Adult angiogenesis, associated with pathologic conditions, is often accompanied by the formation of a fibrinous exudate. This temporary matrix consists mainly of fibrin but is intermingled with plasma proteins and collagen fibers. The formation of capillary structures in a fibrinous matrix in vivo was mimicked by an in vitro model, in which human microvascular endothelial cells (hMVECs) seeded on top of a fibrin-10% collagen matrix form capillarylike tubular structures after stimulation with basic fibroblast growth factor/tumor necrosis factor α (bFGF/TNF-α) or vascular endothelial growth factor (VEGF)/TNF-α. In the fibrin-collagen matrix the metalloproteinase inhibitor BB94 inhibited tubule formation by 70% to 80%. Simultaneous inhibition of plasmin and metalloproteinases by aprotinin and BB94 caused a nearly complete inhibition of tubule formation. Adenoviral transduction of tissue inhibitor of metalloproteinases 1 (TIMP-1) and TIMP-3 into endothelial cells revealed that TIMP-3 markedly inhibited angiogenesis, whereas TIMP-1 had only a minor effect. Immunohistochemical analysis showed the presence of matrix metalloproteinase 1 (MMP-1), MMP-2, and membrane-type 1 (MT1)–MMP, whereas MMP-9 was absent. The endothelial production of these MMPs was confirmed by antigen assays and real-time polymerase chain reaction (PCR). MT1-MMP mRNA was markedly increased in endothelial cells under conditions that induced tubular structures. The presence of MMP-1, MMP-2, and MT1-MMP was also demonstrated in vivo in the newly formed vessels of a recanalized arterial mural thrombus. These data suggest that MMPs, in particular MT-MMPs, play a pivotal role in the formation of capillarylike tubular structures in a collagen-containing fibrin matrix in vitro and may be involved in angiogenesis in a fibrinous exudate in vivo.
Introduction
Angiogenesis in the adult is associated with specific conditions such as tissue ischemia and wound repair, in which the formation of new blood vessels is temporally and spatially controlled. Tissue injury causes damage of blood vessels and the extravasation of plasma proteins, including fibrinogen, which results in the formation of a fibrin clot in the surrounding interstitium. This extracellular matrix of extravasated fibrin entangled with the existing collagen fibers is furthermore composed of a number of proteins such as vitronectin, fibronectin, laminin, hyaluronic acid, and proteoglycans. The fibrinous matrix formed serves as a provisional matrix, into which cells can infiltrate during the subsequent wound healing.1-3
During the formation of a granulation tissue, a dynamic interaction between microvascular endothelial cells (MVECs) and the surrounding extracellular matrix occurs. The cells disrupt existing cell-matrix interactions and locally degrade the surrounding extracellular matrix; they migrate, proliferate, and form new capillarylike tubular structures, which become stabilized in the course of time.4,5 The temporary fibrin matrix can be degraded by plasmin, which is activated from its zymogen by 2 types of plasminogen activators, tissue-type plasminogen activator (t-PA) and urokinase-type plasminogen activator (u-PA).6 The activity of u-PA is directed to the cell surface by a cellular u-PA receptor (u-PAR).7 In vitro8,9 and in vivo10,11 studies have shown that the u-PA/plasmin system plays a critical role in the process of angiogenesis. In addition to the u-PA/plasmin system, matrix metalloproteinases (MMPs) are also involved in the degradation of the extracellular matrix.10-15
A role for both u-PA/plasmin and MMP systems in angiogenesis is supported by the fact that different components in the extracellular matrix are substrates for plasmin as well as for MMPs, including fibrin,16 vitronectin, fibronectin, laminin, gelatins, and proteoglycans, whereas plasmin is unable to degrade collagens.17 Furthermore, endothelial cells at the leading edge of a new blood vessel concomitantly express components of both protease systems,18-20 and their expression is regulated by the same growth factors and cytokines.21-24
MMPs are a family of zinc-dependent enzymes that can be divided into 2 structurally distinct groups, the secreted MMPs and the membrane-type MMPs (MT-MMPs). The MMPs are secreted as inactive zymogens, and in vitro studies have shown that the u-PA/plasmin system located at the cell membrane directly or indirectly activates a number of pro-MMPs, such as pro–MMP-1, pro–MMP-3, pro–MMP-9, pro–MMP-10, and pro–MMP-13.25,26 In addition to zymogen activation, MMP activity is regulated by tissue inhibitors of metalloproteinases (TIMPs), of which to date 4 types have been characterized.27
MT-MMPs, of which 6 types are known, are transmembrane proteins that are activated intracellularly in the secretory pathway by furinlike enzymes.28-30 MT1-MMP is present as an active enzyme on the cell surface where it focuses matrix degradation closely to the cell membrane. MT1-MMP has a direct activity against different extracellular matrix (ECM) proteins,14,29including laminin, fibronectin, vitronectin, collagens, and fibrin. MT1-MMP can activate pro–MMP-2 and pro–MMP-13 at the cell surface. This surface-linked activation mechanism, which is comparable to that of u-PA activation at the u-PAR, localizes MMP activity to the pericellular area. The conversion of latent MMP-2 to active MMP-2 by MT1-MMP can be inhibited by TIMP-2 and TIMP-3, but not by TIMP-1 and TIMP-4.31
A complex interrelationship between the uPA/plasmin and MMP system is not only suggested by the fact that MMPs depend on the uPA/plasmin system for their activation, but also by the fact that MMPs can also control plasminogen activation by u-PA/ u-PAR at the cell surface.32 This suggests that impairment of one protease system influences the activity of the other system.
The formation of new microvessels in vivo can be mimicked in vitro by a model composed of human MVECs (hMVECs) seeded on top of a 3-dimensional matrix and stimulated by a growth factor (basic fibroblast growth factor [bFGF] or vascular endothelial growth factor [VEGF165]) and the cytokine tumor necrosis factor α (TNF-α) to form capillarylike tubular structures.33 In vitro studies using a purified fibrin matrix showed that the formation of capillarylike tubular structures is critically dependent on fibrin degradation initiated by cell-surface localized u-PA and plasminogen activities,34 whereas the contribution of MMPs seems to be of minor importance in this matrix.35 The role of the u-PA/plasmin system may be overemphasized as compared with the MMP system by the use of purified fibrin matrices. A fibrinous exudate in tissue is also intermingled with collagen fibers of the interstitial tissue. Therefore, a fibrin-collagen matrix may be more representative of a fibrinous exudate in pathophysiologic conditions. Here, we studied the involvement of u-PA/plasmin and MMP activities in the formation of capillarylike tubular structures in a fibrin matrix that contained 10% type collagen. We found that MMPs, in particular MT-MMPs, play a major role in this process, with an additional role for the u-PA/plasmin system. Localization studies confirmed the presence of MT1-MMP and several other MMPs in capillarylike structures in vitro and in newly formed vessels in a recanalized mural thrombus in vivo.
Materials and methods
Materials
Penicillin/streptomycin, L-glutamine, and medium M199 with phenol red, Earle balanced salt solution, L-glutamine, and HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) were obtained from Biowitthaker (Verviers, Belgium). Trypsin 1-300 370 USP/mg from ICN (Costa Mesa, CA) and heat-inactivated newborn calf serum from GIBCO BRL (Paisley, Scotland) were used. Human serum was prepared from pooled fresh blood of 10 to 20 healthy donors obtained from a local blood bank. Fibronectin was a gift from Dr J. van Mourik (Central Laboratory of the Blood Transfusion Service, Amsterdam, the Netherlands). A crude preparation of endothelial cell growth factor was prepared from bovine brain.36 Thrombin and heparin (UFH) were obtained from Leo Pharmaceutical Products (Weesp, the Netherlands) and tissue culture plastics from Costar (Cambridge, MA). Human fibrinogen was obtained from Chromogenix AB (Mölndal, Sweden). Human fibrinogen depleted from plasminogen was obtained from Kordia (Leiden, the Netherlands). TNF-α, containing 2.45 × 107 U/mg protein and less than 40 ng lipopolysaccharide per mg protein, was from Dr J. Tavernier (Biogent, Gent, Belgium). Recombinant human bFGF and VEGF165were purchased from PeproTech (Rocky Hill, NJ). Polyclonal antibodies against u-PA, t-PA, and MMP-14 were produced in our laboratory. MMP-1, MMP-2, and MMP-3 antibodies were from Biotrend (Cologne, Germany). An inhibiting mouse monoclonal antibody against the u-PA binding region of u-PAR was a kind gift from Dr H. Weidle (Roche, Mannheim, Germany). Horseradish peroxidase (HRP) conjugates of goat-antirabbit immunoglobulin G (IgG), rabbit-antimouse IgG, and rabbit–anti-CD-31 IgG and rabbit-antihuman fibrin(ogen) were from DAKO (Glostrup, Denmark). Aprotinin was purchased from Pentapharm (Basel, Switzerland), pyrogen-free human serum albumin was from CLB (Amsterdam, the Netherlands), and BB94 was a kind gift from Dr E. A. Bone (British Biotech, Oxford, United Kingdom). Collagen was purchased from Collagen Corporation (Palo Alto, CA).
Cell culture
Human foreskin microvascular endothelial cells (hMVECs) were isolated, cultured, and characterized as previously described.37 Cells were cultured until confluence at 5% CO2/95% air on fibronectin-coated dishes in M199 supplemented with 2 mM L-glutamine, 20 mM HEPES (pH 7.3), 10% heat-inactivated human serum, 10% heat-inactivated newborn calf serum, 150 μg/mL crude endothelial cell growth factor (ECGF), 100 IU/mL penicillin, and 100 mg/mL streptomycin. The endothelial cells were then detached with trypsin/EDTA (ethylenediaminetetraacetic acid) and transferred to new fibronectin-coated dishes at a split ratio of 1:3. Confluent endothelial cells were used at passage 9 to 11.
Formation of capillarylike tubular structure in a 3-dimensional matrix
Three-dimensional matrices were prepared by the addition of 100 μL of a mixture of 0.1 U/mL thrombin and 2 mg/mL fibrinogen or a mixture of collagen (10%) and fibrinogen (90%) solution in a 0.32-cm2 well of 96-well plates. After polymerization the matrices were equilibrated with 0.5 mL M199 containing 10% human serum and 10% newborn calf serum to inactivate residual thrombin. In plasminogen-free conditions plasminogen-free fibrinogen was used, and plasminogen from the sera was removed by equilibration with lysine-Sepharose.
After preparation of the matrices, confluent endothelial cells (0.7 × 105 cells/cm2) were detached from the fibronectin-coated dishes with trypsin/EDTA. The cell suspension was seeded in the culture media on the matrices in a confluent density. After 24 hours the medium was replaced with medium containing different mediators. Every 48 hours the medium was changed and collected, and this was done for a time period of 4 to 6 days. The formation of tubular structures of endothelial cells by invasion into the underlying matrix was analyzed by phase contrast microscopy. The total length of the structures formed was measured in 4 randomly chosen microscopic fields (7.3 mm2/field) by a computer equipped with Optimas image analysis software (Bioscan, Edmonds, WA) connected to a monochrome CCD camera (MX5) and was expressed as millimeter per centimeter squared.33
Histology and immunostaining
Fibrin matrices and human mural thrombi were fixed at 4°C for 3 hours in 2% p-formaldehyde in phosphate-buffered saline (PBS; pH = 7.4) and were embedded in paraffin. The material was cut in sections of 4 μm. All other handling steps and staining occurred as previously described.34 Polyclonal antibodies for MMP-1, MMP-2, MMP-9, MT1-MMP, and fibrin(ogen) and a monoclonal CD31 antibody were used as first antibody. Horseradish peroxidase-conjugated rat-antimouse or goat-antirabbit (1:300) was used as a second antibody, and the sections were counterstained with Mayer hematoxylin.
Assay of MMPs
MMP antigen levels in conditioned media were measured by using commercially available immunoassay kits from Amersham for MMP-1, MMP-2, and MMP-9.
MT1-MMP was determined in cell extracts by the MT1-MMP activity assay (Biotrak; Amersham Biosciences, Buckinghamshire, United Kingdom). To that end, confluent MVECs were washed with cold PBS and lysed on ice for 15 minutes in MMP assay buffer supplemented with 0.25% (vol/vol) Triton X-100 (extraction buffer). The MT1-MMP was captured by a specific antihuman–MT1-MMP antibody, and the activity of MT1-MMP was measured by using modified urokinase as a substrate, and recombinant MT1-MMP was used as a standard.
Real-time polymerase chain reaction (PCR) of MT1-MMP mRNA was performed according to the Taqman method of Applied Biosystems (Perkin Elmer) by using a combination of a forward and backward primer and a specific (6-carboxy-fluorescein/6-carboxy-tetramethyl-rhodamine [FAM/TAMRA]) double-labeled probe. The amplicon lies between nucleotides 861 and 944 in the coding region of the MT1-MMP mRNA. Results were corrected for glyceraldehyde phosphate dehydrogenase (GAPDH) expression. The following sequences were used for MT1-MMP (MMP14): forward primer, 5′-TGGAGGAGACACCCACTTTGA-3′; reverse primer, 5′-GCCACCAGGAAGATGTCATTT-3′; and probe, 5′-CCTGACAGTCCAAGGCTCGGCAGA-3′.
Primers and probe of MT1-MMP were obtained from Isogen (Maarsen, The Netherlands). GAPDH primer-probe combination (VIC-labeled) was purchased from Applied Biosystems (Nieuwerkerk a/d IJssel, The Netherlands).
TIMP-1 antigen was assayed by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Oxon, United Kingdom). Inhibition of MMP-9 by TIMP-1 and TIMP-3 was determined by addition of serial dilutions of 48-hour conditioned media of nontransfected cells and MVECs transduced with Ad.PC10, Ad.TIMP-1, and Ad.TIMP-3 to 12 ng active MMP-9, which was prepared by activation of recombinant pro–MMP-9 (Invitek, Berlin, Germany). The residual MMP-9 activity was determined in an assay using modified urokinase as previously described.38 Furthermore, selective TIMP-3 activity over that of TIMP-1 was assayed by inhibition of the activity of human recombinant MT1-MMP (pro-domain–catalytic domain–hemopexin domain) purchased from Chemicon, (Temecula, CA) and by determining the inhibition of MMP-2 activation by gelatin zymography. The activity of MT1-MMP was measured in an assay using modified urokinase as a substrate (Biotrak; Amersham Biosciences).
Gelatin zymography
Gelatinolytic activities of secreted MMPs were analyzed by zymography on gelatin-containing polyacrylamide gels as described.39 By using this technique both active and latent gelatinases can be visualized. The 48-hour conditioned media of hMVECs or human umbilical vein endothelial cells (HUVECs) cultured in M199 supplemented with 0.03% serum albumin and without or with 10 nmol/L phorbolmyristate acetate (PMA) were used for these assays. Samples were made in 2% (wt/vol) sodium dodecyl sulfate (SDS) and 10% (vol/vol) glycerol and applied to 10% (wt/vol) polyacrylamide gels copolymerized with 0.2% (wt/vol) gelatin. After electrophoresis the gels were washed twice for 15 minutes in 50 mM Tris (tris(hydroxymethyl)aminomethane)/HCl, pH 8.0, containing 5 mM CaCl2, 1 μM ZnCl2, and 2.5% (wt/vol) Triton X-100 to remove the SDS, followed by 2 washes of 5 minutes in 50 mM Tris/HCl, pH 8.0, containing 5 mM CaCl2, and incubated overnight at 37°C. The gels were stained with Coomassie brilliant blue R-250.
Adenoviral transfer of TIMP-1 and TIMP-3 to hMVECs
Replication-defective vectors (E1-deleted, cytomegalovirus [CMV] promoter) encoding human TIMP-1 (Ad.TIMP-1) and TIMP-3 (Ad.TIMP-3) were used for the experiments. As a control vector, an empty vector lacking an expression cassette (Ad.PC10) was used. The vectors were produced as described by Quax et al.40 Confluent endothelial cells were 3 times incubated for 10 minutes with M199 to remove serum components. Thereafter, the hMVECs were incubated with 108 pfu/mL adenovirus in M199 supplemented with 0.1% human serum albumin. After 2 hours the media were removed and cells were incubated overnight with culture medium. For the in vitro angiogenesis assay, the noninfected cells were mixed with 20% infected cells and seeded in confluency on the matrices.
Statistics
Experiments were performed with duplicate wells and expressed as mean ± SD. For statistical analysis the analysis of variance (ANOVA) was used, followed by a modified t test according to Bonferroni.
Results
Plasmin(ogen) dependency of invading endothelial structures in a fibrin matrix
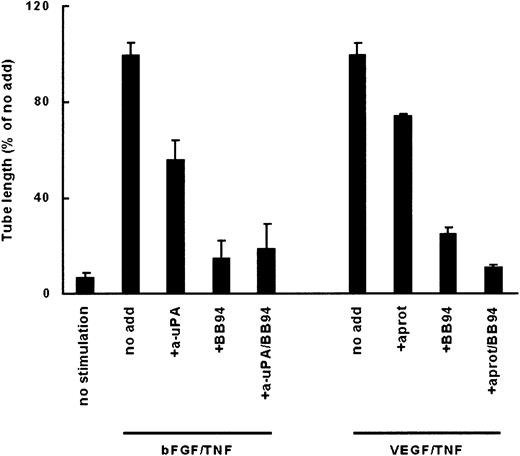
When plasminogen was present in the culture medium, stimulation of the hMVECs with the combination of an angiogenic growth factor and TNF-α (bFGF/TNF-α or VEGF165/TNF-α) induced the formation of tubular structures in a fibrin matrix, as shown for bFGF/TNF-α in Figure 1A-C, in agreement with our previous data. The formation of tubular structures depended on plasmin activity (Figure 1D) and cell-bound u-PA (Figure2).33-35 It was not inhibited by the general metalloproteinase inhibitor BB94 (Batimastat) (Figure 2).
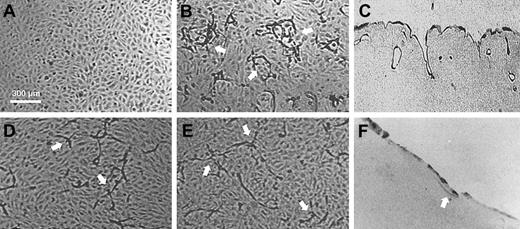
Plasmin(ogen) dependency of capillarylike tubular structure formation in a fibrin matrix by hMVECs.
hMVECs were cultured on top of a 3-dimensional fibrin matrix in M199 with 10% human serum and 10% newborn calf serum (NBCS) and were not stimulated (A) or stimulated with the addition of 10 ng/mL bFGF and 10 ng/mL TNF-α (B). After 4 days of culture, nonphase photomicrographs were taken. Histologic analysis of cross-sections of structures in the underlying fibrin matrix (C,F). Arrows indicate endothelial cells present in the underlying fibrin matrix. Addition of aprotinin (100 U/mL) (D) or depletion of plasminogen from the matrix and the culture media alter the formation of structures (E-F). Scale bar represents 300 μm.
Plasmin(ogen) dependency of capillarylike tubular structure formation in a fibrin matrix by hMVECs.
hMVECs were cultured on top of a 3-dimensional fibrin matrix in M199 with 10% human serum and 10% newborn calf serum (NBCS) and were not stimulated (A) or stimulated with the addition of 10 ng/mL bFGF and 10 ng/mL TNF-α (B). After 4 days of culture, nonphase photomicrographs were taken. Histologic analysis of cross-sections of structures in the underlying fibrin matrix (C,F). Arrows indicate endothelial cells present in the underlying fibrin matrix. Addition of aprotinin (100 U/mL) (D) or depletion of plasminogen from the matrix and the culture media alter the formation of structures (E-F). Scale bar represents 300 μm.
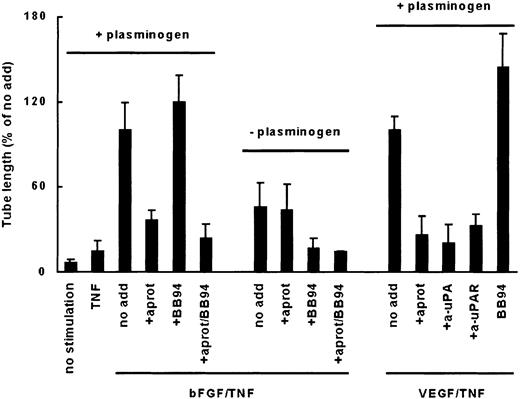
Formation of capillarylike tubular structures in a purified fibrin matrix depends on plasmin activity and not MMP activity.
hMVECs were cultured on top of a 3-dimensional matrix containing M199 supplemented with 10% human serum (HS) and 10% NBCS and stimulated with 10 ng/mL bFGF and 10 ng/mL TNF-α (bFGF/TNF-α) or 50 ng/mL VEGF165 and 10 ng/mL TNF-α (VEGF/TNF-α) in the presence (+ plasminogen) or absence of plasminogen (− plasminogen) with or without (no add) the addition of a blocking polyclonal antibody against u-PA (100 μg/mL), a blocking monoclonal antibody against u-PAR (10 μg/mL), aprotinin (aprot; 100 U/mL), or BB94 (10 μg/mL). After 4 days of culturing, total tube length was measured as described and expressed as percentage of tube length formed by bFGF/TNF-α-stimulated hMVECs or VEGF165/TNF-α-stimulated hMVECs. The data are expressed as mean ± SD of 4 to 17 experiments.
Formation of capillarylike tubular structures in a purified fibrin matrix depends on plasmin activity and not MMP activity.
hMVECs were cultured on top of a 3-dimensional matrix containing M199 supplemented with 10% human serum (HS) and 10% NBCS and stimulated with 10 ng/mL bFGF and 10 ng/mL TNF-α (bFGF/TNF-α) or 50 ng/mL VEGF165 and 10 ng/mL TNF-α (VEGF/TNF-α) in the presence (+ plasminogen) or absence of plasminogen (− plasminogen) with or without (no add) the addition of a blocking polyclonal antibody against u-PA (100 μg/mL), a blocking monoclonal antibody against u-PAR (10 μg/mL), aprotinin (aprot; 100 U/mL), or BB94 (10 μg/mL). After 4 days of culturing, total tube length was measured as described and expressed as percentage of tube length formed by bFGF/TNF-α-stimulated hMVECs or VEGF165/TNF-α-stimulated hMVECs. The data are expressed as mean ± SD of 4 to 17 experiments.
In the absence of plasminogen or plasmin activity, the formation of tubular structures was impaired (Figure 1E-F). A reduced number of elongated cordlike structures were formed, consisting of sprouting cells, which invaded the matrix superficially and did not form a substantial lumen (63% ± 6% reduction of the length of structures as compared with tubule formation in the presence of plasminogen, n = 11). In contrast to its effect in plasminogen-rich fibrin matrices, BB94 completely inhibited the formation of these structures in plasminogen-deficient fibrin matrices (Figure 2).
Capillarylike tubular structure formation in fibrin/collagen matrix in vitro involves both u-PA/plasmin and MMP activity
Subsequently, 3-dimensional fibrin matrices containing 10% collagen were used to determine whether, in addition to the role of plasmin, the MMP system would also contribute to the formation of capillarylike tubular structures in a more complex matrix. On this matrix the outgrowth of structures was only partially reduced by plasmin inhibition (35% ± 13%) as is shown for VEGF/TNF-α-stimulated cells in Figure3. Inhibition of MMP activity by the addition of BB94 (10 ng/mL) caused an 80% ± 7% inhibition of tube formation (n = 6, Figure 3). An almost complete inhibition of tube formation (85% ± 5%) was obtained by simultaneous inhibition of both MMP and plasmin activity (Figure 3). Similar results were obtained when cells were stimulated with bFGF/TNF-α and blocking anti–u-PA antibodies were used instead of aprotinin (not shown).
Formation of capillarylike tubular structures in a fibrin-collagen matrix depends on plasmin and MMP activity.
hMVECs were cultured on top of a 3-dimensional matrix fibrin matrix containing 10% collagen in M199 supplemented with 10% HS and 10% NBCS and stimulated with 50 ng/mL VEGF165 and 10 ng/mL TNF-α (VEGF/ TNF-α) with or without (no add) the addition of an aprotinin (aprot; 100 U/mL) or BB94 (10 μg/mL). After 4 days of culturing, total tube length was measured as described and expressed as percentage of tube length formed by hMVECs stimulated with VEGF165/TNF-α. The data are expressed as mean ± SD of 3 experiments with duplicate wells.
Formation of capillarylike tubular structures in a fibrin-collagen matrix depends on plasmin and MMP activity.
hMVECs were cultured on top of a 3-dimensional matrix fibrin matrix containing 10% collagen in M199 supplemented with 10% HS and 10% NBCS and stimulated with 50 ng/mL VEGF165 and 10 ng/mL TNF-α (VEGF/ TNF-α) with or without (no add) the addition of an aprotinin (aprot; 100 U/mL) or BB94 (10 μg/mL). After 4 days of culturing, total tube length was measured as described and expressed as percentage of tube length formed by hMVECs stimulated with VEGF165/TNF-α. The data are expressed as mean ± SD of 3 experiments with duplicate wells.
Adenoviral TIMP-3 gene transfer impairs capillarylike tubular structure formation
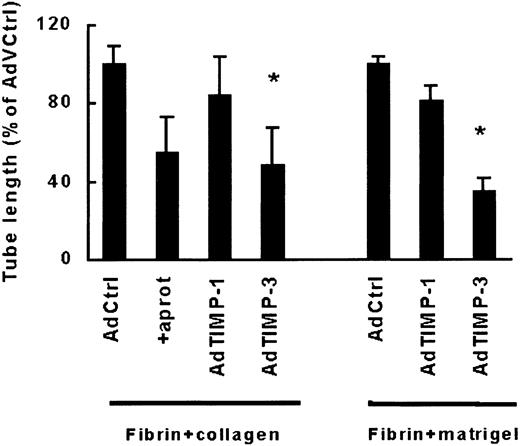
Because tube formation in a fibrin matrix containing 10% collagen was markedly inhibited by a metalloproteinase inhibitor, we evaluated the effects of 2 physiologic tissue inhibitors of matrix metalloproteinases (TIMPs), TIMP-1 and TIMP-3. hMVECs were infected with 108 pfu replication-deficient adenovirus expressing human TIMP-1 (Ad.TIMP-1), TIMP-3 (Ad.TIMP-3), or a control adenovirus (Ad.PC10) and seeded on top of a fibrin-10% collagen matrix. The overexpression of TIMP-1 was verified by ELISA. MVECs transduced with control adenovirus, Ad.TIMP-1, and Ad.TIMP-3 produced 10, 1366, and 11 ng/48 hours per 105 cells TIMP-1, respectively. Overexpression of either TIMP-1 or TIMP-3 was demonstrated by the ability of the transduced cells to inhibit exogenously added recombinant MMP-9. Residual activity of 12 ng active MMP-9 was reduced to 12% to 18% or 15% to 26% after incubation with a 50- to 100-fold dilution of the conditioned medium of Ad.TIMP-1– or Ad.TIMP-3–transduced MVECs, whereas it remained 94% to 100% active after incubation with the same dilutions of conditioned medium of Ad.PC10-transduced or nontransduced MVECs.
Overexpression of TIMP-3 clearly inhibited tube formation in VEGF165/TNF-α-stimulated MVECs (52% ± 19%, n = 6) (Figure 4). On the contrary, overexpression of TIMP-1 had only a minor inhibitory effect on tubule formation (16% ± 20%, n = 6). To compare the contribution of more complex matrix proteins in a fibrinous environment, we performed similar experiments with a fibrin matrix that contained 10% matrigel. Again TIMP-3 was more effective than TIMP-1 (65% ± 7% versus 9% ± 8% inhibition, respectively, n = 4).
TIMP-3 impairs capillarylike tubular structure formation.
hMVECs were infected with control virus Ad.PC10 (AdCtrl), a virus containing TIMP-1 (AdTIMP-1) or TIMP-3 (AdTIMP-3) as described and were cultured on top of a 3-dimensional matrix containing fibrin and 10% collagen in M199 supplemented with 10% HS and 10% NBCS and stimulated with 50 ng/mL VEGF165and 10 ng/mL TNF-α. After 4 days of culturing, total tube length was measured as described and expressed as percentage of tube length formed by VEGF165/TNF-α-stimulated hMVECs infected with control virus. The data are expressed as mean ± SD of 6 experiments with duplicate wells for fibrin-collagen matrices. *P < .05 as compared with AdPC10.
TIMP-3 impairs capillarylike tubular structure formation.
hMVECs were infected with control virus Ad.PC10 (AdCtrl), a virus containing TIMP-1 (AdTIMP-1) or TIMP-3 (AdTIMP-3) as described and were cultured on top of a 3-dimensional matrix containing fibrin and 10% collagen in M199 supplemented with 10% HS and 10% NBCS and stimulated with 50 ng/mL VEGF165and 10 ng/mL TNF-α. After 4 days of culturing, total tube length was measured as described and expressed as percentage of tube length formed by VEGF165/TNF-α-stimulated hMVECs infected with control virus. The data are expressed as mean ± SD of 6 experiments with duplicate wells for fibrin-collagen matrices. *P < .05 as compared with AdPC10.
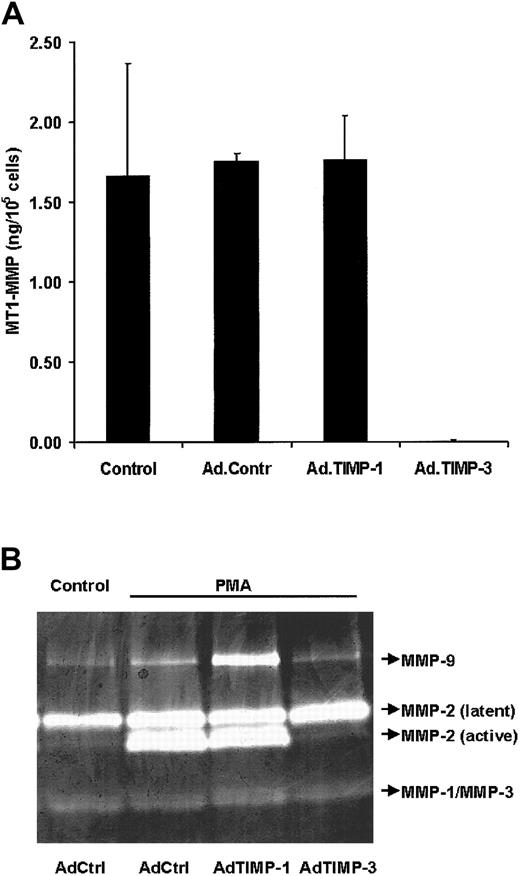
TIMP-3 inhibits MT1-MMP and the conversion of pro–MMP-2 into MMP-2
TIMP-1 and TIMP-3 inhibit a number of MMPs, but they differ in their ability to inhibit MT-MMPs.31 MT1-MMP activity is completely inhibited by TIMP-3 produced by MVECs but not by TIMP-1 (Figure 5A). Because MT-MMPs can activate MMP-2, we evaluated whether TIMP-1 and TIMP-3 overexpression affected the activation of MMP-2 by human endothelial cells. When hMVECs were stimulated by bFGF/TNF-α or VEGF165/TNF-α, an increase in the active form of MMP-2 was observed. This activation was reduced in cells that were overexpressing TIMP-3, whereas TIMP-1 overexpression had no significant effect (not shown). Similar results were obtained when confluent HUVECs, which produce high amounts of MMP-2 after incubation with 10 nM PMA, were infected with a control virus (Ad.PC10), a virus containing TIMP-1 and a virus containing TIMP-3 (Figure 5B). This finding suggests that MT-MMPs are involved in the activation of MMP-2.
MT1-MMP activity is inhibited by TIMP-3.
(A) Direct assay of MT1-MMP activity. Confluent hMVECs were incubated with 108 pfu/mL adenovirus in M199/0.1% human serum albumin, ie, AdPC10 (Ad.Contr), Ad.TIMP-1, or Ad.TIMP-3. MT1-MMP activity was assayed in cell extracts as described in “Materials and methods.” The data represent the mean ± range of 2 independent cultures. (B) Inhibition of MMP-2 activation by TIMP-3. Confluent HUVECs were incubated with 108 pfu/mL AdPC10 (AdCtrl), AdTIMP-1, or AdTIMP-3. After 2 hours the media were removed and cells were incubated overnight with culture medium. Thereafter, cells were washed with M199 containing 0.03% human serum albumin to remove serum components and incubated with this media containing no stimulus or 10 ng/mL PMA. After 48 hours the conditioned media were collected and gelatin zymography was performed as described. The same results were observed in 4 different experiments.
MT1-MMP activity is inhibited by TIMP-3.
(A) Direct assay of MT1-MMP activity. Confluent hMVECs were incubated with 108 pfu/mL adenovirus in M199/0.1% human serum albumin, ie, AdPC10 (Ad.Contr), Ad.TIMP-1, or Ad.TIMP-3. MT1-MMP activity was assayed in cell extracts as described in “Materials and methods.” The data represent the mean ± range of 2 independent cultures. (B) Inhibition of MMP-2 activation by TIMP-3. Confluent HUVECs were incubated with 108 pfu/mL AdPC10 (AdCtrl), AdTIMP-1, or AdTIMP-3. After 2 hours the media were removed and cells were incubated overnight with culture medium. Thereafter, cells were washed with M199 containing 0.03% human serum albumin to remove serum components and incubated with this media containing no stimulus or 10 ng/mL PMA. After 48 hours the conditioned media were collected and gelatin zymography was performed as described. The same results were observed in 4 different experiments.
Localization and production of MMPs during tube formation in a fibrin/collagen matrix
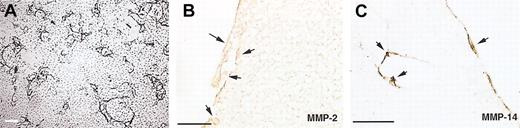
MMP-2, MMP-9, and MT1-MMP (MMP-14) can play pivotal roles in angiogenesis.41-43 By immunohistochemical analysis the presence of MMP-2, MMP-14, and MMP-1 was shown in endothelial cells on and in the fibrin/collagen matrix, but these cells did not show staining for MMP-9 (shown for MMP-2 and MT1-MMP in Figure6). The production of MMP-1 and MMP-2 and the absence of MMP-9 secretion were confirmed by ELISA. Nonstimulated and VEGF/TNF-α-stimulated cells produced 2.6 ± 1.2 and 10.4 ± 1.0 ng/105 cells MMP-1, and 52 ± 19 and 51 ± 22 ng/105 cells MMP-2, whereas no MMP-9 production was detectable (mean ± range, 2 independent cultures). Real-time PCR of MT1-MMP showed a low expression of MT1-MMP mRNA in nonstimulated hMVECs and a 11- to 14-fold higher expression of MT1-MMP mRNA in bFGF/TNF-α-stimulated cells (30 cycli, corrected for GAPDH mRNA expression).
Localization of MMPs in endothelial cells present in the capillarylike tubular structures in a fibrin-collagen matrix.
hMVECs were cultured on top of a 3-dimensional fibrin matrix containing 10% collagen in M199 with 10% human serum and 10% NBCS and were stimulated with 50 ng/mL VEGF165 and 10 ng/mL TNF-α. After 4 days of culture, nonphase micrographs were taken (A), the matrices were fixed and embedded as described, and 4-μm cross-sections perpendicular to the matrix surface were cut and stained for MMP-2 (B) and MT1-MMP (MMP-14) (C). Immunohistochemistry was performed as described. Arrows indicate areas of positive staining. Scale bar = 300 μm (A) or 50 μm (B-C).
Localization of MMPs in endothelial cells present in the capillarylike tubular structures in a fibrin-collagen matrix.
hMVECs were cultured on top of a 3-dimensional fibrin matrix containing 10% collagen in M199 with 10% human serum and 10% NBCS and were stimulated with 50 ng/mL VEGF165 and 10 ng/mL TNF-α. After 4 days of culture, nonphase micrographs were taken (A), the matrices were fixed and embedded as described, and 4-μm cross-sections perpendicular to the matrix surface were cut and stained for MMP-2 (B) and MT1-MMP (MMP-14) (C). Immunohistochemistry was performed as described. Arrows indicate areas of positive staining. Scale bar = 300 μm (A) or 50 μm (B-C).
Localization of MMPs in a recanalized mural thrombus
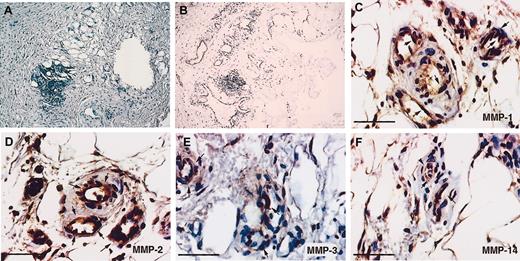
To verify whether the MMPs observed in tubular structures in vitro were also present in comparable in vivo conditions, we studied sections of a recanalized human arterial mural thrombus. The fibrin-containing thrombus (Figure 7A) contained new vascular structures, which were visualized by immunohistochemistry using antibodies against CD31 (Figure 7B). Immunohistochemical analysis showed the clear presence of MMP-1, MMP-2, MMP-3, and MT1-MMP associated with endothelial cells of the neovessels. In addition to the endothelial cells, inflammatory and smooth muscle cells also stained positive for MMPs (Figure 7D-F). Negative controls (same procedure without the primary antibody) showed no staining.
Localization of MMPs in neovessels in a human recanalized mural thrombus.
Immunohistochemistry was performed on paraffin sections of a mural thrombus, as described in “Material and methods.” Fibrin(ogen) present in the thrombus was stained with a polyclonal antibody against fibrin(ogen) (A), and endothelial cells in newly formed vessels were stained with an anti–CD-31 antibody (B). The endothelial cells of the newly formed microvessels were stained for MMP-1 (C), MMP-2 (D), MMP-3 (E), and MT1-MMP (MMP-14) (F). Scale bars = 50 μm. Results are representative of the organized plaques of 2 patients. Arrows indicate areas of positive staining.
Localization of MMPs in neovessels in a human recanalized mural thrombus.
Immunohistochemistry was performed on paraffin sections of a mural thrombus, as described in “Material and methods.” Fibrin(ogen) present in the thrombus was stained with a polyclonal antibody against fibrin(ogen) (A), and endothelial cells in newly formed vessels were stained with an anti–CD-31 antibody (B). The endothelial cells of the newly formed microvessels were stained for MMP-1 (C), MMP-2 (D), MMP-3 (E), and MT1-MMP (MMP-14) (F). Scale bars = 50 μm. Results are representative of the organized plaques of 2 patients. Arrows indicate areas of positive staining.
Discussion
In this study we have shown that capillarylike tubular structure formation in a fibrin matrix containing collagen is largely driven by matrix metalloproteinases. A complete arrest of tubular structure formation was accomplished by simultaneous inhibition of MMPs and plasmin. Overexpression of TIMP-3 markedly inhibited the formation of capillarylike structures, whereas overexpression of TIMP-1 had only a limited effect. This finding suggests that membrane-type matrix metalloproteinases (MT-MMPs) are more important then secreted matrix metalloproteinases (MMPs). Immunohistochemical analysis confirmed the presence of MT1-MMP on endothelial cells in capillarylike tubular structures in vitro and in neovessels of a recanalized thrombus in vivo.
We and other investigators have previously shown that the invasion of human microvascular endothelial cells into a purified fibrin matrix is critically dependent on u-PA/plasmin activity.8,9,33,34 In vivo a fibrinous exudate is enriched with other plasma proteins, such as fibronectin, laminin, vitronectin, and proteoglycans, which are substrates for both MMPs as well as plasmin.1-3Furthermore, it is intermingled with collagen fibers from the interstitium, which cannot be degraded by plasmin.17 We have now demonstrated that invasion and tube formation of these cells in a fibrin-collagen matrix is largely dependent on MMP activity.
During the process of capillarylike tubular structure formation, MMPs play an essential, though not a solitary role, because complete arrest of tube formation could be accomplished only by the addition of inhibitors of both plasmin and MMP activities. Alternatively, u-PA/plasmin plays an essential role during angiogenesis in a pure fibrin matrix in vitro8,33 and after myocardial infarction in vivo.10 The latter may relate to the activation of MMPs by u-PA/plasmin or to a role of u-PA/plasmin in the invasion of leukocytes, which contribute to the angiogenesis process by releasing angiogenesis-stimulating factors.44 45
Several MMPs have been implicated as playing an essential role in angiogenesis. A role during tumor-induced angiogenic switch has been proposed for MMP-2,41 MMP-7,46 and MMP-9.41 In addition to secreted matrix metalloproteases, a role for membrane-type matrix metalloproteases has been shown in embryologic and corneal angiogenesis43 and in the process of fibroblast invasion into a 3-dimensional matrix.47 48The data presented here indicate that MMPs also play an important role in repair-associated angiogenesis during wound healing.
Our first observation that MMPs are involved in the formation of tubular structures was based on the use of BB94, a broadly acting metalloproteinase inhibitor, which not only inhibits MMPs but also members of the ADAMs (with adisintegrin and ametalloproteinase domain) and ADAM-TS (contain varying numbers ofthrombospondin domains) family.49Involvement of MMPs could be demonstrated by the inhibition by TIMPs, which are the physiologic inhibitors of MMPs. TIMP-1 inhibits many of the secreted MMPs, whereas TIMP-3 in addition also inhibits MT-MMPs.31 Our observation that TIMP-3 was much more potent in inhibiting endothelial tube formation than TIMP-1 indicates that MT-MMPs play a crucial role in this process.
The presence of MT1-MMP was demonstrated in VEGF/TNF-α-stimulated endothelial cells present in and on the fibrin-collagen matrix. MT1-MMP mRNA was even considerably increased in the hMVECs exposed to tube-inducing conditions. Furthermore, MT1-MMP was demonstrated in the new vascular structures of a recanalized mural thrombus, indicating that also in vivo this protease accompanies newly formed vessels. At present, we cannot exclude the possibility that other MT-MMPs are also involved in angiogenesis. Future studies have to elucidate to what extent these proteases play a role during the onset of tube formation and during the continuation of angiogenesis in tissue repair.
TIMP-3 inhibits MT-MMPs and thereby also inhibits the conversion of pro–MMP-2 to active MMP-2.31 Multimolecular complexes containing MT1-MMP, MMP-2, and other proteins are formed on invadopodia and probably in focal adhesion sites.50,51 Such focal adhesion sites are considered to play an important role in cell migration and invasion into a matrix.52 However, it cannot be excluded that MT1-MMP acts independently of MMP-2. Although the involvement of MT1-MMP is very likely, it should be noted that several other metalloproteinases are inhibited by TIMP-3 and not by TIMP-1, in particular aggrecanases-1 and -2 (ADAMTS-4 and -5), ADAMTS-1 and TNF-α-converting enzyme (TACE; ADAM-17).53-55 Although a major involvement of these proteases in capillary-tube formation seems unlikely under our experimental conditions, we cannot exclude that these or other members of the ADAM or ADAMTS family may contribute to this process in addition to MT1-MMP. Besides the direct effect by protease inhibition, other more indirect effects of MT-MMPs may be involved. The effect of TIMP-3 on cell death by inducing apoptosis has been demonstrated in proliferating tumor cells and smooth muscle cells.56-58 As such, apoptosis may contribute to the reduction of tube formation in addition to the inhibition of MMPs. However, under our experimental conditions no proliferation of endothelial cells occurred.34 Furthermore, tube formation was largely inhibited by BB94. Therefore, we favor it to suggesting that the predominant effect of TIMP-3 on the formation of capillarylike structures by hMVECs in a fibrin-collagen matrix involves inhibition of MT-MMPs. In line with this suggestion it has been reported that specific inhibitory antibodies against MT1-MMP impair both endothelial migration and phorbol ester-induced matrix invasion.59
The u-PA/plasmin is involved in the activation of MMPs, and MMPs control plasminogen activation at the cell surface.25,26,28 This suggests a complex interrelationship. In contrast to several other MMPs, MT1-MMP does not depend on activation of plasmin, but it is activated by an intracellular furinlike activation mechanism.30 As such MT1-MMP is present as an active enzyme on the cell surface, where it has direct activity against different ECM proteins, including fibronectin, vitronectin, collagens, and fibrin.14 In plasmin-deficient conditions, MT1-MMP can degrade the extracellular matrix and mediate cell migration and invasion either directly or via activation of MMPs. Indeed Hiraoka et al14 have shown that MT1-MMP acts as a pericellular fibrinolysin, mediating the invasion of HUVECs into a plasminogen-free fibrin matrix.14
To summarize, our in vitro data provide further insight into the proteolytic mechanisms involved in angiogenesis in a fibrinous exudate. Capillarylike tubular structure formation in a collagen-containing fibrin matrix is largely driven by matrix metalloproteinases and, more specifically, by membrane-type metalloproteinase(s). It is only partially mediated by u-PA/plasmin activity. The present findings predict that influencing angiogenesis in a fibrinous exudate, either in a wounded area or in a fibrinous tumor stroma, may require agents that act both on the plasminogen activator/plasmin system and the MMP system.
The authors thank Margreet de Vries for excellent technical assistance.
Prepublished online as Blood First Edition Paper, September 19, 2002; DOI 10.1182/blood-2002-05-1593.
Supported by the Netherlands Heart Foundation (grant M93.001) and the Dutch Cancer Foundation (grant TNOP 97-1511).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Victor W. M. van Hinsbergh, Gaubius Laboratory TNO-PG, Zernikedreef 9, PO Box 2215, 2301 CE Leiden, the Netherlands; e-mail: vwm.vanhinsbergh@pg.tno.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal