Neuropilin-1 (NP-1) is a receptor for vascular endothelial growth factor-165 (VEGF165) and acts as a coreceptor that enhances the function of VEGF165 through VEGF receptor-2 (VEGFR-2). Studies using transgenic and knock-out mice of NP-1 indicated that this molecule is important for vascular development as well as neuronal development. We recently reported that clustered soluble NP-1 phosphorylates VEGFR-2 on endothelial cells with a low dose of VEGF165 and rescues the defective vascularity of the NP-1−/− embryo in vitro and in vivo. Here we show that NP-1 is expressed by CD45+ hematopoietic cells in the fetal liver, can bind VEGF165, and phosphorylates VEGFR-2 on endothelial cells. CD45+NP-1+ cells rescued the defective vasculogenesis and angiogenesis in the NP-1−/− P-Sp (para-aortic splanchnopleural mesodermal region) culture, although CD45+NP-1− cells did not. Moreover, CD45+NP-1+ cells together with VEGF165 induced angiogenesis in an in vivo Matrigel assay and cornea neovascularization assay. The extracellular domain of NP-1 consists of “a,” “b,” and “c” domains, and it is known that the “a” and “c” domains are necessary for dimerization of NP-1. We found that both the “a” and “c” domains are essential for such rescue of defective vascularities in the NP-1 mutant. These results suggest that NP-1 enhances vasculogenesis and angiogenesis exogenously and that dimerization of NP-1 is important for enhancing vascular development. In NP-1−/− embryos, vascular sprouting is impaired at the central nervous system (CNS) and pericardium where VEGF is not abundant, indicating that NP-1–expressing cells are required for normal vascular development.
Introduction
Blood vessels are formed by 2 different steps called vasculogenesis (formation of new blood vessels de novo) and angiogenesis (branching of pre-existing blood vessels).1Over the last several years, a great deal of progress in defining vasculogenesis and angiogenesis has been made by isolating factors including a family of vascular endothelial growth factors (VEGFs), angiopoietins, and ephrins.2,3 The gene targeting strategy revealed that these factors including counter receptors are important molecules for the development of the vascular system. Among them, VEGFs and cognate receptor tyrosine kinases, tyrosine-kinase VEGF receptor-1 (VEGFR-1) and tyrosine-kinase VEGF receptor-2 (VEGFR-2), which are specifically expressed on the surface of endothelial cells (ECs), have been demonstrated to be required for both vasculogenesis and angiogenesis.4-7
The major components of a blood vessel are endothelial cells and mural cells such as smooth muscle cells and pericytes. Therefore, the interaction between endothelial cells and mural cells has been studied in an attempt to elucidate vascular development; however, hematopoietic cells have been suggested to be one of the regulators of the development of blood vessels. Recently, we reported that angiopoietin-1, a ligand for TIE2, is produced by hematopoietic stem cells and that it regulates the formation of the vascular network in the restricted region in vivo.8 Moreover, VEGF and placental growth factor (PlGF) expressed in erythroblasts have been suggested to be involved in blood vessel formation,9 and macrophages and mast cells have been reported to be involved in tumor angiogenesis.10 During embryogenesis and especially organogenesis, tissue-specific cells or cells other than hematopoietic cells may affect vascular development. Therefore, to gain an understanding of the molecular mechanism of vessel formation, it is important to analyze the interactions between various kinds of cells and endothelial cells.
Neuropilin-1 (NP-1) is unique among the many blood vessel–related proteins. NP-1 was initially described as a cell surface glycoprotein expressed on axons in the developing nervous system11 and was shown to be a receptor for semaphorin/collapsin,12,13a family of transmembrane and secreted glycoproteins that act as mediators of neuronal guidance.12,14 Extracellular domain of NP-1 is composed of complement (“a”, CUB), coagulation factor V/VIII (“b”), and MAM (“c”) homology subdomains. Secreted collapsin-1 (SemaIIIA) binds to NP-1 on axons, repels neurons, and induces the collapse of neuronal growth cones. Other than the neuronal system, NP-1 was identified as another VEGFR that binds the 165 amino acid form of VEGF (VEGF165) but not the 121 amino acid form of VEGF (VEGF121).15 When NP-1 is coexpressed on VEGFR-2+ ECs, the binding to VEGF165 and chemotactic activity by VEGF165 for these cells are enhanced compared with those of ECs expressing VEGFR-2 alone; this suggests that in ECs, NP-1 acts as a coreceptor for VEGFR-2. Moreover, SemaIIIA inhibits the motility of porcine aortic ECs depending on whether NP-1 is expressed by disrupting the formation of lamellipodia and inducing depolymerization of F-actin.16 VEGF165 and SemaIIIA are competitive inhibitors of each other in binding, in EC motility, and in the collapse assay of dorsal root ganglia,16suggesting that the 2 ligands have overlapping NP-1 binding sites, possibly the b/coagulation factor homology domain.17
Many studies have recently shown that modification of theNP-1 gene altered vascular development in vivo. Overexpression of the NP-1 gene in mice led to the formation of excess capillaries and blood vessels in addition to a malformed heart.18 Targeted disruption of NP-1 led to embryonic lethality and vascular defects such as impairment of neural vascularization, transposition of large vessels, and insufficient development of vascular networks in the yolk sac.19,20Furthermore, we previously demonstrated that soluble clustered NP-1 enhanced vasculogenesis and angiogenesis in vitro in the NP-1−/− P-Sp (para-aortic splanchnopleural mesodermal region) culture and in vivo in the NP-1−/−embryo.21 Phosphorylation of VEGFR-2 on ECs that had been sorted from an NP-1−/− embryo was enhanced by simultaneous application of VEGF165 and soluble NP-1, compared with that by application of VEGF165alone.21 It has been demonstrated that stromal cells,22 tumor cells,15 and mesenchymal cells18 express NP-1. These results suggest that the NP-1 on such cells acts on ECs exogenously. We found that CD45+hematopoietic cells in the murine fetal liver and adult bone marrow express NP-1. In this study, we show that the NP-1 on hematopoietic cells plays a key role in the development of endothelial cells and blood vessel formation.
Materials and methods
Animals
C57BL/6 mice were purchased from Japan SCL (Shizuoka, Japan). NP-1 heterozygous mutant mice19 and green fluorescence protein (GFP) transgenic mice (gift from Dr Masaru Okabe, Osaka University, Japan)23 were housed in environmentally controlled rooms of our facility at Kumamoto University School of Medicine under the guidelines of Kumamoto University for animal and recombinant DNA experiments. Genotype analysis of the neuropilin mutants was performed by polymerase chain reaction (PCR) as described by Kitsukawa et al.19
RT-PCR analysis
The RNeasy Mini kit (Qiagen, Hilden, Germany) was used for isolation of total RNA from whole embryos, CD45+ cells, or CD45− cells. Total RNA was reverse transcribed using the reverse transcription (RT) for PCR kit (Clontech, Palo Alto, CA). The cDNA was amplified using Advantage Polymerase Mix (Clontech) in a GeneAmp PCR system model 9700 (Perkin-Elmer, Norwalk, CT) by 30 cycles. The sequences of the gene-specific primers for RT-PCR were as follows: 5′-mNP-1 (ACTGACAGCGCAATAGCAAAAGAAG), 3′-mNP-1 (TCGGACAAATCGAGTATCAGTGGT), 5′-mVEGFR-1 (CTTCCTACAGCACAGCAGATGTGAA), 3′-mVEGFR-1 (CACGTTTACAATGAGAGTGGCAGTG), 5′-mVEGFR-2 (TACACAATTCAGAGCGATGTGTGGT), 3′-mVEGFR-2 (CTGGTTCCTCCAATGGGATATCTTC), 5′-mVEGF165 (CTTTACTGCTGTACCTTCACCATGC), 3′-mVEGF165 (AACAAGGCTCACAGTGATTTTCTGG), 5′-G3PDH (TGAAGGTCGGTGTGAACGGATTTGGC), and 3′-G3PDH (CATGTAGGCCATGAGGTCCACCAC). Each cycle consisted of denaturation at 94°C for 30 seconds and annealing/extension at 70°C for 4 minutes.
Immunohistochemistry
Immunohistochemical analyses on tissue sections and culture dishes were performed as previously described.24 The tissue fixation procedures were the same as those described by Yoshida et al.25 The fixed specimens were embedded in polyester wax and sectioned at 8 μm. An anti–PECAM-1 antibody (Pharmingen, San Diego, CA), anti-CD45 antibody (Pharmingen), anti–VEGFR-2 antibody (Pharmingen), anti–vascular endothelial–cadherin (anti–VE-cadherin) antibody (Pharmingen), and anti–NP-1 polyclonal antibody18 were used in this assay. In brief, anti–PECAM-1 antibody and anti-CD45 antibody were developed with horseradish peroxidase–conjugated antirat immunoglobulin G (IgG) antibody (Biosource, Camarillo, CA), and an anti–NP-1 polyclonal antibody was developed with horseradish peroxidase–conjugated antirabbit IgG antibody (Biosource). In the final step of staining, samples were soaked with phosphate-buffered saline (PBS) containing 250 μg/mL diaminobenzidine (Dojin Chemicals, Kumamoto, Japan) in the presence of 0.05% NiCl2 for 10 minutes, and then hydrogen peroxidase was added for the enzymatic reaction. Finally, the sections were observed and photographed under a microscope (IX-70, Olympus, Tokyo, Japan).
VEGF binding assay and flow cytometry
Fluorokine biotinylated VEGF (R&D Systems, Minneapolis, MN) was used for this binding assay. This assay was performed according to the protocol provided by R&D Systems. In brief, cells from E12.5 fetal liver were stained with a phycoerythrin (PE)–conjugated anti-CD45 monoclonal antibody (mAb) (Pharmingen) and an allophycocyanin (APC)–conjugated anti-B220 mAb (Pharmingen), and then CD45+B220+ cells were sorted using FACS Vantage (Becton Dickinson, San Jose, CA). A total of 10 μL biotinylated VEGF reagent was added to 25 μL of the CD45+B220+cells (4 × 106 cells per milliliter). As a negative control, an identical sample of cells was stained with 10 μL biotinylated negative control reagent. The cells were incubated for 60 minutes on ice. Then, 10 μL avidin–fluorescein isothiocyanate (FITC) reagent was added to each sample, and the reaction mixture was incubated for an additional 30 minutes on ice. After incubation, the cells were washed twice with 2 mL of 1 × RDF1 buffer (wash buffer) to remove unreacted avidin-FITC, and then the cells were resuspended in 0.2 mL of 1 × RDF1 buffer for flow cytometric analysis. The stained cells were analyzed and sorted using FACS Vantage. Total RNA from the sorted CD45+B220+VEGFR+ cells was isolated using the RNeasy Mini kit (Qiagen). Then, RT-PCR using various VEGFR-specific primers was performed as described above.
Cell preparation, immunoprecipitation, and immunoblotting
Whole-mount E12.5 embryos were dissociated using Dispase II (Boehringer Mannheim, Mannheim, Germany) and Collagenase S-1 (Nitta Gelatin, Osaka, Japan) and drawn through a 23-gauge needle. Cells were stained with anti–VEGFR-2–PE (Pharmingen) and anti–PECAM-1–biotin (Pharmingen). Biotinylated antibodies were visualized with APC-conjugated streptavidin (Pharmingen). Then, VEGFR-2+PECAM-1+ ECs were sorted using FACS Vantage. Sorted VEGFR-2+PECAM-1+ cells were cultured on OP9 cells in 10% fetal calf serum (FCS) (JRH Bioscience, Lenexa, KS) and 10−5 M 2-Mercaptoethanol (2-ME) (GIBCO, Gaithersburg, MD) containing RPMI 1640 (GIBCO) with 5 ng/mL VEGF (PeproTech, London, United Kingdom) and with or without CD45+NP-1+ cells and VEGFR-2–Fc fusion protein. After culture for 10 days, cells were stained with an anti–PECAM-1 antibody (Pharmingen) to visualize the existence of ECs. Aggregates consisting of more than 10 cells expressing PECAM-1 were counted as a cluster.
For immunoprecipitation and immunoblotting, sorted VEGFR-2+PECAM-1+ cells were cultured on a fibronectin (FN)–coated dish in 10% FCS supplemented with 10 ng/mL VEGF (PeproTech). After 7 days, culturing cells were washed twice with PBS and incubated in culture in serum-free medium for 12 hour before stimulation. CD45+ cells from E12.5 fetal liver of NP-1 wild and mutant embryos were incubated with VEGF and/or NP-1 Flag, semaphorin IIIA, neutralizing VEGF antibody (Oncogene, Boston, MA) for 30 minutes on ice and then were washed twice with PBS to remove unreacted VEGF. Then, VEGFR-2+PECAM-1+ ECs were stimulated with CD45+NP-1+ cells or CD45+NP-1− cells for 10 minutes at 37°C. The cells were solubilized with lysis buffer (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4; 137 mM NaCl; 5 mM EDTA [ethylenediaminetetraacetic acid]; 1 mM Na3VO4, 1% Triton X-100; and protease inhibitors). Immunoprecipitation and immunoblotting were performed as previously described.26
Production of recombinant fusion protein
Recombinant fusion proteins of the extracellular domain of murine surface molecules and human IgG (Fc fragment) and Flag epitope were designed. The VEGFR-2–Fc and NP-1 Flag were produced by COS7 cells in serum-free conditioned medium as previously described21 and purified over a protein A column (Bio-Rad, Richmond, CA) and anti-Flag M2 column (Scientific Imaging System; Eastman Kodak, Rochester, NY). This purity was assessed by Coomassie brilliant blue staining of 7.5% sodium dodecyl sulfate (SDS) gels.
Production of recombinant semaphorin IIIA
Construction of semaphorin IIIA expression vector (pCAG-semIIIA) was previously described.18 In brief, the full length of semaphorin IIIA cDNA was ligated into the EcoRI site of the COS cell expression vector pCAGGS. COS cells were transfected with pCAG-semIIIA using LipofectAMINE (GIBCO) and cultured in Dulbecco modified Eagle medium (DMEM) containing 5% FCS (JRH Bioscience) for 4 days at 37°C. Then culture supernatant was collected.
In vitro culture of P-Sp
The stromal cell line, OP9,27 was maintained in α-modified minimum essential media (α-MEM) (GIBCO) supplemented with 20% FCS (JRH Bioscience). Explants of E9.5 P-Sp containing a part of the omphalomesenteric artery (OA) were cultured on OP9 stromal cells in 10% FCS and 10−5M (2-ME) (GIBCO) containing RPMI 1640 (GIBCO). After culture for 14 days, an anti–PECAM-1 antibody (Pharmingen) was used to visualize the existence of ECs.
Quantitative analysis of vascular areas
The method of immunohistochemistry in the P-Sp culture using PECAM-1 antibodies was performed as described above. After PECAM-1 immunohistochemical staining, images were integrated using a color chilled 3CCD camera (Hamamatsu Photonics, Shizuoka, Japan). Image processing software (NIH image 1.62/Power Macintosh G3: National Institutes of Health, Bethesda, MD) was used to determine alterations in the size of vascular areas. Three vascular areas from each P-Sp explant were measured under each culture condition. The values of all parameters are shown as the mean and standard deviation. Pvalues were calculated by 2-tailed Student t test analysis.
In vivo neovascularization using Matrigel
Preparation, injection, and processing of Matrigel (Becton Dickinson) were performed as described by Passaniti et al28 with some modifications. Briefly, 8-week-old C57BL mice were injected subcutaneously with 0.5 mL Matrigel and 40 units of heparin per milliliter (Sigma), 20 ng/mL VEGF (PeproTech), and 5 × 104 CD45+B220+ cells from the fetal liver of E12.5 NP-1 wild or mutant embryos. Five days later, the mice were killed and Matrigel plugs were removed, weighed, and processed for histology or hemoglobin concentration determination. For histologic analysis, plugs were fixed by 4% paraformaldehyde and embedded in polyester wax and sectioned at 8 μm. Then sections were stained with anti–PECAM-1 antibody (Pharmingen) or anti–VEGFR-2 antibody (Pharmingen). For hemoglobin determination, which correlates with the number of blood vessels, plugs were homogenized in 1 mL distilled water. Hemoglobin concentration was determined by the Drabkin method using a Drabkin reagent kit (Sigma).
Mouse cornea neovascularization assay
The corneal assay was performed as previously described.29 30 In brief, under sterile conditions, slow-release pellets were prepared incorporating VEGF (PeproTech) alone, PBS alone, VEGF+CD45+NP-1+ cells, and VEGF+CD45+NP-1− cells into a casting solution of ethynil-vinyl copolymer (Elvax-40, DuPont, Wilmington, DE) in 10% methylene chloride. After anesthesia with sodium pentobarbital (Dainippon Pharmaceutical, Osaka, Japan), pellets were implanted into the corneal micropocket in male 8-week-old C57BL/6 mice. The corneas of all mice were routinely examined by slitlamp biomicroscopy on postoperative days 5 or 6 after pellet implantation. After taking photographs, organs were excised, embedded in polyester wax, and sectioned at 10 μm. Staining of sections was performed as described above using anti–PECAM-1 antibody (Pharmingen), anti–VEGFR-2 antibody (Pharmingen), or anti–VE-cadherin antibody (Pharmingen).
Expression of mutant neuropilin-1 proteins in L cells
L cells transfected with the cDNA of various mouse NP-1 mutants were produced as described previously.31 In brief, the myc-tag sequence, GGEQKLISEEDL, was introduced in such cDNA as follows. An XbaI site was added to the 3′-end of the coding region of NP-1 by PCR, and then the XbaI-myc-tag-stop codon adapter was ligated. In all mutant NP-1 cDNAs, the signal sequence was retained intact. To isolate cells that stably expressed truncated NP-1, L cells, a mouse fibroblastic cell line, were cotransfected with the truncated NP-1 cDNAs and pST-neoB32 according to the calcium phosphate method and selected with Geneticin (GIBCO).
Results
NP-1 expression on hematopoietic cells
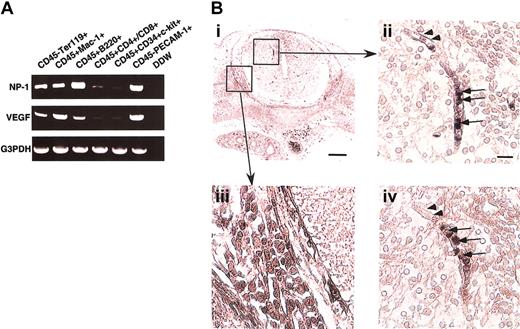
To determine whether hematopoietic cells (HCs) express NP-1, several fractions of CD45+ cells from murine fetal liver at E12.5 were sorted, and the expression of NP-1 and VEGF165mRNA was examined by RT-PCR. In the fetal liver, high NP-1 expression was detected in CD45+B220+ B lymphocytes, CD45+Mac-1+ monocytes, and CD45−Ter119+ erythrocytes. In contrast, NP-1 was less expressed in CD45+CD4+/CD8+ T lymphocytes and CD45+CD34+c-kit+ stem cells (Figure1A). In the case of VEGF165expression, high VEGF165 expression was detected in CD45+Mac-1+ monocytes and CD45−Ter119+ erythrocytes as previously reported.9 Next, we investigated whether nonendothelial cells expressing NP-1 were located at the site of vascular sprouting, focusing on the central nervous system (CNS) where vascular defects had been observed in NP-1−/− embryos. In the CNS, round cells in the lumen expressed NP-1 (Figure 1Bii; arrows), and these round cells were in the hematopoietic lineage as confirmed by their expression of CD45 in the serial sections (Figure 1Biv; arrows). ECs (arrowheads in Figure 1Bii) and neuronal cells in dorsal root ganglia (DRG; Figure 1Bi,iii) were also NP-1+ as previously reported.19 21
NP-1 expression on hematopoietic cells.
(A) RT-PCR analysis of NP-1 and VEGF-A expression in various fractions of hematopoietic cells from the fetal liver. High NP-1 expression was detected in CD45+B220+ and CD45+Mac-1+ cells; however, very low NP-1 expression was detected in the fraction of stem cells marked as CD45+CD34+c-kit+ cells. RNA from CD45–PECAM-1+ ECs was used for positive control. (B) Histologic analysis of NP-1 expression on hematopoietic cells in embryo. (i) Transversal section of spinal cord stained with anti–NP-1 antibody. Boxes are viewed in higher power in panels ii and iii. (ii) Round cells in the lumen of a vessel (arrows) and ECs lining the vessel (arrowheads) are positively stained with anti–NP-1 antibody. (iii) Neuronal cells in the DRG are positively stained with anti–NP-1 antibody. (iv) Serial section of panel ii stained with anti-CD45 antibody. Round cells in the vessel as observed in panel ii are positively stained with anti-CD45 antibody. Positively stained cells are visualized as dark blue products. Isotype-matched control Ig for anti-CD45 or anti–NP-1 antibody did not show any staining. Scale bar indicates 50 μm (i,iii); 20 μm (ii,iv).
NP-1 expression on hematopoietic cells.
(A) RT-PCR analysis of NP-1 and VEGF-A expression in various fractions of hematopoietic cells from the fetal liver. High NP-1 expression was detected in CD45+B220+ and CD45+Mac-1+ cells; however, very low NP-1 expression was detected in the fraction of stem cells marked as CD45+CD34+c-kit+ cells. RNA from CD45–PECAM-1+ ECs was used for positive control. (B) Histologic analysis of NP-1 expression on hematopoietic cells in embryo. (i) Transversal section of spinal cord stained with anti–NP-1 antibody. Boxes are viewed in higher power in panels ii and iii. (ii) Round cells in the lumen of a vessel (arrows) and ECs lining the vessel (arrowheads) are positively stained with anti–NP-1 antibody. (iii) Neuronal cells in the DRG are positively stained with anti–NP-1 antibody. (iv) Serial section of panel ii stained with anti-CD45 antibody. Round cells in the vessel as observed in panel ii are positively stained with anti-CD45 antibody. Positively stained cells are visualized as dark blue products. Isotype-matched control Ig for anti-CD45 or anti–NP-1 antibody did not show any staining. Scale bar indicates 50 μm (i,iii); 20 μm (ii,iv).
NP-1 on hematopoietic cells binds VEGF165 and induces phosphorylation of VEGFR-2 on ECs
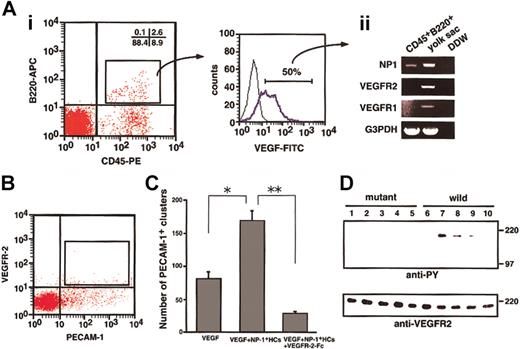
To determine whether NP-1 on CD45+ HCs binds VEGF165, we performed flow cytometric analysis. As shown in Figure 1, a portion of the CD45+ HCs expressed NP-1. It was previously reported that monocytes such as Mac-1+ cells express VEGFR-1.33 34 According to our analysis, CD45+B220+ HCs did not express VEGFR-1 or VEGFR-2 (data not shown). Therefore, in the binding assay for VEGF165, we used CD45+B220+ cells so that we could study the binding of VEGF165 to NP-1 other than VEGFR-1 and VEGFR-2. First, we obtained CD45+B220+ cells (2.6%, Figure 2Ai) from fetal livers and incubated the cells with biotinylated VEGF165. The binding of VEGF165 to cells was visualized by staining with streptavidin-FITC (Figure 2Ai). Interestingly, most CD45+B220+ cells were bound to VEGF165. We isolated these VEGF165receptor-positive cells (approximately 50% of VEGF-binding cells, as shown in Figure 2Ai), and the mRNA from these cells was analyzed for the expression of VEGF receptors by RT-PCR. As expected, CD45+B220+ cells expressed NP-1 (Figure 2Aii) but did not express other VEGF165 receptors. Therefore, we concluded that CD45+B220+ B lymphocytes express NP-1, which binds with VEGF165.
To examine whether NP-1 on CD45+ HCs directly stimulates VEGFR-2 on ECs, we sorted VEGFR-2+PECAM-1+ ECs from E12.5 embryos (Figure 2B) and cultured them on OP9 cells in the presence or absence of VEGF, NP-1+ HCs, and VEGFR-2–Fc. After 10 days of culturing, cultured cells were fixed and stained with anti–PECAM-1 antibody to examine the proliferation of ECs. VEGFR-2+PECAM-1+ ECs from embryos formed vascular structure (data not shown) in the presence of 5 ng/mL VEGF. On addition of CD45+ HCs from fetal liver of wild-type embryos to this culture, the number of PECAM-1+ EC clusters significantly increased (Figure 2C). This effect was completely abolished by addition of VEGFR-2–Fc (Figure 2C). CD45+cells from NP1 mutant embryos did not enhance the proliferation of ECs in the presence of 5 ng/mL VEGF (data not shown). These findings indicate that CD45+NP-1+ HCs enhance the proliferation of individual ECs through VEGFR-2.
CD45+ cells isolated from the fetal liver bind VEGF165 and phosphorylate VEGFR-2 on ECs.
(A) Cells obtained from an E12.5 fetal liver were stained with anti-CD45–PE and anti-B220–APC and analyzed by flow cytometry. Some cells (2.6%) were positively stained by both antibodies (i), and sorted CD45+B220+ cells were then stained with VEGF-biotin and streptavidin-FITC. Many of the cells shifted to the right (blue line; positive cells). Fifty percent (50%) of the cells over the negative gate (i; right histogram) expressed NP-1 alone among VEGF165 receptors (ii). (B) E12.5 embryos were dissociated and stained with PE-conjugated anti–PECAM-1 and biotin-conjugated anti–VEGFR-2 mAbs. Biotin was developed to avidin-allophycocyanin. PECAM-1+VEGFR-2+ cells indicated by the box were sorted by using FACS Vantage. (C) PECAM-1+VEGFR-2+ cells were cultured with OP9 cells in the presence or absence of VEGF, NP-1+ HCs, and VEGFR-2–Fc. The number of PECAM-1+ endothelial clusters was scored. *P, **P < .05. (D) Cell lysates of PECAM-1+VEGFR-2+ cells that had been stimulated by various factors or cells were immunoprecipitated with anti–VEGFR-2 antibody and then subjected to Western blotting using an antiphosphotyrosine mAb (anti-PY). The PECAM-1+VEGFR-2+ cells were incubated with the CD45+ cells from fetal livers of NP-1 mutant (lanes 1-5) or wild-type (lanes 6-10) embryos with or without the indicated factors in each lane; no factor (lanes 1, 6); 10 ng/mL VEGF (lanes 2, 7); 10 ng/mL VEGF plus 20 μg/mL NP-1 Flag (lanes 3, 8); 10 ng/mL VEGF plus 300 ng/mL SemaIIIA (lanes 4, 9); 10 ng/mL VEGF plus 1 μg/mL anti-VEGF neutralizing antibody (lanes 5, 10). Phosphorylation of VEGFR-2 was induced by the addition of CD45+NP1+ cells mixed with 10 ng/mL VEGF (lane 7), and it was specifically blocked by NP-1 Flag or SemaIIIA or anti-VEGF neutralizing antibody (lanes 8-10). The addition of CD45+NP1− cells mixed with VEGF barely induced phosphorylation of VEGFR-2 on ECs (lane 2). “wild” indicates cells from the wild type, and “mutant” indicates cells from the mutant embryo of NP-1 from the same litter.
CD45+ cells isolated from the fetal liver bind VEGF165 and phosphorylate VEGFR-2 on ECs.
(A) Cells obtained from an E12.5 fetal liver were stained with anti-CD45–PE and anti-B220–APC and analyzed by flow cytometry. Some cells (2.6%) were positively stained by both antibodies (i), and sorted CD45+B220+ cells were then stained with VEGF-biotin and streptavidin-FITC. Many of the cells shifted to the right (blue line; positive cells). Fifty percent (50%) of the cells over the negative gate (i; right histogram) expressed NP-1 alone among VEGF165 receptors (ii). (B) E12.5 embryos were dissociated and stained with PE-conjugated anti–PECAM-1 and biotin-conjugated anti–VEGFR-2 mAbs. Biotin was developed to avidin-allophycocyanin. PECAM-1+VEGFR-2+ cells indicated by the box were sorted by using FACS Vantage. (C) PECAM-1+VEGFR-2+ cells were cultured with OP9 cells in the presence or absence of VEGF, NP-1+ HCs, and VEGFR-2–Fc. The number of PECAM-1+ endothelial clusters was scored. *P, **P < .05. (D) Cell lysates of PECAM-1+VEGFR-2+ cells that had been stimulated by various factors or cells were immunoprecipitated with anti–VEGFR-2 antibody and then subjected to Western blotting using an antiphosphotyrosine mAb (anti-PY). The PECAM-1+VEGFR-2+ cells were incubated with the CD45+ cells from fetal livers of NP-1 mutant (lanes 1-5) or wild-type (lanes 6-10) embryos with or without the indicated factors in each lane; no factor (lanes 1, 6); 10 ng/mL VEGF (lanes 2, 7); 10 ng/mL VEGF plus 20 μg/mL NP-1 Flag (lanes 3, 8); 10 ng/mL VEGF plus 300 ng/mL SemaIIIA (lanes 4, 9); 10 ng/mL VEGF plus 1 μg/mL anti-VEGF neutralizing antibody (lanes 5, 10). Phosphorylation of VEGFR-2 was induced by the addition of CD45+NP1+ cells mixed with 10 ng/mL VEGF (lane 7), and it was specifically blocked by NP-1 Flag or SemaIIIA or anti-VEGF neutralizing antibody (lanes 8-10). The addition of CD45+NP1− cells mixed with VEGF barely induced phosphorylation of VEGFR-2 on ECs (lane 2). “wild” indicates cells from the wild type, and “mutant” indicates cells from the mutant embryo of NP-1 from the same litter.
To examine the role of NP-1 on CD45+ in the activation of VEGFR-2, we studied the phosphorylation of VEGFR-2 on ECs. For this experiment, we sorted VEGFR-2+PECAM-1+ ECs from E12.5 embryos (Figure 2B) and cultured on a fibronectin-coated dish. After 7 days of culturing, ECs were starved under serum-free condition for 12 hours and subsequently challenged with CD45+hematopoietic cells mixed with VEGF165 in the presence or absence of an NP-1 Flag or semaphorin IIIA or anti-VEGF neutralizing antibody. Cell lysates were immunoprecipitated with an anti–VEGFR-2 antibody and then subjected to Western blotting using an antiphosphotyrosine mAb (4G10). Phosphorylation of VEGFR-2 was induced by adding CD45+NP1+ cells mixed with VEGF165, and it was specifically blocked by the NP-1 Flag or semaphorin IIIA or neutralizing VEGF antibody, although the addition of CD45+NP1− cells mixed with the same amount of VEGF as above did not induce phosphorylation of VEGFR-2 on ECs (Figure 2D). We confirmed that the mRNA expression of angiogenic factors such as VEGFs and angiopoietin-1 and -2 did not differ between the CD45+NP1+ cells and CD45+NP1− cells (data not shown). Therefore, we concluded that NP-1 on hematopoietic cells together with VEGF165 activates VEGFR-2 on ECs exogenously.
Hematopoietic cells expressing NP-1 induce vascular development in in vitro P-Sp culture
We previously reported that soluble clustered NP-1 enhanced vascular development.21 Because some CD45+cells expressed NP-1, we hypothesized that CD45+ cells work as soluble NP-1 receptors, because hematopoietic cells can freely move through the vessels. To address this hypothesis, we used the P-Sp culture system.24 As previously reported, this P-Sp culture system supports the simultaneous growth of ECs and hematopoietic cells.24 Especially in the case of ECs, the process of sheet formation occurs first (vascular bed), and subsequently the spreading ECs form a network (vascular network). We found that inhibition of VEGF signaling in this culture system using soluble Flk-1/VEGFR-2 led to complete lack of EC development,24 inhibition of angiopoietin signaling in this culture system using soluble TIE2 led to suppress the network formation,24 and addition of an excess amount of VEGF in the culture enhanced sheet formation.21 In the case of hematopoietic cells, the hematopoietic stem cells that develop first on the sheet formation of ECs migrate into the network area of ECs and proliferate and differentiate on the network area.24Recently, we reported the critical role of angiopoietin-1 produced from hematopoietic stem cells in promoting the formation of this network.8 Therefore, if the NP-1 on hematopoietic cells works like exogenously added soluble NP-1, such hematopoietic cells should rescue the defective vascularity observed in the P-Sp culture of NP-1−/− embryos. Therefore, we tested whether CD45+ cells expressing NP-1 could rescue the defective vascular formation in the culture of NP-1−/− P-Sp explants.
In NP-1−/− embryos, formation of both the vascular bed and network was defective (Figure 3Ai). The addition of CD45+NP-1+ cells from the fetal liver of E12.5 green mice expressing GFP ubiquitously (5 × 103 cells per well) rescued the defective formation of the vascular bed and network (Figure 3Aii). The exogenously added hematopoietic cells did not differentiate to endothelial cells as confirmed by their morphology (Figure 3Aiv). On the other hand, the addition of CD45+cells from the fetal liver of E12.5 NP-1−/− embryos did not effectively rescue the defective vascular formation (Figure 3Aiii). Quantitative analyses show that vascular areas were increased in the presence of CD45+NP-1+ cells (Figure 3B).
Effect of hematopoietic cells expressing NP-1 on vascular development in the P-Sp culture.
(A) The development of ECs in P-Sp cultures from E9.5 NP-1−/− embryos. Culture plates were fixed after 14 days of culture and stained with anti–PECAM-1 mAb. PECAM-1+ cells are visualized as dark blue products. (i) In mutant embryo, formation of the vascular bed and network is defective. (ii) The addition of CD45+ cells from the fetal liver of E12.5 GFP embryo (5 × 103 cells per well), which are also positive for NP-1, rescued the defective formation of the vascular bed (vb) and network (vn); however, these cells did not differentiate to endothelial cells as confirmed by their morphology (iv). (iii) The addition of CD45+ cells from the fetal liver of E12.5 NP-1−/− embryo did not rescue the defective vascular formation. (iv) Localization of GFP+ HCs in panel ii. Formation of vb and vn observed in panel ii is comparable to that observed in the culture using P-Sp explants from wild-type embryo. Scale bar indicates 200 μm. (B) Comparison of the area of the vascular bed. The area of the vascular beds in the images in panel A was determined by NIH image 1.62 software. The vascular area per explant is as follows: (i) 0.3 ± 0.1 mm2; (ii) 20.5 ± 3.2 mm2; (iii) 3.5 ± 0.5 mm2. Each result was obtained from 3 independent experiments and is expressed as the mean ± SD.
Effect of hematopoietic cells expressing NP-1 on vascular development in the P-Sp culture.
(A) The development of ECs in P-Sp cultures from E9.5 NP-1−/− embryos. Culture plates were fixed after 14 days of culture and stained with anti–PECAM-1 mAb. PECAM-1+ cells are visualized as dark blue products. (i) In mutant embryo, formation of the vascular bed and network is defective. (ii) The addition of CD45+ cells from the fetal liver of E12.5 GFP embryo (5 × 103 cells per well), which are also positive for NP-1, rescued the defective formation of the vascular bed (vb) and network (vn); however, these cells did not differentiate to endothelial cells as confirmed by their morphology (iv). (iii) The addition of CD45+ cells from the fetal liver of E12.5 NP-1−/− embryo did not rescue the defective vascular formation. (iv) Localization of GFP+ HCs in panel ii. Formation of vb and vn observed in panel ii is comparable to that observed in the culture using P-Sp explants from wild-type embryo. Scale bar indicates 200 μm. (B) Comparison of the area of the vascular bed. The area of the vascular beds in the images in panel A was determined by NIH image 1.62 software. The vascular area per explant is as follows: (i) 0.3 ± 0.1 mm2; (ii) 20.5 ± 3.2 mm2; (iii) 3.5 ± 0.5 mm2. Each result was obtained from 3 independent experiments and is expressed as the mean ± SD.
Hematopoietic cells expressing NP-1 induce angiogenesis in in vivo Matrigel assay
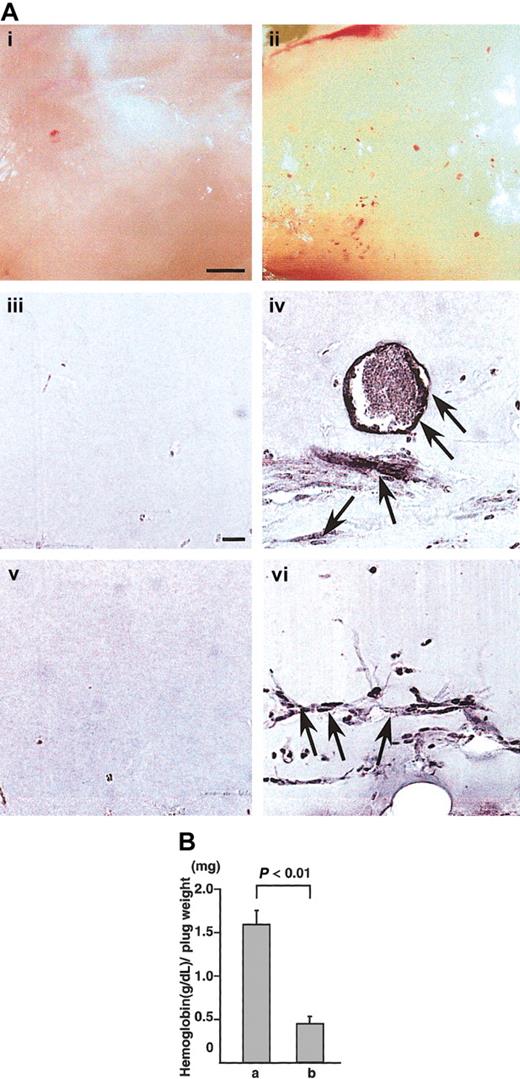
To confirm whether CD45+ hematopoietic cells induce angiogenesis in vivo, we used the Matrigel assay system28 and cornea neovascularization assay system,29,30 which have been established as a method for evaluating angiogenesis in vivo.28 In Matrigel assay, appearance of Matrigel containing CD45+B220+cells from the E12.5 fetal liver of wild-type mice together with VEGF165 was yellow (Figure 4Aii). In the section, PECAM-1+ (Figure 4Aiv) or VEGFR-2+ (Figure 4Avi) ECs formed blood vessels in the Matrigel. On the other hand, appearance of Matrigel containing CD45+B220+cells from NP1 mutants together with VEGF165 was white (Figure 4Ai). In the section, we did not detect PECAM-1+(Figure 4Aiii) or VEGFR-2+ (Figure 4Av) ECs. Figure 4B shows the hemoglobin content normalized to the weight of the Matrigel plugs. As previously reported,28 the hemoglobin content correlates with the number of blood vessels in the plugs. By this method, CD45+NP-1+ cells with VEGF165 induced significantly more blood vessels in the Matrigel plugs compared with CD45+NP-1− cells with VEGF165.
Hematopoietic cells expressing NP-1 induce angiogenesis in in vivo Matrigel assay.
(A) Matrigels containing CD45+B220+ cells from E12.5 fetal liver of wild type (ii,iv,vi) or CD45+B220+ cells from E12.5 fetal liver of NP-1 mutants (i,iii,v) were injected subcutaneously near the abdominal midline of 8-week-old C57BL mice. (i-ii) Gross appearance of Matrigels on day 5. (iii-vi) Histologic analysis of sections from the Matrigels. ECs were visualized by the staining with anti–PECAM-1 antibody (iii-iv) or anti–VEGFR-2 antibody (v,vi). Scale bar indicates 500 μm (i-ii) and 50 μm (iii-vi). Arrows indicate newly formed vessels in the Matrigel plugs. (B) Values represent the concentration of hemoglobin (g/dL) per Matrigel plug weight (mg) ± SE for 6 assays. “a” is CD45+ NP-1+ cells with VEGF165; “b,” CD45+ NP-1–cells with VEGF165.
Hematopoietic cells expressing NP-1 induce angiogenesis in in vivo Matrigel assay.
(A) Matrigels containing CD45+B220+ cells from E12.5 fetal liver of wild type (ii,iv,vi) or CD45+B220+ cells from E12.5 fetal liver of NP-1 mutants (i,iii,v) were injected subcutaneously near the abdominal midline of 8-week-old C57BL mice. (i-ii) Gross appearance of Matrigels on day 5. (iii-vi) Histologic analysis of sections from the Matrigels. ECs were visualized by the staining with anti–PECAM-1 antibody (iii-iv) or anti–VEGFR-2 antibody (v,vi). Scale bar indicates 500 μm (i-ii) and 50 μm (iii-vi). Arrows indicate newly formed vessels in the Matrigel plugs. (B) Values represent the concentration of hemoglobin (g/dL) per Matrigel plug weight (mg) ± SE for 6 assays. “a” is CD45+ NP-1+ cells with VEGF165; “b,” CD45+ NP-1–cells with VEGF165.
Hematopoietic cells expressing NP-1 induce angiogenesis in corneal neovascularization assay
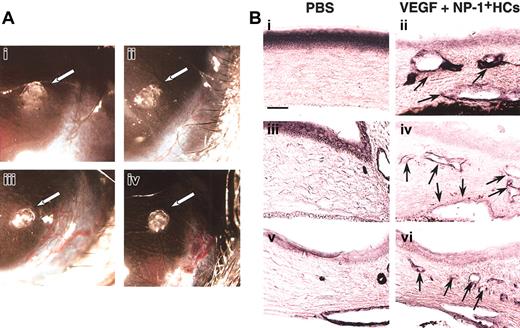
For further confirmation of the effect of CD45+NP-1+ HCs in vivo for vascular formation, we used corneal neovascularization assay. In this assay (Figure5), CD45+ cells from the E12.5 fetal liver of wild-type embryos together with a low dose of VEGF (20 ng/mL) induced neovascularization (Figure 5Aiii) as well as a high dose of VEGF (100 ng/mL) as a positive control (Figure 5Aiv). On the other hand, pellet alone or CD45+ cells from the E12.5 fetal liver of NP-1 mutants together with a low dose of VEGF (20 ng/mL) did not induce neovascularization (Figure 5Ai-ii). In the section, we confirmed that pellet containing a low dose of VEGF and CD45+ cells from wild-type embryos induced blood vessels composed of PECAM-1+ (Figure 5Bii), VEGFR-2+(Figure 5Biv), or VE-cadherin+ (Figure 5Bvi) ECs in cornea and that pellet containing PBS alone did not (Figure 5Bi,iii,v).
Hematopoietic cells expressing NP-1 induce angiogenesis in corneal neovascularization assay.
(A) Gross appearance of neovascularization in the cornea. Pellets containing control buffer (i) or a low-dose VEGF (20 ng/mL) with CD45+NP-1− hematopoietic cells (ii) did not induce corneal neovascularization; on the other hand, pellets containing a low-dose VEGF with CD45+NP-1+hematopoietic cells (iii) or high-dose VEGF (100 ng/mL) (iv) induced corneal neovascularization. (B) Histologic analysis in mouse cornea. Sections were stained with anti–PECAM-1 (i-ii), anti–VEGFR-2 (iii-iv), or anti–VE-cadherin (v-vi) antibody, and positive cells are visualized as dark blue products. Pellets containing a low dose of VEGF and CD45+ cells (ii,iv,vi) induced the blood vessel formation composing with PECAM-1+ (ii), VEGFR-2+ (iv), or VE-cadherin+ (vi) ECs into cornea; however, pellets containing PBS alone did not (i,iii,v). Arrows indicate newly formed vessels into cornea. Scale bar indicates 50 μm.
Hematopoietic cells expressing NP-1 induce angiogenesis in corneal neovascularization assay.
(A) Gross appearance of neovascularization in the cornea. Pellets containing control buffer (i) or a low-dose VEGF (20 ng/mL) with CD45+NP-1− hematopoietic cells (ii) did not induce corneal neovascularization; on the other hand, pellets containing a low-dose VEGF with CD45+NP-1+hematopoietic cells (iii) or high-dose VEGF (100 ng/mL) (iv) induced corneal neovascularization. (B) Histologic analysis in mouse cornea. Sections were stained with anti–PECAM-1 (i-ii), anti–VEGFR-2 (iii-iv), or anti–VE-cadherin (v-vi) antibody, and positive cells are visualized as dark blue products. Pellets containing a low dose of VEGF and CD45+ cells (ii,iv,vi) induced the blood vessel formation composing with PECAM-1+ (ii), VEGFR-2+ (iv), or VE-cadherin+ (vi) ECs into cornea; however, pellets containing PBS alone did not (i,iii,v). Arrows indicate newly formed vessels into cornea. Scale bar indicates 50 μm.
In these 2 types of in vivo analyses, induction of angiogenesis by CD45+ cells with a low dose of VEGF was almost completely suppressed by simultaneous addition of soluble NP-1 Flag, SemaIIIA, or neutralizing antibody against VEGF (data not shown).
Dimerization of neuropilin-1 is important for inducing angiogenesis
Previously we reported that the dimer of soluble NP-1 induces the phosphorylation of VEGFR-2 and enhanced vascular development, although the monomer of soluble NP-1 did not.21 It is known that the “a” and “c” domains of the NP-1 protein are required for dimerization of NP-1.31We previously constructed L cells that expressed various portions of the extracellular domain of NP-1.31 Upon the addition of L cells that possessed only the “a” domain (data not shown) or “a” and “b” domains of the NP-1 protein (Figure 6A-C) to the NP-1−/− P-Sp culture (2 × 103 cells per well) after 4 days of starting culture, the defective vascularity was not rescued at either the areas adherent to (Figure 6B) or the areas nonadherent to the L cells (Figure 6C). On the other hand, upon the addition of L cells that possessed the “a,” “b,” and “c” domains (Figure 6D-F) to the NP-1−/− P-Sp culture (2 × 103 cells per well) at the same schedule as above, the defective vascularity was rescued at the area adherent to the L cells (Figure 6E); however, it was not rescued at the areas that were nonadherent to the L cells (Figure 6F).
Dimerization of NP-1 is important for inducing endothelial cell growth.
ECs developed in P-Sp cultures were stained with anti–PECAM-1 mAb. PECAM-1+ cells are visualized as dark blue products. Upon the addition of L cells that possessed only the “a” domain (data not shown) or “a” and “b” domains of the NP-1 protein (A-C) to an NP-1−/− P-Sp culture (2 × 103 cells per well) on the fourth P-Sp culture day, the defective vascularity was not rescued at either the areas that were adherent to the L cells (red dashed line [B]) or the areas that were not adherent to the L cells (C). Small numbers of ECs make cordlike structures, indicated by arrow in panels B and C. Panels B and C are higher magnifications of the areas indicated by the boxes in panel A. On the other hand, upon the addition of L cells that possessed the “a,” “b,” and “c” domains (D-F) to an NP-1−/− P-Sp culture (2 × 103 cells per well) on the fourth P-Sp culture day, the defective vascularity was rescued at the areas that were adherent to the L cells (red dashed line [E]); however, it was not rescued at the areas that were not adherent to the L cells (F). Sheetlike structures of ECs (white dashed line) and network formation of ECs (yellow dashed line) are observed (E). Panels E and F are higher magnifications of the areas indicated by the boxes in panel D. Scale bar indicates 400 μm (A,D) and 200 μm (B-C,E-F).
Dimerization of NP-1 is important for inducing endothelial cell growth.
ECs developed in P-Sp cultures were stained with anti–PECAM-1 mAb. PECAM-1+ cells are visualized as dark blue products. Upon the addition of L cells that possessed only the “a” domain (data not shown) or “a” and “b” domains of the NP-1 protein (A-C) to an NP-1−/− P-Sp culture (2 × 103 cells per well) on the fourth P-Sp culture day, the defective vascularity was not rescued at either the areas that were adherent to the L cells (red dashed line [B]) or the areas that were not adherent to the L cells (C). Small numbers of ECs make cordlike structures, indicated by arrow in panels B and C. Panels B and C are higher magnifications of the areas indicated by the boxes in panel A. On the other hand, upon the addition of L cells that possessed the “a,” “b,” and “c” domains (D-F) to an NP-1−/− P-Sp culture (2 × 103 cells per well) on the fourth P-Sp culture day, the defective vascularity was rescued at the areas that were adherent to the L cells (red dashed line [E]); however, it was not rescued at the areas that were not adherent to the L cells (F). Sheetlike structures of ECs (white dashed line) and network formation of ECs (yellow dashed line) are observed (E). Panels E and F are higher magnifications of the areas indicated by the boxes in panel D. Scale bar indicates 400 μm (A,D) and 200 μm (B-C,E-F).
These results indicated that dimerization of NP-1 on the cell surface is important for inducing angiogenesis at the site where NP-1+ cells and ECs colocalize.
Discussion
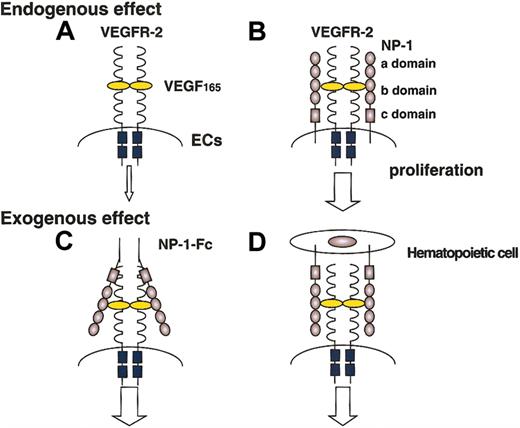
In this report, we found that the NP-1 expressed on hematopoietic cells regulates vascular development by exogenously affecting the stimulation of VEGFR-2. Moreover, we found that this kind of exogenous effect was exerted not only by hematopoietic cells but also by stromal cells (data not shown) and L cells (fibroblastic cell lines). Previously we reported that the addition of the soluble form of clustered NP-1 with a low dose of VEGF165 effectively induced phosphorylation of VEGFR-2 compared with the addition of a low dose of VEGF165 alone and that the defective vasculature of NP-1 mutant mice was rescued by injection of soluble clustered NP-1 in utero.21 Taking together our present report and previous findings, we confirmed that NP-1 on hematopoietic cells other than endothelial cells can induce vascular development by working as a soluble clustered NP-1 (Figure 7).
Effect of NP-1 expressed on ECs and non-ECs on the proliferation of ECs.
The proliferation of ECs expressing NP-1 together with VEGFR-2 (B) is enhanced compared with that of ECs expressing VEGFR-2 alone (A). Soluble clustered NP-1 effectively enhances the signaling of VEGFR-2 (C). These findings suggest that hematopoietic cells expressing NP-1 also enhance the signaling of VEGFR-2 on ECs (D).
Effect of NP-1 expressed on ECs and non-ECs on the proliferation of ECs.
The proliferation of ECs expressing NP-1 together with VEGFR-2 (B) is enhanced compared with that of ECs expressing VEGFR-2 alone (A). Soluble clustered NP-1 effectively enhances the signaling of VEGFR-2 (C). These findings suggest that hematopoietic cells expressing NP-1 also enhance the signaling of VEGFR-2 on ECs (D).
Previously, we reported the biologic differences between the monomer and dimer types of soluble NP-1.21 Monomer-type NP-1 bound and saturated VEGF165 and inhibited VEGF-VEGFR signaling. On the other hand, dimer-type NP-1 enhanced VEGF165-mediated VEGFR-2 phosphorylation. This biologic effect corresponds to that of the physiological soluble NP-1. Naturally occurring soluble NP-1 contains only the “a” and “b” domains of NP-1 and lacks the “c” domain.35 Because the “a” and “c” domains of NP-1 have been suggested to be important for dimerization,31 such natural soluble NP-1 exists as a monomer form. In this case, such spliced NP-1 has been suggested to inhibit tumor angiogenesis by saturating VEGF.36 Our analyses with the in vivo Matrigel and cornea neovascularization assay as well as the in vitro P-Sp culture clearly showed that exogenous NP-1 on hematopoietic cells enhanced vascular development. However, it was unclear whether NP-1 on hematopoietic cells had to undergo dimerization to enhance vascular development. Therefore, we generated several sublines of L cells harboring mutated NP-1, such as NP-1 with the “a” domain alone, NP-1 with the “a” and “b” domains, and NP-1 with the “a”, “b”, and “c” domains, and then added the cells into a P-Sp culture of an NP-1 mutant. The results clearly showed that only the dimer form of exogenous NP-1 on the cell surface could rescue the defective vasculature of the NP-1 mutant. Although it is not clarified whether NP-1 on hematopoietic cells is always undergoing dimerization, we may conclude that at least the dimer form of NP-1 on the cell surface can exogenously stimulate VEGFR-2 on endothelial cells. Moreover, we could not deny the possibility that hematopoietic cells release the dimer form of soluble NP-1 by proteolytic cleavage. However, we could not detect soluble NP-1 in the culture supernatant of hematopoietic cells (data not shown). Therefore, it seems that membrane-bound NP-1 on hematopoietic cells stimulates VEGFR2 on ECs.
In this study, we used the B220+ B-lymphocyte lineage for the analysis of binding with VEGF165 and phosphorylation of VEGFR-2. The reason we selected this lineage is that this lineage expresses NP-1 but does not express other VEGF receptors, which was confirmed by RT-PCR analysis. If we had used a myeloid lineage (Mac-1+) or erythroid lineage (Ter119+), the abundant endogenous production of VEGF might alter the phosphorylation of VEGFR-2. However, such myeloid and erythroid cells also express NP-1 on their surface (Figure 1A); therefore, they might be able to effectively support vascular development through an autocrine loop in vivo.
The other question that we have to address is the effect of NP-1 on hematopoiesis, because NP-1 expression is observed in mature hematopoietic lineages other than hematopoietic stem cell populations during embryogenesis. The number of hematopoietic cells and maturation of hematopoietic cells in the fetal liver of NP-1 mutant embryos, however, did not differ from those in the wild-type embryos (data not shown). Moreover, we analyzed the role of NP-1 in hematopoiesis using hematopoietic cells from wild-type and NP-1 mutant embryos in an in vitro colony assay (culture colony-forming unit [CFU-c]), and we did not detect a large difference in the number of CFU-c or any difference in lineage commitment (data not shown). Therefore, we concluded that the NP-1 on hematopoietic cells does not alter hematopoiesis, at least during embryogenesis. Recently, it was shown that NP-1 was expressed by human dendritic cells (DCs) and T cells both in vitro and in vivo.37 The initial contact between DCs and T cells led to NP-1 polarization on T cells and induced proliferation of T cells. In our experiments, abundant expression of NP-1 was detected in CD45+B220+ cells (B-cell fraction); on the other hand, T cells (CD45+CD4+/CD8+) in fetal liver did not express NP-1 highly. By contrast, in adult bone marrow, abundant expression of NP-1 was detected in T-cell fractions (data not shown). Therefore, correlation between expression and function of NP-1 on T and B cells should be addressed in the future.
It has been reported that the mesenchymal cells surrounding ECs18 and stromal cells22 also express NP-1. Moreover, such mesenchymal cells are one of the major sources of VEGF production. This indicates that mesenchymal cells that coexpress VEGF and NP-1 enhance vascular development locally. This hypothesis is supported by 2 analyses reported by Kitsukawa et al18 and the present results. When NP-1 was overexpressed under the transcriptional control of the actin promoter, excess capillaries and blood vessels were observed in such transgenic mice.18 Our study also showed that ectopic overexpression of NP-1 on OP9 stromal cells enhanced vascular development in P-Sp culture (data not shown). Taken together, the mesenchymal cells surrounding a blood vessel are one of the key regulators of normal vascular development. However, our discovery that the NP-1 on cells other than ECs has the capacity to induce vascular development came from in vitro findings. Therefore, it is not clear whether this effect of NP-1 is actually involved in normal vascular development. The best way to observe the function of NP-1 on nonendothelial lineages, especially hematopoietic cells, is to construct and analyze the vascularity in mice harboring anNP-1 gene with a mutation in hematopoietic cells alone using a promoter gene that is specifically expressed in hematopoietic cells. However, such promoter gene is not currently available; therefore, instead of gene targeting, we are currently planning to transplant NP-1 null hematopoietic cells from NP-1−/− fetal liver into irradiated recipient mice. Using these chimeric mice having NP-1−/− hematopoietic cells, we will study whether NP-1 on hematopoietic cells contributes to new vessel formation under physiological conditions such as wound healing and ontogeny of tumor.
Recently, double NP-1/NP-2 knock-out mice have been reported to show more severe defects of vascular formation than other NP-1 or NP-1 single knock-outs.38 Although in this study we did not examine the role of NP-2 in VEGF-induced angiogenesis, a recent report showed that VEGF-induced cellular response was lacking in ECs expressing NP-2 alone.39 In addition, NP-2 seems to have a minor contribution in embryonic vasculogenesis and angiogenesis, because NP-2 expression is not detected in the heart or capillaries of embryo40 and NP-2 knock-out mice are viable and fertile.41 Moreover, in NP-1 knock-out mice, the NP-2 expression was not compensated in hematopoietic cells (data not shown). However, the precise role of NP-2 in angiogenesis is still unknown. Therefore, effects for vascular formation by hematopoietic cells from single NP-1 or NP-2 knock-out mice or double NP-1/NP-2 mutant mice should be compared in the future. Finally, we suggest that induction of the NP-1 gene in particular locations might be a useful gene therapy in concert with VEGF administration for ischemic diseases.
The authors thank Drs Hiroaki Kodama and Masaru Okabe for providing us with OP9 stromal cells and GFP transgenic mice, respectively. We also thank Dr Shin-Ichiro Hayashi for critical technical advice.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-01-0119.
Supported by Grants-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, and Culture of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nobuyuki Takakura, Department of Stem Cell Biology, Cancer Research Institute, Kanazawa University, Takaramachi13-1, Kanazawa 920-0934, Japan; e-mail:ntakaku@kenroku.kanazawa-u.ac.jp.


![Fig. 6. Dimerization of NP-1 is important for inducing endothelial cell growth. / ECs developed in P-Sp cultures were stained with anti–PECAM-1 mAb. PECAM-1+ cells are visualized as dark blue products. Upon the addition of L cells that possessed only the “a” domain (data not shown) or “a” and “b” domains of the NP-1 protein (A-C) to an NP-1−/− P-Sp culture (2 × 103 cells per well) on the fourth P-Sp culture day, the defective vascularity was not rescued at either the areas that were adherent to the L cells (red dashed line [B]) or the areas that were not adherent to the L cells (C). Small numbers of ECs make cordlike structures, indicated by arrow in panels B and C. Panels B and C are higher magnifications of the areas indicated by the boxes in panel A. On the other hand, upon the addition of L cells that possessed the “a,” “b,” and “c” domains (D-F) to an NP-1−/− P-Sp culture (2 × 103 cells per well) on the fourth P-Sp culture day, the defective vascularity was rescued at the areas that were adherent to the L cells (red dashed line [E]); however, it was not rescued at the areas that were not adherent to the L cells (F). Sheetlike structures of ECs (white dashed line) and network formation of ECs (yellow dashed line) are observed (E). Panels E and F are higher magnifications of the areas indicated by the boxes in panel D. Scale bar indicates 400 μm (A,D) and 200 μm (B-C,E-F).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/5/10.1182_blood-2002-01-0119/3/m_h80533936006.jpeg?Expires=1769743404&Signature=IzeQMAzOnwMHK0WlSW7iqYhoKIdw1rQbadFKtlFuN3bo9fgzjWRfiQQ6oqkHJwH5osW7zTqNWa0Dnr8862o8AJY-Ygf79xdpEaBn0y4NCEB5dXWXPnPFFv2IRwczi4X8D8uqA2r9FVkCM6TXKyxisKrbOHXF5eFeE-4g1NyJ49GdZ2cIS6Ix4GKzLIUiwNhoMaXMO~748qpow4mp1Yx7tmwOTIRP-CxGZYE4R3CMWN7iV1DOkcOmbM2IZDGURdwFFWh~aqBlbPm69T59c3SAL4tugx8tYqiZKYYHcLGlgSBL9K~ahhUaY8cwewOkFneV0f4qdb~qx9MGxwFAbdTapA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal