These results suggest that the nonadherent cells were in a less mature stage than adherent cells, and that Notch signaling might affect the precursor cells after they lose their bipotentiality to differentiate into osteoclasts and dendritic cells, and inhibit terminal differentiation into osteoclasts.
Notch signaling also affects stromal cells, and reduces their ability to support osteoclastogenesis
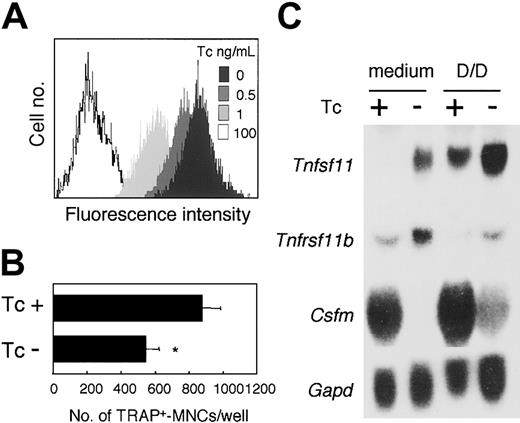
Osteoclast formation is known to be supported by osteoblasts/stromal cells via a mechanism involving the production of M-CSF and RANKL. ST2 cells expressed Notch1,Notch2, and Notch3, and Notch ligands,Delta1 and Jagged1 (Figure 2A). Therefore, to examine the effect of Notch signaling on the ability of cells to support osteoclast development, we established an ST2 cell subline (ST2NIC) carrying an active form of the Notch1(aNotch1) gene regulated under the Tc-Off system. In the ST2NIC cells, the expression of the aNotch1 gene was induced in the absence of Tc (Figure 8A, upper panel). We cocultured BM cells with ST2NIC stromal cells in the presence of DEX and 1,25(OH)2D3 for 6 days. Osteoclast formation was reduced in the absence compared with the presence of Tc (Figure 8A).
Effect of inducible aNotch1 in ST2 stromal cells on their ability to support osteoclastogenesis.
After culturing for 72 hours at the indicated concentration of Tc, the expression of aNotch in ST2NIC stromal cells was assessed by measuring the fluorescence intensity of GFP (A). (B) Freshly prepared BM cells were cultured for 6 days on ST2NIC with Tc (100 ng/mL) or the same volume of EtOH in the presence of 1.25(OH)2D3 (10−8 M) and DEX (10−7 M). After 6 days of culturing, TRAP+multinucleated osteoclasts were counted. Bars indicate means ± SD of triplicate cultures. *Indicates significantly different from the corresponding cultures with EtOH (P < .001). (C) After culturing for 4 days with Tc (100 ng/mL) or EtOH in the presence of 1,25(OH)2D3 and DEX (D/D), Northern hybridization analysis of Csfm (M-CSF), Tnfsf11(RANKL), and Tnfrsf11b (OPG) gene expression in ST2NIC was performed. Gapd gene expression is shown as a loading control in the bottom blot.
Effect of inducible aNotch1 in ST2 stromal cells on their ability to support osteoclastogenesis.
After culturing for 72 hours at the indicated concentration of Tc, the expression of aNotch in ST2NIC stromal cells was assessed by measuring the fluorescence intensity of GFP (A). (B) Freshly prepared BM cells were cultured for 6 days on ST2NIC with Tc (100 ng/mL) or the same volume of EtOH in the presence of 1.25(OH)2D3 (10−8 M) and DEX (10−7 M). After 6 days of culturing, TRAP+multinucleated osteoclasts were counted. Bars indicate means ± SD of triplicate cultures. *Indicates significantly different from the corresponding cultures with EtOH (P < .001). (C) After culturing for 4 days with Tc (100 ng/mL) or EtOH in the presence of 1,25(OH)2D3 and DEX (D/D), Northern hybridization analysis of Csfm (M-CSF), Tnfsf11(RANKL), and Tnfrsf11b (OPG) gene expression in ST2NIC was performed. Gapd gene expression is shown as a loading control in the bottom blot.
To clarify the reason for this effect, we performed Northern blot hybridization analysis of the gene expression of M-CSF (Csfm), RANKL (Tnfsf11), and OPG (Tnfrsf11b), which are known to be produced by stromal cells.38 42 Addition of DEX and 1,25(OH)2D3 to the cultures enhanced the level of expression of Tnfsf11, and reduced the expression ofTnfrsf11b in ST2 cells. Surprisingly, in the absence of Tc, the levels of expression of Tnfsf11 and Tnfrsf11bwere slightly enhanced, whereas that of Csfm was dramatically reduced in ST2NIC cells (Figure 8B). Thus, Notch signaling enhanced the production of not only RANKL, but also OPG, a decoy receptor for RANKL. Moreover, because M-CSF production was significantly reduced in the stromal cells, the ability of these cells to support osteoclastogenesis might have been reduced. Taken together, our findings suggest that Notch signaling might regulate osteoclastogenesis negatively by affecting both osteoclast precursors and stromal cells.
Discussion
In this study, we showed that immobilized Delta-1 inhibited osteoclastogenesis from precursors in the BM, spleen, and peritoneal cavity, and from a macrophagelike cell line. The immobilized Delta-1–FL appeared to function as an agonist for Notch receptor(s), and to direct its signal to osteoclast precursors. Signaling via Notch receptor(s) on ST2 stromal cells also reduced their ability to support osteoclast development.
In the presence of recombinant M-CSF and RANKL, osteoclastogenesis from the freshly prepared cells tested in this study was inhibited by immobilized Delta-1–FL (Figure 1). However, these cells consisted of a variety of hematopoietic cells and stromal cells, which express Notch and Notch ligand genes. To identify the target cells of Delta-1, we used a cloned macrophagelike cell line.43 Our finding that the osteoclast formation of C7-TY cells was also inhibited by immobilized Delta-1–FL suggested that the signaling via immobilized Delta-1–FL might affect osteoclast precursors directly.
Two laboratories have reported the effects of Notch signaling using the same cell line, but their results were not consistent.52-54 Therefore, we assessed the effects of Notch signaling on osteoclast precursors prepared from mouse BM cells. Freshly prepared BM cells contained less than 2% Mac-1 and c-Fms double-positive cells, whereas in contrast, 94% of dish-adherent cells cultured with M-CSF expressed both molecules on their surface. Corresponding to the increase of the proportion of these cells, the frequency of osteoclast precursors was increased from 1/60 in freshly prepared BM cells to more than 1/3 in the cells cultured with M-CSF for 4 days. The osteoclast formation of M-CSF–cultured adherent cells was more strongly inhibited by immobilized Delta-1–FL compared with that of freshly prepared BM cells. These results also suggest that the inhibition by immobilized Delta-1–FL might be directed to osteoclast precursors. C7-TY cells and M-CSF–cultured adherent cells, in which osteoclastogenesis was inhibited by immobilized Delta-1–FL, expressed mainly Notch-1 and Notch-2 (Figures 2A, 5). It has been reported that Delta-1 can bind both Notch-1 and Notch-2, and that both Notch receptors regulate the same signaling pathway.55 56 Therefore, we conclude that immobilized Delta-1 mainly inhibited the formation of osteoclasts through both Notch-1 and Notch-2.
Immobilized Delta-1–FL induced Hes1 gene expression in M-CSF–stimulated BM cells (Figure 3), indicating that immobilized Delta-1–FL is an agonistic ligand for Notch receptor(s). Opposite effects of the immobilized and soluble forms of Delta-1-FL were detected on the expression of c-Fms (Figure 6). Therefore, it is possible that soluble Delta-1–FL might function as an antagonistic ligand, although soluble Delta-1–FL did not inhibit Hes1gene expression (Figure 3). Since Shimizu and colleagues reported that soluble Delta-1 weakly activated Notch signaling, while it also functioned as an antagonistic ligand when present together with full-length Notch ligands,57 the effect of soluble Notch ligands is not obvious. Varnum-Finney and colleagues also reported that the extracellular domain of immobilized Notch ligands inducedHes1 transcription, and the Notch function was inhibited by soluble Notch ligands,58 suggesting that truncated Notch ligands might function like full-length ligands by immobilization on plastic dishes.
It still remains unclear whether immobilized Delta-1–FL inhibited osteoclastogenesis from nonadherent cells cultured with M-CSF. This cell fraction contained cells that have the potential to differentate into osteoclasts at a frequency of 1/2.3, and half of them expressed both Mac-1 and c-Fms on their surfaces. One possibility is that nonadherent cells are in a less mature stage than adherent cells, and that the Notch signaling might affect the precursor cells after they lose their bipotentiality to differentiate into osteoclasts and dendritic cells. We found that nonadherent cells had the potential to differentiate into osteoclasts and dendritic cells, whereas adherent cells lacked the potential to differentiate into dendritic cells (Figure 7B). Given that osteoclasts and dendritic cells have been reported to be derived from common precursors,27,28 the adherent cells cultured with M-CSF might be more mature than the nonadherent cells. It has been reported that the regulation of hematopoietic cells by Notch signaling occurs mainly in cells at immature stages, such as hematopoietic stem cells,59,60and common progenitors to B- and T-cell lineages.21 61 In osteoclast formation, however, Notch signaling may preferentially affect terminal differentiation.
Another possibility is that nonadherent cells may be of a different lineage than adherent cells, and nonadherent cells may not be sensitive to Notch signaling. However, this is not likely, because although Notch-signaling genes were expressed on M-CSF–induced adherent cells but not nonadherent cells on the second day of culturing (Figure 5), these genes gradually came to be expressed on nonadherent cells, and on the fourth day the levels of expression on nonadherent cells became comparable with those on adherent cells (Figure 5). One possible alternative explanation is that the Delta-1–FL coated on the dishes might be unstable, and function only at the initiation of cultures. This can be ruled out because the binding of this ligand to Notch receptors remained essentially unchanged for a week (S.S., unpublished observation, April 2002).
One possible reason for the partial inhibition of freshly prepared BM cells by immobilized Delta-1–FL is that they might contain few cells corresponding to the adherent cells precultured with M-CSF. The inhibition of the adherent cells from BMMCs as well as PECs was almost complete. Immobilized Delta-1–FL reduced the expression of the M-CSF receptor c-Fms on the surface of M-CSF–induced adherent cells (Figure6A). Moreover, immobilized Delta-1-FL inhibited osteoclast formation from C7-TY cells in the absence of M-CSF (Figure 2B). These results suggest that immobilized Delta-1–FL might affect the signaling via not only c-Fms, but also RANK. Therefore, we examined the effect of immobilized Delta-1–FL on the expression of the genesTnfr11a (for RANK), Mitf and Bcl2, which are essential for the formation of osteoclasts, andIfnb (which encodes interferon [IFN]-β) andIfng (which encodes IFN-γ), which are all negative regulators of osteoclastogenesis.31,32,62 However, the expressions of these genes were not changed by incubation with immobilized Delta-1 (data not shown). Other groups have shown that Notch signaling did not change the expression of the Pu.1gene, which is also essential for osteoclast development.63 To further clarify the mechanism, further studies will be needed.
In osteoclastogenesis, the osteoclast precursor-osteoblast/stromal cell interaction is crucial.34 Stromal cells that produce RANKL and M-CSF construct the microenvironment for hematopoiesis including osteoclast differentiation.37,38,40 Stromal cells also express Notch and Notch ligands, suggesting that Notch signaling might regulate the function of stromal cells. We established an ST2 stromal cell line (ST2NIC) carrying a constitutively active form of Notch regulated by the Tc-Off system.50 51 Because Notch signaling is activated in the ST2NIC cells without extracellular stimulation, we were able to assess the direct effect of Notch signaling on the stromal cells. The results indicated that the Notch signaling induced the expression of the gene for RANKL and OPG, and severely reduced Csfm (M-CSF) expression, resulting in reduction of the ability of the ST2NIC cell line to support osteoclastogenesis (Figure 8). Thus, Notch signaling may affect stromal cells as well as osteoclast precursors, and thereby function as a negative regulator of osteoclast development.
In this study, we demonstrated that Notch signaling negatively regulated osteoclastogenesis. In osteoclast precursors, the signaling reduced the expression of c-Fms on the cell surface and inhibited terminal differentiation into osteoclasts. In stromal cells, Notch signaling regulated the expression of the genes for M-CSF, RANKL, and OPG, and reduced the ability to undergo osteoclastogenesis. Our findings and those of a recent report showing that osteoblast differentiation was positively regulated by Notch signaling64 suggest that the balance between osteoclasts and osteoblasts in the bone might be regulated by Notch signaling.
We thank Dr Hermann Bujard (Heidelberg University), Dr Hitoshi Niwa (RIKEN Center for Developmental Biology, Kobe, Japan), and Dr Ryoichiro Kageyama (Kyoto University) for plasmids; Dr Kazuya Yamanishi and Dr Masayuki Takahashi (Otsuka Pharmaceutical), Dr Tetsuo Sudo (Toray Industries), and Dr Shin-Ichi Nishikawa (Kyoto University) for reagents. We also thank Dr Toshiyuki Shibahara for maintenance of the mice and Ms Toshie Shinohara for secretarial assistance.
Prepublished online as Blood First Edition Paper, October 31, 2002; DOI 10.1182/blood- 2002-06-1740.
Supported by grants from the Special Coordination Funds of the Ministry for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science, and Technology, the Japanese Government; and from the Molecular Medical Science Institute, Otsuka Pharmaceutical.
S.S. is employed by Asahi Kasei Corporation, which produced recombinant proteins of soluble human Notch ligands studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Takayuki Yamada, Division of Immunology, Department of Molecular and Cellular Biology, School of Life Science, Faculty of Medicine, Tottori University, Yonago, Tottori 683-8503, Japan; e-mail: yamad@grape.med.tottori-u.ac.jp.