Congenital blood disorders are common and yet clinically challenging globin disorders. Gene therapy continues to serve as a potential therapeutic method to treat these disorders. While tremendous advances have been made in vivo, gene delivery protocols and vector prototypes still require optimization. Alternativecis-acting promoter elements derived from VL30 retroelements have been effective in expressing tissue-specific transgene expression in vivo in nonerythroid cells. VL30 promoter elements were isolated from ELM-I-1 erythroid progenitor cells upon erythropoietin (epo) treatment. These promoters were inserted into a VL30-derived expression vector and reintroduced into the ELM-I-1 cells. β-Galactosidase reporter gene activity from the ELM 5 clone, a BVL-1–like VL30 promoter, was capable of expressing sustained levels of the transgene expression over a 16-week assay period. These findings delineate the potential utility of these retroelement promoters as transcriptionally active, erythroid-specific, long terminal repeat (LTR) components for current globin vector constructs.
Introduction
Retroviral and lentiviral model systems are close to being utilized for hemoglobinopathy human gene therapy trials.1 Incorporation of cis-acting regulatory components of the globin gene, including the locus control region (LCR), initially caused aberrant RNA processing. Modifications have allowed significant levels of recombinant globin gene expression to be achieved.2-4 Retroviral vectors using the promoter from the red cell membrane protein ankyrin have shown position-independent, copy number–dependent expression of the γ-globin gene in vivo.5 However, significant obstacles still face the contemporary constructs, including silencing regions within the vectors' long terminal repeat (LTR) regions and position effect variegation (PEV).6 Removal of silencing regions within the vectors' promoter (U3), repeat (R), and primer binding site (PBS) and insertion of insulators have not entirely prevented transcriptional silencing7 and PEV,6 respectively. Optimization of specific globin vector designs is requisite to allow for therapeutically beneficial and sustainable globin gene expression.
A variation on virally derived LTR vector components is the use of retrotransposon-based VL30 U3 promoter sequences. Mouse VL30s include more than 100 different promoter variations within the endogenous genome, have limited sequence homology to murine retroviruses,8 and are activated by transformation,9,10 growth factors,11,12steroids,13 and cytokines.14 VL30 promoters have been characterized for tissue-specific expression in vitro15,16 and in vivo.17 Hematopoietic VL30 elements14 and specialized erythropoietin (epo)–responsive erythroid progenitor cell lines have been probed.20 However, these clones have not been assessed in the context other than their native configuration. Therefore, VL30 promoter elements within a heterologous construct were reintroduced into erythroid progenitor cells. These expression assays determined that the ELM 5 (BVL-1–like) VL30 clone provided sustained reporter gene expression in erythroid progenitor cells in vitro.
Study design
VL30 promoter selections
Electroporation into ELM-I-1 erythroid progenitor cells
Linearized constructs with the novel promoters, ELM 5 and MEL/ELM, and control promoter, NVL3, within the LTRs of the VL30-derived VLIBAG cassette (17) and the retroviral vector RVBAG were electroporated into the ELM cells (10 × 106 to 20 × 106) using a voltage of 300 V, resistance of 13 Ω, and a capacitance of 200 F. Mass cell cultures of transfected cells were grown under selective G418 growth conditions (0.8 mg/mL).
X-gal staining of VLIBAG-transfected cells
Transfected cells were stained with X-gal (X-galactopyranoside 1 mg/mL in N,N,-dimethyl formamide) along with X-gal staining solution (20 mM K3Fe(CN)6; 20 mM K4Fe(CN)6 3H20; 1.5 mM MgCl2 in phosphate-buffered saline [PBS], pH 7.4) and incubated for 20 hours at 37°C.21
ONPG hydrolysis of cell lysates from stably transfected ELM cells
Transfected ELM cells (2.5 × 105 to 4 × 105/mL) were grown for 3 days in uninduced (0.55 U/mL) and epo-induced (10 U/mL) growth conditions in T75 Falcon flasks (Fisher, Pittsburgh, PA) for 3 days. Cell lysates were monitored through duplicate assays performed in triplicate. Using the β-Gal Assay Kit (Invitrogen, Carlsbad, CA), the specific activity of the cell lysates (nanomoles of o-nitrophenyl-β-D-galactopyranoside [ONPG] hydrolyzed per milligram of total protein) from each vector construct was calculated and normalized to the total protein concentration (Pierce, Rockford, IL). The uninduced VLIBAG vector's expression was defined from each time point as 1 nmol of ONPG hydrolyzed per milligram of protein in 30 minutes at 37°C.
Statistical analysis of expression assays
Raw data from each assay were assessed through a paired correlated groups Student t test using the InStat program (GraphPad Software, Version 3.01). Probability (P) values that were less than .05 were considered significant.
Results and discussion
VL30 promoter isolations
Degenerate VL30-specific primers were used to reverse transcribe (RT) a single-stranded 3′ U3 promoter complementary DNA (cDNA)20 (Figure 1A). Polymerase chain reaction (PCR) was used to synthesize and amplify double-stranded cDNA clones from MEL and ELM-I-1 cell RNA. VL30 promoter clones were identified and classified.20 The MEL/ELM clone was conserved between both erythroid progenitor cell lines and was grouped with members of the VL30 subgroup IV.21 The other 5 promoters were categorized in subgroup III.21 Clone ELM 5 showed homology (97%) to a previously identified VL30 element, BVL-1 (GenBank no. X17124),14,22and contained a putative erythropoietin-activated Jak2/STAT5 recognition site.23 Sequences of the ELM 5 and MEL/ELM clones encoded 3 GATA-1 sites20 and 2 B10 Ras-responsive elements.12 Confirmatory ribonuclease protection of the various VL30 promoters verified the expression of the isolated VL30 promoters.20
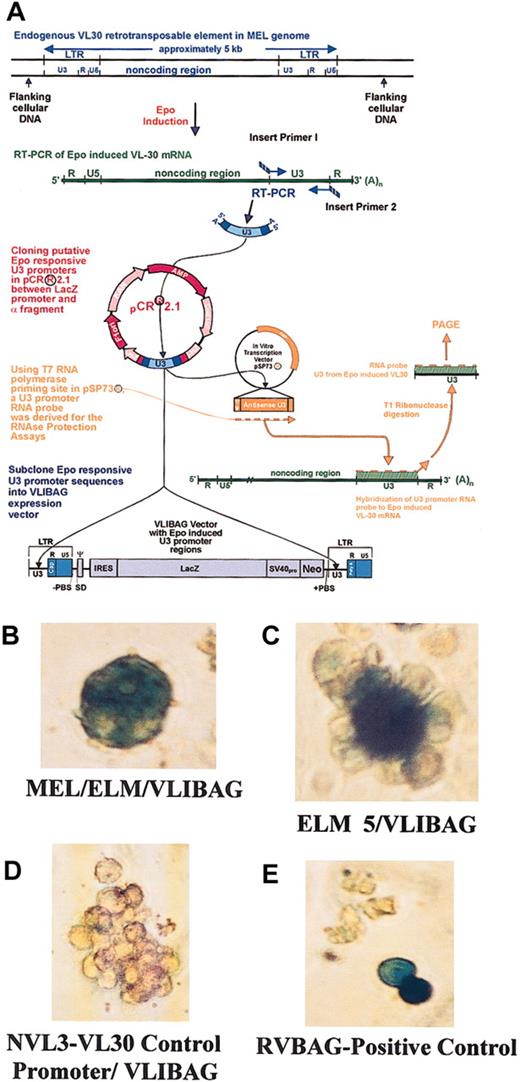
Endogenous ELM 5 and MEL/ELM promoters expressing reporter gene activity in transfected ELM cells.
ELM VL30 promoters were isolated, confirmed, and quantified for their epo responsiveness20 and cloned into the LTRs of the expression vector, VLIBAG (A). Mass cultures of ELM transfectants carrying the endogenously expressed promoters MEL/ELM (B) and ELM 5 (C) within the heterologous cassette, VLIBAG, were histochemically stained with X-gal after 2 weeks of selective growth conditions. The unexpressive negative control transfectant, VLIBAG, with the NVL3 promoter is shown (D) as well as the positive control RVBAG retroviral vector (E). Original magnification, × 200 (B-E).
Endogenous ELM 5 and MEL/ELM promoters expressing reporter gene activity in transfected ELM cells.
ELM VL30 promoters were isolated, confirmed, and quantified for their epo responsiveness20 and cloned into the LTRs of the expression vector, VLIBAG (A). Mass cultures of ELM transfectants carrying the endogenously expressed promoters MEL/ELM (B) and ELM 5 (C) within the heterologous cassette, VLIBAG, were histochemically stained with X-gal after 2 weeks of selective growth conditions. The unexpressive negative control transfectant, VLIBAG, with the NVL3 promoter is shown (D) as well as the positive control RVBAG retroviral vector (E). Original magnification, × 200 (B-E).
The VL30 promoters MEL/ELM and ELM 5 were cloned into the promoter regions of the VL30-derived expression vector, VLIBAG (GenBank no.AF062997), for electroporation into the ELM cells (Figure 1A). A fibroblast-specific VL30 promoter, NVL3,17,24 as well as the positive control, the retroviral vector RVBAG,25 were also electroporated into the ELM cells. Transfected cells were grown in selective media and initially stained with X-gal 2 weeks after transfection to confirm the constructs' reporter gene expression (Figure 1B-E). In contrast to the control NVL3 promoter, MEL/ELM and ELM 5 constructs showed insignificant reporter gene expression levels in transformed fibroblast cell lines (data not shown).
Transfected ELM cell ONPG hydrolysis assays
The transfected ELM cells were grown simultaneously under uninduced (0.5 U/mL epo) and epo-induced (10 U/mL epo) growth conditions. The specific activity for each of the transfected cells was monitored over 2-week intervals for 16 weeks.
The NVL3 control promoter in the VLIBAG construct showed little activity throughout the time-course assay (Figure2). The specific activity of the positive control vector, RVBAG, was greatest after 2 weeks in culture and showed minimal expression by the 12th week (Figure 2). ELM cells transfected with the novel MEL/ELM promoter showed significant β-galactosidase expression during the fourth and sixth weeks after transfection. However, this construct also expressed minimal activity by the 16th week (Figure 2). ELM 5–transfected cells showed sustained β-galactosidase activity (Figure 2), averaging 18.64 nmol of hydrolyzed ONPG in epo-induced growth conditions. Six of the 8 time points—week 4 (P = .0441), week 8 (P = .019), week 10 (P = .0086), week 12 (P = .0007), week 14 (P = .049), and week 16 (P = .037)—reflected a significant enhancement of reporter gene activity (Figure 2). Even though the epo induction was modest, 1.2- to 1.7-fold (data not shown), the ELM 5 promoter within the VL30 vector was able to direct sustained expression throughout the 16-week time course in either growth condition.
Beta-galactosidase (β-galactosidase)–specific activity of BVL-1–like, ELM 5 promoter shows sustained expression patterns in transfected ELM clones.
The VL30 promoters ELM 5, MEL/ELM, negative control NVL3 promoter within VLIBAG, and the positive retroviral control vector, RVBAG, were transfected into ELM-I-1 cells and monitored for β-galactosidase reporter gene activity through ONPG hydrolysis assays over a 16-week time course. β-Galactosidase reporter gene activity was monitored in cell lysates from all 4 constructs in epo-induced (10 U/mL epo) growth conditions. The transfected BVL-1–like ELM 5 vector showed sustained β-galactosidase activity and modest epo induction (*P < .05, n = 6 [error bars]) on weeks 4, 8, 10, 12, 14, and 16.
Beta-galactosidase (β-galactosidase)–specific activity of BVL-1–like, ELM 5 promoter shows sustained expression patterns in transfected ELM clones.
The VL30 promoters ELM 5, MEL/ELM, negative control NVL3 promoter within VLIBAG, and the positive retroviral control vector, RVBAG, were transfected into ELM-I-1 cells and monitored for β-galactosidase reporter gene activity through ONPG hydrolysis assays over a 16-week time course. β-Galactosidase reporter gene activity was monitored in cell lysates from all 4 constructs in epo-induced (10 U/mL epo) growth conditions. The transfected BVL-1–like ELM 5 vector showed sustained β-galactosidase activity and modest epo induction (*P < .05, n = 6 [error bars]) on weeks 4, 8, 10, 12, 14, and 16.
In summary, erythroid-specific VL30 promoters were isolated, confirmed for their apparent epo-responsive behavior,20 and cloned into an expression vector, VLIBAG. Reintroduction of the novel constructs into the ELM cells revealed that the ELM 5 (BVL-1–like) promoter was able to express itself consistently during the 16-week assay period. This promoter was able to direct sustainable expression levels throughout the time course assay and continued to express itself past 16 weeks in culture. The incorporation of this promoter within the flanking LTR regions of contemporary vectors may allow for sustained therapeutic levels of transgenic globin expression.
We thank James Grunkemeyer, Daniel Meehan, and Dominic Cosgrove from Boys Town National Research Hospital for providing the VLIBAG vector and technical assistance and thank Mike Hendrickson for assistance in preparing the figures for this document.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-07-2105.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joseph A. Knezetic, Department of Biomedical Sciences, Creighton University, 2500 California Plaza, Omaha, NE; e-mail: joek@creighton.edu.

![Fig. 2. Beta-galactosidase (β-galactosidase)–specific activity of BVL-1–like, ELM 5 promoter shows sustained expression patterns in transfected ELM clones. / The VL30 promoters ELM 5, MEL/ELM, negative control NVL3 promoter within VLIBAG, and the positive retroviral control vector, RVBAG, were transfected into ELM-I-1 cells and monitored for β-galactosidase reporter gene activity through ONPG hydrolysis assays over a 16-week time course. β-Galactosidase reporter gene activity was monitored in cell lysates from all 4 constructs in epo-induced (10 U/mL epo) growth conditions. The transfected BVL-1–like ELM 5 vector showed sustained β-galactosidase activity and modest epo induction (*P < .05, n = 6 [error bars]) on weeks 4, 8, 10, 12, 14, and 16.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/5/10.1182_blood-2002-07-2105/3/m_h80533928002.jpeg?Expires=1769939183&Signature=xqbCnjqLcBP1H6ZZdcDTnXDtyFCClU8~l6x3WLaglXTYAVordlLj4BQf0leCNDpyikU5i1WyBRDJgEZ~cspxjfZBHwvTtHPDRJkgb720GxC1Gv5~qjhPG8bGvRfe~mMdNIqpyeFmeGemq4n2xI~okHmK88xm2jgqJqjJmlOXnvXs~zTuJdGzPRB0ngF8wmqd3j7kJoR3d28AxVVL2TlE4tfZ9pdQiDbiYzubxPDodhRPu1gpDSGokYNEHh5jVqcc1u9qGyLPg3iJ5BvFTTSGSn26PVVXTghSKQpDln7ZPBFOnfTVs-7CUBLLJARj1o2Tx~0XBIX9lQSMcas4unjF2A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
