Two patients with severe Crohn disease, defined by a Crohn Disease Activity Index (CDAI) higher than 250 despite anti–tumor necrosis factor α (TNF-α), were treated by intense immune suppression and autologous hematopoietic stem cell transplantation (HSCT). Stem cells were mobilized from the peripheral blood using cyclophosphamide (2.0 g/m2) and granulocyte colony-stimulating factor (G-CSF; 5 μg/kg/d), enriched ex vivo by CD34+ selection, and reinfused after immune conditioning with cyclophosphamide (200 mg/kg) and equine anti–thymocyte globulin (ATG; 90 mg/kg). Patients have remained in remission (CDAI < 100) for 1 year since HSCT. We conclude that further HSCT studies for severe Crohn disease appear warranted.
Introduction
Crohn disease (CD) is presumed to be an immune-mediated disease of the intestinal tract. It is associated with significant morbidity including fistulas, abscesses, eye manifestations, skin illnesses, arthritis, hepatobiliary complications, the need for recurrent surgery, severe abdominal pain with drug addiction, and eventually after multiple operations, a short-bowel syndrome requiring home parenteral nutrition. There is little in the literature available on mortality data related to CD, but a large series by Farmer et al showed a 6% mortality attributable to CD.1 The mortality rate for selective patients with refractory and severe disease is probably higher.
Crohn disease is treated by immune-modulating or immune-suppressive therapies. These treatments include local anti-inflammatory agents such as 5-aminosalicylic acid products; broad immune suppression such as corticosteroids, azathioprine, or methotrexate; cytokine suppression such as antibody to tumor necrosis factor alpha (TNF-α); and antibiotics such as ciprofloxacin and metronidazole, which might work by decreasing the putative antigen exposure to the intestine.
Study design
Patients signed informed consent under institutional review board– and United States Food and Drug Administration–approved study IDE 7846. Candidates for HSCT had to have failed corticosteroids, 5 aminosalicylic acid, metronidazole, azathioprine, and the TNF-α inhibitor infliximab. Failure being defined as a Crohn disease activity index (CDAI) higher than 250 with the patient being considered for surgical excision because of active disease. Patients' stem cells were mobilized by administration of cyclophosphamide (2.0 g/m2 intravenously) and granulocyte colony-stimulating factor (G-CSF; 5 μg/kg/d) beginning 48 hours after cyclophosphamide infusion. Stem cells were enriched by CD34+ selection using immunoselection (Isolex, Baxter, Chicago, IL). The immune suppressive regimen was cyclophosphamide (200 mg/kg; divided 50 mg/kg on days −5, −4, −3, and −2), and equine anti–thymocyte globulin (ATG; 90 mg/kg; divided 30 mg/kg on days −4, −3, and −2). Methylprednisolone (1.0 g) was administered 30 minutes prior to each dose of ATG.
From admission until discharge supportive care included fluconazole, ciprofloxacin, oral acyclovir, and metronidazole. When neutropenia developed, cefepime was added independent of fever. Leukocyte-depleted, irradiated, and cytomegalovirus (CMV)–safe blood products were administered to maintain hemoglobin levels higher than 80 g/L (8.0 g/dL) and platelet counts higher than 20 000/μL. For 6 months after HSCT, patients were placed on prophylactic fluconazole, acyclovir, and trimethaprim sulfamethoxazole.
Results and discussion
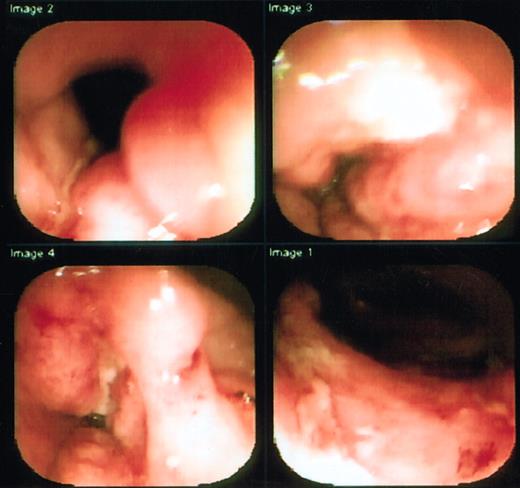
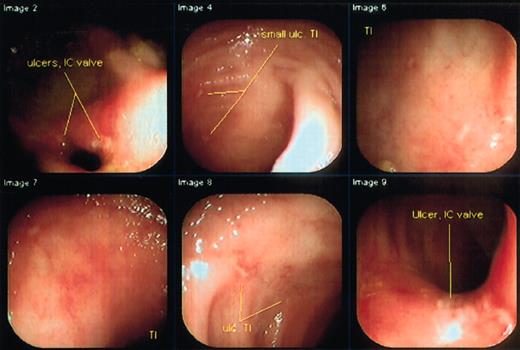
Two patients were treated. The first patient had continuous disease for 6 years prior to HSCT, the second, for 10 years. After mobilization, both patients continued to have active disease without significant change in diarrhea or pain. The first patient, a 22-year-old woman, had a CDAI of 308, and was extremely ill, addicted to opiates, and receiving 6-mercaptopurine (6-MP) and corticosteroids at time of entry. She had undergone prior resection of part of her ileum and colon and had been on total parenteral nutrition for 2 years. She was having 25 liquid stools per 24 hours, severe abdominal pain despite continuous infusion narcotics, arthralgias, anal fissures, and anal and vaginal fistulas. Physical examination was remarkable for tender vulvar edema, large tender perianal skin tags, and bilateral lower abdominal tenderness. Entry colonoscopy and small-bowel series revealed severe ileal and colonic ulcerating disease (Figure1). Following HSCT, she has entered a medication-free remission on a normal diet, although occasional diarrhea has occurred. A follow-up colonoscopy showed marked improvement, although there remained slight ulceration at the ileocecal valve area (Figure 2). She is no longer receiving corticosteroids or any therapy for Crohn disease, and her narcotics are being tapered through a methadone program (Table1).
Pretransplantation colonoscopy of first patient shows deep serpiginous ulcers in transverse colon.
The colonoscope was unable to transverse the inflammed and narrowed segment.
Pretransplantation colonoscopy of first patient shows deep serpiginous ulcers in transverse colon.
The colonoscope was unable to transverse the inflammed and narrowed segment.
Colonoscopy of the first patient shows marked improvement 6 months after transplantation.
Full colonoscopy was easily performed showing only a few superficial ulcerations in the area of the ileocecal valve.
Colonoscopy of the first patient shows marked improvement 6 months after transplantation.
Full colonoscopy was easily performed showing only a few superficial ulcerations in the area of the ileocecal valve.
The second HSCT was performed on a 16-year-old boy who had been continuously ill for 6 years. His CDAI was 267 prior to the transplantation. His colonoscopy showed severe disease especially in his transverse colon. He was maintained with tube feeding, methotrexate, 6-MP, and 5-aminosalicylic acid. Entry colonoscopy and small-bowel series revealed severe Crohn colitis, transverse colonic stricture, and multiple small-bowel skip areas of inflammation. Physical examination was remarkable for a midabdominal tender mass. Following HSCT, he has remained in an asymptomatic remission on no medications (Table 2). Pretransplantation transverse colon stricture and abdominal mass have both disappeared. He has returned to school full-time and is active in contact sports.
The duration of neutropenia (absolute neutrophil count [ANC] < 500/μL) was 7 and 8 days for the first and second patient, respectively. Both patients had culture-negative fever for 48 hours while neutropenic. The transplantations were otherwise uncomplicated with no unexpected toxicity. No late opportunistic infections have occurred.
Autologous HSCT has been suggested as a method to reintroduce tolerance based on successful treatment of some animal autoimmune diseases, and recent reports of clinical results for HSCT of autoimmune diseases such as systemic lupus erythematosus.2-5However, an autoimmune etiology for CD remains controversial and unproven. No intestinal self-antigen (initiating or spread epitope) has been identified. On the other hand, several animal gene knock-out models suggest that inflammatory bowel disease may be a result of immune dsyregulation between TH1 and TH2 cytokines. Deficiency of multiple TH2 cytokines may cause colitis. Interleukin-10 (IL-10)–deficient mice develop acute and chronic colitis.6 IL-2–deficient,7double-mutant IL-2– and IL-4–deficient, and transforming growth factor beta–deficient8 mice also develop colitis. When raised in a germ-free environment these gene knock-out mice remain disease free. Animal models, therefore, demonstrate the need for both cytokine imbalance and gut bacterial flora as disease triggers.
Histologically, CD generally manifests as a TH1 delayed-type hypersensitivity of the gut wall with granuloma formation.9 Failure to induce disease by immunization with intestinal autoantigens and murine models of cytokine gene knock-out dsyregulation suggests that CD is a TH1 immune-mediated imbalance perhaps caused by a delayed-type hypersensitivity against intestinal flora. High-dose immune ablative therapy and HSCT may reset the immune balance resulting in regeneration of a normal phenotype and prolonged disease remission.
There have been 6 patients with CD reported who underwent allogeneic bone marrow transplantation for other reasons.10 Of these patients, one was in remission at the time of the transplantation and remained in remission for 15 years in spite of discontinuation of immunosuppression. At the time of transplantation, 3 of the 5 with active CD went into remission for 6 to 10 years and remained in remission at the time of the report. The fourth had significant fistulous disease in the year following the transplantation, requiring ileal resection. The fifth died from sepsis 3 months following the transplantation. Another report of a patient with an allogeneic bone marrow transplantation described improvement in CD following the transplantation, at least for the short term.11 A patient with coincidental CD undergoing autologous bone marrow transplantation for non-Hodgkin lymphoma went into remission from both his lymphoma and CD for 7 years and remained in remission at the time of the report.12 Finally, a patient with incidental Crohn colitis who underwent an autologous bone marrow transplantation was reported to enter a symptomatic remission, although there remained colonic inflammation on colonoscopy.13
To our knowledge, this is the first report of HSCT being performed for the indication of CD. Both cases were severe and refractory to currently available therapies prior to transplantation. Both patients are now clinically asymptomatic off all therapies. It is unknown if a similar beneficial response could be obtained without infusion of stem cells or without purging or CD34+ selection of the reinfused graft. Whether persistent subclinical pathologic inflammation will gradually resolve or become clinically active in the future is also unknown. Although follow-up is short, the patients have demonstrated remarkable improvements, becoming medication- and nearly symptom-free for the first time since disease onset. Further studies of HSCT in refractory Crohn disease appear warranted.
Prepublished online as Blood First Edition Paper, October 10, 2002; DOI 10.1182/blood-2002-07-2122.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Richard K. Burt, Division of Immunotherapy, Northwestern University Medical School, 320 East Superior, Searle Bldg, Rm 3-489, Chicago, IL 60611; e-mail: rburt@nwu.edu.