We previously identified a novel common virus integration site, Evi11, by means of retroviral insertional mutagenesis. We demonstrated that the gene encoding the peripheral cannabinoid receptor (Cb2) is the potential target, suggesting that Cb2 is a proto-oncogene. To elucidate a role for this G protein–coupled receptor (GPCR) in leukemic transformation we generated a Cb2-EGFP cDNA construct that was introduced into 32D/G-CSF-R cells. These cells require interleukin 3 (IL-3) to proliferate in vitro, whereas in the presence of granulocyte–colony-stimulating factor (G-CSF) they differentiate toward mature neutrophils. We demonstrate that 32D/G-CSF-R/Cb2-EGFP cells migrate in a transwell assay in reponse to the Cb2 ligand 2-arachidonoylglycerol (2-AG), indicating that the fusion protein was functional. When cultured in the presence of G-CSF neutrophilic differentiation of Cb2-EGFP–expressing 32D/G-CSF-R cells was completely blocked. Moreover, a Cb2-specific antagonist fully recovered the G-CSF–induced neutrophilic differentiation of 32D/G-CSF-R/Cb2-EGFP cells. To investigate which signal transduction pathway(s) may be involved in the block of neutrophilic maturation, differentiation experiments were carried out using specific inhibitors of signaling routes. Interestingly, full rescue of G-CSF–induced neutrophilic differentiation was observed when cells were cultured with the mitogen-induced extracellular kinase (MEK) inhibitors, PD98059 or U0126, and partial recovery was detected with the phosphoinositide 3-kinase (PI3-K) inhibitor LY-294002. These studies demonstrate that the Cb2 receptor is an oncoprotein that blocks neutrophilic differentiation when overexpressed in myeloid precursor cells. Cb2 appears to mediate its activity through MEK/extracellular signal-related kinase (ERK) and PI3-K pathways.
Introduction
Cb2, the gene encoding the peripheral cannabinoid receptor, has previously been identified as a common virus integration site (cVIS) in retrovirally induced leukemias, suggesting that it is a proto-oncogene.1,2 The peripheral as well as the central cannabinoid receptor (Cb1) are 7 transmembrane proteins and belong to the family of G protein–coupled receptors (GPCRs).3,4 Previous studies have shown that the rank order of Cb2 mRNA in hematopoietic cells is as follows: B cells > natural killer cells > monocytes > neutrophils > T8 cells > T4 cells.5,6Moreover, we and others have shown that Cb2 protein is normally expressed in areas enriched for B lymphocytes, that is, in the marginal zone of the spleen, the cortex of lymph nodes, the nodular corona of Peyer patches, and the mantle zones of secondary follicles in tonsils3,6-8 (N. Rayman and R.D., unpublished observation, June 2002). We recently described a role of the Cb2 receptor in migration of hematopoietic cells.9 Using transwell assays we observed strong migration of Cb2-expressing spleen cells in response to 2-arachidonoylglycerol (2-AG), the endogenous ligand for the Cb2 receptor. This migration could be completely abolished by a Cb2-specific antagonist, SR144528, whereas the Cb1-specific antagonist SR141716 had no effect. Immunophenotyping revealed that the 2-AG–responding cells express B220, CD19, immunoglobulin M (IgM), and IgD, suggesting a role for this GPCR in chemoattraction, mobilization, and/or activation of splenic B lymphocytes during the immune response.9
In acute myeloid leukemia (AML), immature myeloid precursor cells accumulate in marrow and blood (for review see Lowenberg et al10). Although several genes involved in leukemia development have been identified by cloning of breakpoints at chromosomal translocations,11-13 little is known about the genetic defects and aberrant signaling causing a block of myeloid differentiation. Retroviral insertional mutagenesis has been extensively used by different research groups in the past 2 decades and several novel disease genes involved in leukemia and lymphoma development have been identified by means of this procedure.2,14-17 Our previous observation that theCb2 gene is a frequent proviral target in Cas-Br-M MuLV–induced myeloid leukemias suggests a role for this receptor in myeloid transformation.1,2 18
In the present study we investigated whether overexpression of the Cb2 receptor in myeloid precursor cells could interfere with neutrophilic development, the major characteristic of myeloid leukemia. For this purpose we generated a Cb2-EGFP fusion construct that was cloned into the viral vector pLNCX and introduced into cells from the 32D/G-CSF receptor (32D/G-CSF-R) cell line. This cell system has been demonstrated to be a powerful in vitro model to study the molecular mechanisms involved in granulocytic differentiation.19-21 32D/G-CSF-R cells proliferate in vitro in the presence of interleukin 3 (IL-3) and are capable of fully differentiating toward mature neutrophils upon granulocyte–colony-stimulating factor (G-CSF) stimulation. Cb2 expression levels are low in the 32D/G-CSF-R cells, as has been shown by radiolabeled ligand binding studies.9 To determine exogenous Cb2 protein levels, Cb2-EGFP fusion constructs were generated and transfected into 32D/G-CSF-R cells. After demonstrating that the Cb2-EGFP fusion protein was expressed on the surface membrane of 32D/G-CSF-R cells and fully functional, we investigated whether overexpression of Cb2 could affect G-CSF–induced neutrophilic differentiation. We studied whether activation of the receptor by agonists or inactivation by antagonists could influence neutrophilic development. Using specific signal transduction inhibitors, we investigated through which intracellular signaling pathway Cb2 might alter normal development. We demonstrate that (1) G-CSF–induced granulocytic differentiation of 32D/G-CSF-R is fully blocked by Cb2-EGFP overexpression, (2) addition of the Cb2-specific antagonist SR144528 fully recovers neutrophilic differentiation, and (3) the differentiation arrest conferred by the Cb2 receptor may involve activation of MEK/ERK (mitogen-induced extracellular kinase/extracellular signal-related kinase) and phosphoinositide 3-kinase (PI3-K) signaling routes.
Materials and methods
Cannabinoid ligands, cytokines, and inhibitors of intracellular signaling
The Cb2 cannabinoid ligand 2-arachidonoylglycerol (2-AG) was obtained from Sigma (Zwijndrecht, The Netherlands). Cb1-specific antagonist SR141716 and Cb2-specific antagonist SR144528 were kindly donated by Dr Casellas (Sanofi Recherche, Montpellier, France). Murine IL-3 was obtained from an IL-3–producing Chinese hamster ovary (CHO) cell line and G-CSF was from Amgen (Thousand Oaks, CA). Dibutyryl cyclic adenosine monophosphate (AMP) (dbcAMP), LY-294002 (PI3-K inhibitor), SB203580 (p38/MAPK [mitogen-activated protein kinase] inhibitor), and U0126 (MEK inhibitor) were from Kordia Life Science (Leiden, The Netherlands), whereas PD98059 (MEK inhibitor) was obtained from Omnilabo International (Breda, The Netherlands). The inhibitors were dissolved in dimethyl sulfoxide (DMSO) and added to the cultures at concentrations indicated and refreshed daily.
In vitro proliferation and neutrophilic differentiation of 32D/G-CSF-R cells
The 32D/G-CSF-R cell line19 was cultured in RPMI 1640 medium (Life Technologies, Breda, The Netherlands) supplemented with penicillin (100 IU/mL), streptomycin (100 ng/mL), 10% fetal calf serum (FCS), and murine IL-3 (10 ng/mL) or human G-CSF (100 ng/mL). Cell counting was performed using a CASY1/TTC cell counter (Schärfe System, Reutlingen, Germany) and the cell density was readjusted to 2 × 105 cells/mL daily. Morphologic analysis was determined by microscopy on May-Grünwald-Giemsa–stained cytospins (Shandon Holland, Amsterdam, The Netherlands).
Cb2-EGFP expression construct and infection of 32D/G-CSF-R cells
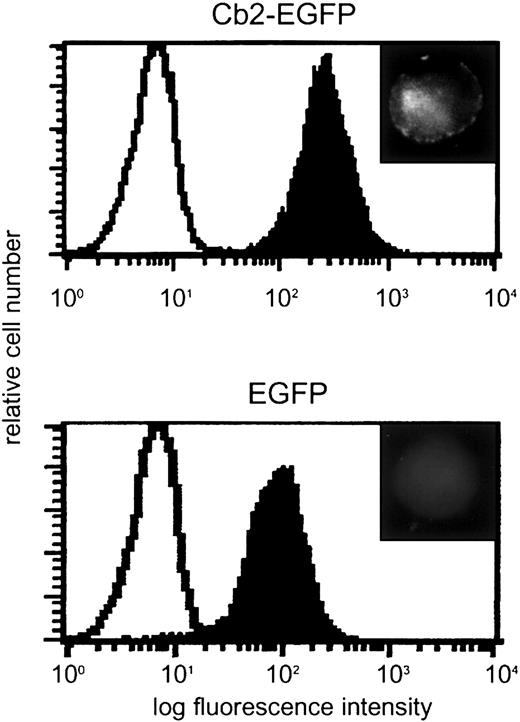
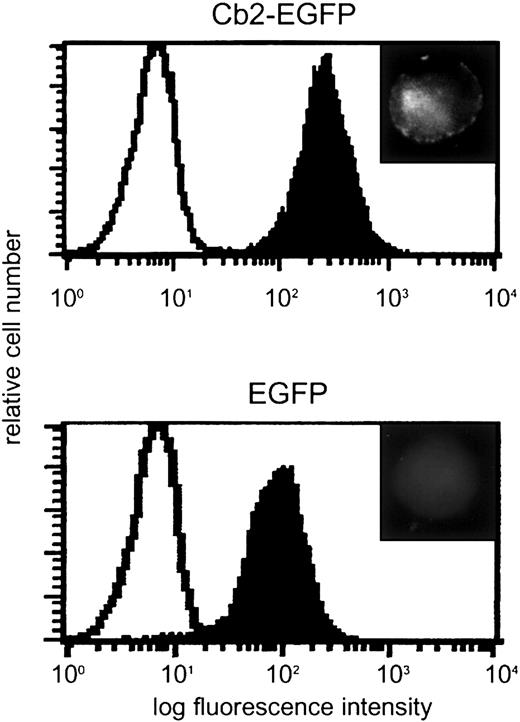
The primers 5′-CAAAGCCCATCCATGGAG-3′ and 5′-AAGGATCCGTGGTTTTCACATCAG-3′ were used to amplify the coding region of Cb2, mutate the TAG stop codon, and introduce aBamHI restriction site at the 3′ end. The polymerase chain reaction (PCR) product was cloned in TA vector and subcloned as anEcoRI/BamHI fragment into pEGFP-N1.Cb2-EGFP fusion construct was obtained byEco47III/NotI digestion of this latter construct and cloned as a blunt fragment into the HpaI site of pLNCX (Clontech, Palo Alto, CA). The Cb2-EGFP fusion construct was verified by nucleotide sequencing. The expression constructs were transfected into type E Phoenix cells (gift from G. Nolan, Stanford, CA) and the viral supernatants were used for infection of 32D/G-CSF-R cells. Single clones were obtained using limiting dilution in 96-well microtiter trays (Becton Dickinson, Mountain View, CA) and infected clones were selected on 0.8 mg/mL G418 (Gibco, Breda, The Netherlands). To determine Cb2-EGFP mRNA expression, Northern blot analysis was performed.2 Blots were hybridized using aNotI/EcoRI enhanced green fluorescence protein (EGFP) cDNA probe of 760 bp. Cb2-EGFP fusion protein expression was analyzed by Leica DMRXA microscopy (Leica Microsystems, Rijswijk, The Netherlands) and flow cytometric analysis of EGFP fluorescence (Figure1).
Cb2-EGFP and EGFP expression in 32D/G-CSF-R cells.
Flow cytometric analysis of a 32D/G-CSF-R clone overexpressing Cb2-EGFP and a 32D/G-CSF-R control clone overexpressing EGFP. Upper right inserts show cell fluorescence distribution in the transfected cells by microscopy. Original magnification of insets × 63.
Cb2-EGFP and EGFP expression in 32D/G-CSF-R cells.
Flow cytometric analysis of a 32D/G-CSF-R clone overexpressing Cb2-EGFP and a 32D/G-CSF-R control clone overexpressing EGFP. Upper right inserts show cell fluorescence distribution in the transfected cells by microscopy. Original magnification of insets × 63.
Flow cytometric analysis
32D/G-CSF-R/Cb2-EGFP and 32D/G-CSF-R/EGFP clones were analyzed by flow cytometric analysis (FACScan flow cytometer, Becton Dickinson). Cells (1 × 106) were washed twice with phosphate buffered saline (PBS) and resuspended in 500 μL Hanks balanced salt solution (HBSS) containing 0.5% bovine serum albumin (BSA). Dead cells were excluded from the analysis by addition of 7-AAD (7-aminoactinomycin D; Eugene, Leiden, The Netherlands). EGFP fluorescence was determined and the amount of protein was related to the peak channel (Figure 1).
Migration assay
Migration assays were performed using 5-μm pore size and 6.5-mm diameter transwells (Corning Costar, Amsterdam, The Netherlands) as previously described.9 In brief, cells were washed twice with HBSS medium, resuspended in 100 μL of migration medium (Iscoves modified Dulbecco medium [IMDM] plus 0.5% BSA) and placed in the upper chamber of the transwells. In the lower chamber, 600 μL migration medium with or without 300 nM 2-AG was placed. After 4 hours of incubation at 37°C and 5% CO2, the upper chamber was removed and the numbers of migrated cells were determined using a CASY1/TTC cell counter (Schärfe System). Cb1- and Cb2-specific antagonists (100 nM) were added to the upper chamber when tested.
Tritiated thymidine (3H-TdR) incorporation assay
DNA synthesis was determined by tritiated thymidine (3H-TdR) incorporation as described before.22Briefly, 1 × 104 cells were incubated in 100 μL RPMI 1640 medium supplemented with 10% FCS, murine IL-3 (10 ng/mL) or human G-CSF (100 ng/mL), and in the presence or absence of 2-AG (300 nM), Cb1- or Cb2-specific antagonist (100 nM). A quantity of 0.25 μCi (0.00925 MBq) 3H-TdR (Amersham International, Amersham, United Kingdom) was added to each well 4 hours before cell harvesting. Cells were harvested on nitrocellulose using a filtermate 196 harvester (Packard Instruments, Meriden, CT) and3H-TdR incorporation was measured by Topcount liquid scintillation counter (Packard Instruments).
Results
32D/G-CSF-R/Cb2-EGFP cells migrate in response to the natural Cb2 ligand 2-AG
32D/G-CSF-R cells were infected with retrovirus carryingCb2-EGFP or EGFP constructs. Following G418 selection, 8 Cb2-EGFP– and 4 EGFP-expressing 32D/G-CSF-R clones were obtained. Expression of Cb2-EGFP or EGFP RNA in those 32D/G-CSF-R–transfected cells was demonstrated by Northern blot analysis (data not shown). Cb2-EGFP and EGFP protein expression was assessed by means of fluorescence microscopy and flow cytometric analysis. Figure 1 shows 2 representative clones. Although high levels of EGFP were present in both clone types, membrane fluorescence was only observed on Cb2-EGFP–expressing cells (Figure 1, inserts).
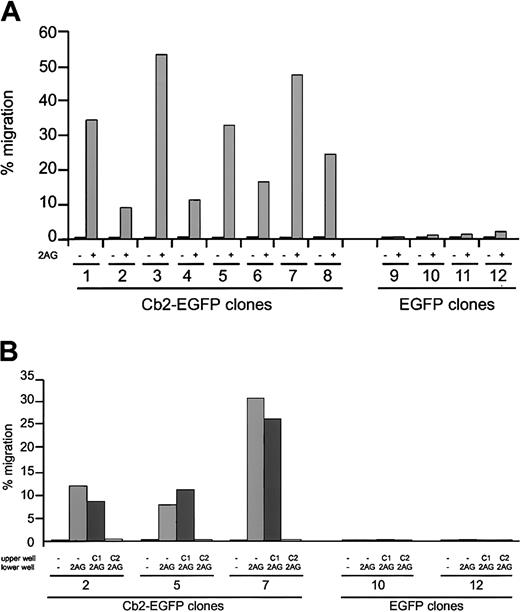
To investigate whether the Cb2-EGFP fusion protein had retained the normal Cb2 function, migration assays were performed. Figure2A shows that the 8 32D/G-CSF-R/Cb2-EGFP clones migrate with high efficiency following 2-AG exposure, whereas the 4 EGFP control clones did not migrate in response to 2-AG. The migration levels of the Cb2-EGFP–expressing clones are comparable to those observed previously using Cb2-expressing 32D/G-CSF-R cells.9 Moreover, 2-AG–induced migration could be completely blocked by the addition of Cb2-specific antagonist SR144528 to the upper chamber in a transwell assay, whereas the Cb1-specific antagonist SR141716 had no effect (Figure 2B). These experiments indicate that EGFP fused to the C-terminus of Cb2 does not interfere with the normal function of the peripheral cannabinoid receptor.
In vitro migration of Cb2-EGFP–expressing cells following 2-AG stimulation.
(A) Cb2-EGFP–expressing 32D/G-CSF-R cells (clones 1-8) and EGFP control 32D/G-CSF-R cells (clones 9-12) were exposed to 300 nM 2-AG (+) or nothing (−) in a transwell assay. The y-axis shows percentage of migration from an input of 2 × 105 cells. (B) Effect of the Cb1-specific antagonist SR141716 (C1) or Cb2-specific antagonist SR155528 (C2) on 2-AG–induced migration of 3 Cb2-EGFP–expressing clones (nos. 2, 5, and 7) and 2 EGFP control clones (nos. 10 and 12). The y-axis shows percentage of migration from an input of 2 × 105 cells.
In vitro migration of Cb2-EGFP–expressing cells following 2-AG stimulation.
(A) Cb2-EGFP–expressing 32D/G-CSF-R cells (clones 1-8) and EGFP control 32D/G-CSF-R cells (clones 9-12) were exposed to 300 nM 2-AG (+) or nothing (−) in a transwell assay. The y-axis shows percentage of migration from an input of 2 × 105 cells. (B) Effect of the Cb1-specific antagonist SR141716 (C1) or Cb2-specific antagonist SR155528 (C2) on 2-AG–induced migration of 3 Cb2-EGFP–expressing clones (nos. 2, 5, and 7) and 2 EGFP control clones (nos. 10 and 12). The y-axis shows percentage of migration from an input of 2 × 105 cells.
Overexpression of Cb2-EGFP in 32D/G-CSF-R cells blocks G-CSF–induced neutrophilic differentiation
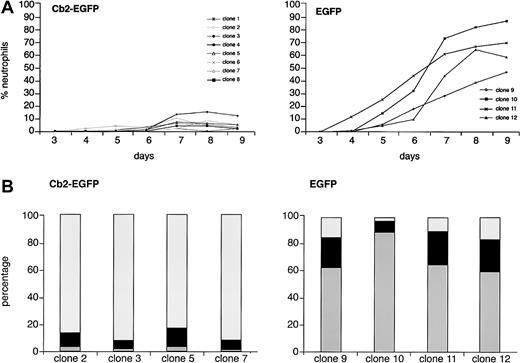
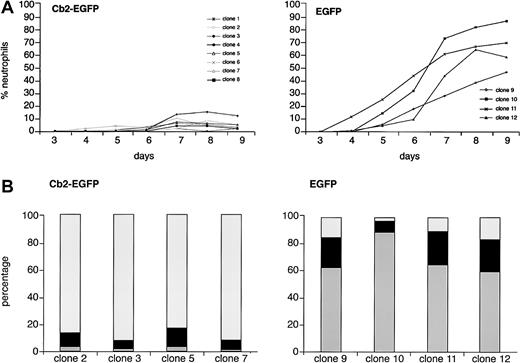
To study whether overexpression of the Cb2 receptor in 32D/G-CSF-R cells affects neutrophilic development, 8 Cb2-EGFP–expressing clones and 4 EGFP control clones were cultured in the presence of G-CSF. Morphologic analysis showed a complete block of neutrophilic differentiation of 32D/G-CSF-R/Cb2-EGFP clones cultured with G-CSF (Figure 3A). Differential countings on day 9 of culture revealed that the majority of these cells presented a blastlike morphology (Figure 3B). In contrast, EGFP-expressing 32D/G-CSF-R control cells fully matured toward neutrophilic granulocytes in response to G-CSF (Figure 3A-B). Flow cytometric analysis revealed high Cb2-EGFP or EGFP fluorescence during the 9 days of culture in the presence of G-CSF (data not shown).
G-CSF–induced differentiation response of Cb2-EGFP– and EGFP-expressing 32D/G-CSF-R clones.
(A) There were 8 Cb2-EGFP–expressing clones and 4 EGFP control clones cultured for 9 days in the presence of G-CSF (see “Materials and methods”). The y-axis shows the percentage of neutrophils and the x-axis shows the days of culture. (B) Differential countings of 4 Cb2-EGFP–expressing clones and 4 EGFP control clones at day 9 of G-CSF culture. White represents blast cells, black represents intermediately matured granulocytic forms, and gray indicates terminally differentiated neutrophilic granulocytes.
G-CSF–induced differentiation response of Cb2-EGFP– and EGFP-expressing 32D/G-CSF-R clones.
(A) There were 8 Cb2-EGFP–expressing clones and 4 EGFP control clones cultured for 9 days in the presence of G-CSF (see “Materials and methods”). The y-axis shows the percentage of neutrophils and the x-axis shows the days of culture. (B) Differential countings of 4 Cb2-EGFP–expressing clones and 4 EGFP control clones at day 9 of G-CSF culture. White represents blast cells, black represents intermediately matured granulocytic forms, and gray indicates terminally differentiated neutrophilic granulocytes.
Strikingly, the maturation arrest of 32D/G-CSF-R/Cb2-EGFP cells already occurred without the addition of Cb2 ligand. Inclusion of 2-AG to the G-CSF–supplemented cultures had no additional effect on granulocytic differentiation (data not shown).
Cb2-specific antagonist reestablishes G-CSF–induced neutrophilic differentiation of Cb2-EGFP–expressing 32D/G-CSF-R clones
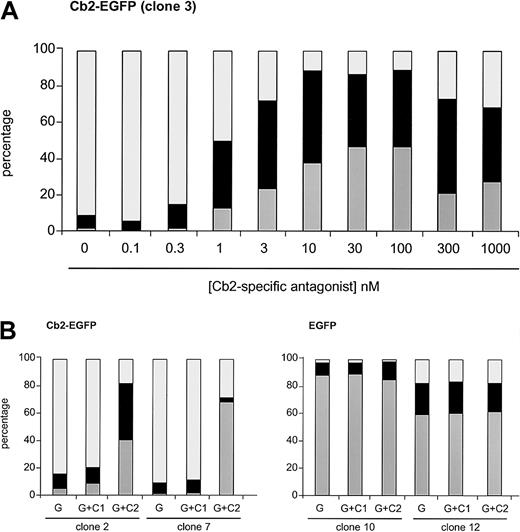
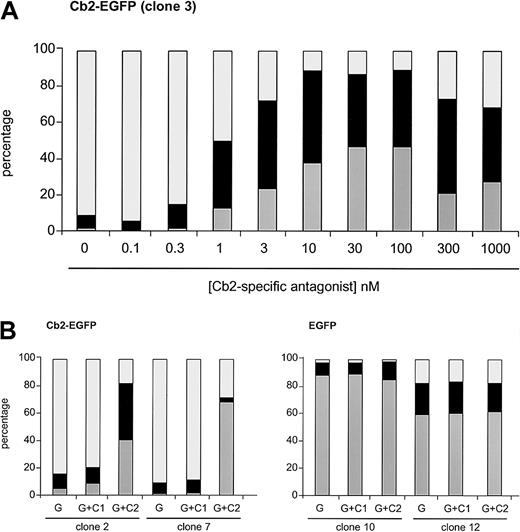
We next studied whether interfering with the Cb2 receptor using the cannabinoid antagonists would restore the neutrophilic differentiation of Cb2-EGFP–expressing clones. The Cb2-specific antagonist SR144528 was added at different concentrations to G-CSF–supplemented cultures in 2 different Cb2-EGFP clones. One representative experiment is shown in Figure4A, demonstrating that 1 nM of the Cb2-specific antagonist was already capable of partially restoring differentiation of Cb2-EGFP–overexpressing cells, whereas higher concentrations resulted in a complete recovery of neutrophilic differentiation of these cells.
Effect of the Cb2-specific antagonist SR144528 on G-CSF–induced differentiation.
(A) Cb2-specific antagonist titration experiment when added to the G-CSF cultures in differentiation assays. Countings were carried out at day 9 of culture. (B) Effect of Cb1-specific (C1) and Cb2-specific (C2) antagonist (100 nM) on 2 Cb2-EGFP and 2 EGFP clones cultured for 9 days in the presence of G-CSF (G). White represents blast cells, black represents intermediate forms, and gray indicates neutrophils.
Effect of the Cb2-specific antagonist SR144528 on G-CSF–induced differentiation.
(A) Cb2-specific antagonist titration experiment when added to the G-CSF cultures in differentiation assays. Countings were carried out at day 9 of culture. (B) Effect of Cb1-specific (C1) and Cb2-specific (C2) antagonist (100 nM) on 2 Cb2-EGFP and 2 EGFP clones cultured for 9 days in the presence of G-CSF (G). White represents blast cells, black represents intermediate forms, and gray indicates neutrophils.
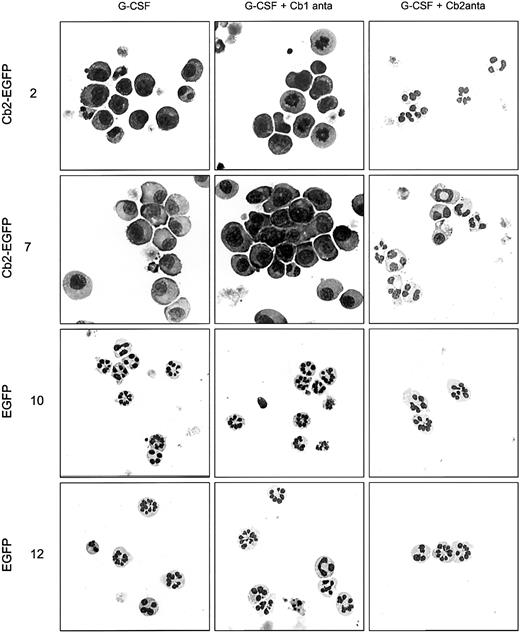
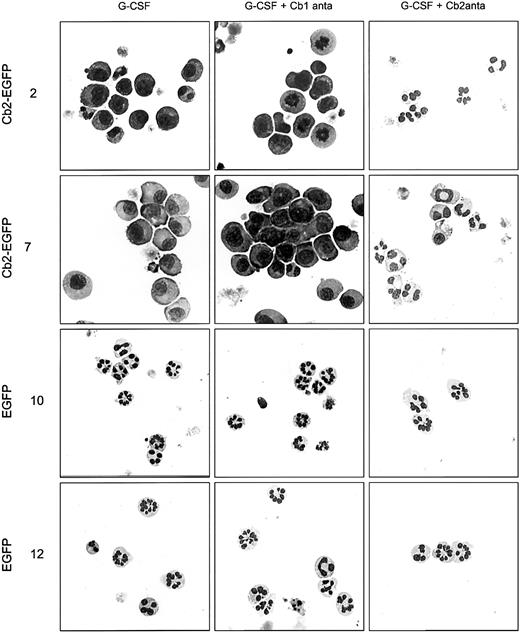
All 8 32D/G-CSF-R/Cb2-EGFP clones, of which 2 are shown in Figure4B and Figure 5, revealed a complete recovery of neutrophilic differentiation when cultured with G-CSF and 100 nM of Cb2-specific antagonist SR144528. In contrast, the Cb1-specific antagonist SR141716 (100 nM) did not restore granulocytic maturation of 32D/G-CSF-R/Cb2-EGFP cells (Figures 4B, 5). Neither Cb1- nor Cb2-specific antagonists exerted differentiation-inducing effects on EGFP-expressing control clones (Figures 4B, 5).
Cell morphology of 32D/G-CSF-R cells cultured in different conditions.
Morphologic analysis of 2 Cb2-EGFP–expressing clones and 2 EGFP control clones cultured for 9 days in the presence of G-CSF, G-CSF plus Cb1-specific antagonist, or G-CSF plus Cb2-specific antagonist. Original magnifications × 63.
Cell morphology of 32D/G-CSF-R cells cultured in different conditions.
Morphologic analysis of 2 Cb2-EGFP–expressing clones and 2 EGFP control clones cultured for 9 days in the presence of G-CSF, G-CSF plus Cb1-specific antagonist, or G-CSF plus Cb2-specific antagonist. Original magnifications × 63.
The G-CSF–induced proliferative response of Cb2-EGFP– expressing 32D/G-CSF-R cells is slightly altered
Since Cb2-EGFP–expressing 32D/G-CSF-R clones are blocked in neutrophilic differentiation, we wondered whether the blasts that accumulate when cultured with G-CSF still had proliferative capacity. Although the 8 Cb2-EGFP–expressing and the 4 EGFP-expressing 32D/G-CSF-R clones show a similar G-CSF proliferative curve, the data suggest that Cb2-EGFP–expressing 32D/G-CSF-R clones stop growing at a later time point than control clones (Figure6A). Indeed, 3H-TdR incorporation experiments carried out with cells harvested at day 7 of G-CSF culture showed that Cb2-EGFP–expressing 32D/G-CSF-R clones were still proliferating, whereas EGFP-expressing clones were not (Figure6B). Moreover, Cb2-EGFP–expressing 32D/G-CSF-R cells cultured with G-CSF plus the Cb2-specific antagonist SR1445283 harvested at day 7 behaved like EGFP-transfected control cells; that is, they did not incorporate 3H-TdR. The same cells cultured in the presence of the Cb1-specific antagonist SR141716 did not lose their proliferative response (data not shown). Cells from Cb2-EGFP–expressing or control clones harvested at day 8 or 9 of culture in the presence of G-CSF did not show any 3H-TdR incorporation (data not shown).
G-CSF and IL-3 proliferative response of Cb2-EGFP– and EGFP-expressing 32D/G-CSF-R clones.
(A) Different proliferation curves of 8 Cb2-EGFP–expressing clones and 4 EGFP control clones stimulated with G-CSF. The y-axis indicates the number of cells × 105/mL. (B) [3H]-TdR incorporation of Cb2-EGFP–expressing cells or EGFP control cells at day 7 of culture in G-CSF (G) with or without the Cb2-specific antagonist (C2). The y-axis represents [3H]-TdR incorporation in counts per minute. (C) Proliferation curves of 8 Cb2-EGFP– and 4 EGFP-expressing clones stimulated with IL-3 during 9 days of culture. The y-axis indicates the number of cells × 105/mL.
G-CSF and IL-3 proliferative response of Cb2-EGFP– and EGFP-expressing 32D/G-CSF-R clones.
(A) Different proliferation curves of 8 Cb2-EGFP–expressing clones and 4 EGFP control clones stimulated with G-CSF. The y-axis indicates the number of cells × 105/mL. (B) [3H]-TdR incorporation of Cb2-EGFP–expressing cells or EGFP control cells at day 7 of culture in G-CSF (G) with or without the Cb2-specific antagonist (C2). The y-axis represents [3H]-TdR incorporation in counts per minute. (C) Proliferation curves of 8 Cb2-EGFP– and 4 EGFP-expressing clones stimulated with IL-3 during 9 days of culture. The y-axis indicates the number of cells × 105/mL.
No significant differences were found in the IL-3 response of Cb2-EGFP–expressing clones versus EGFP-expressing clones (Figure 6C). No effect of 2-AG or of the antagonists was observed on the IL-3–induced proliferative response of Cb2-EGFP– or EGFP-expressing 32D/G-CSF-R clones (data not shown).
A potent Cb2 agonist is present in the culture medium
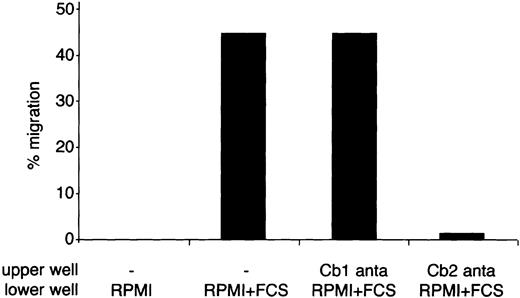
The 2-AG, a potent Cb2 ligand, is usually required to induce migration of Cb2-expressing cells (Figure 2 and Alberich Jorda et al9). However, the addition of 2-AG was not required in the G-CSF–containing cultures to block G-CSF–induced neutrophilic differentiation. We hypothesized that a ligand activating the Cb2 receptor had been present in the cultures. This potent agonist could either have been present in the serum added to the cultures or endogenously produced by the cells (autocrine stimulation). To test whether the serum contained a Cb2-specific agonist, we assessed the effect of the culture medium on migration of 32D/G-CSF-R/Cb2-EGFP cells. Cb2-EGFP–expressing cells showed a significant migration when RPMI plus 10% FCS was added to the lower chamber in a transwell assay (Figure 7). Moreover, this migration could be completely blocked by addition of Cb2-specific antagonist SR1445283 to the upper well, but not by addition of the Cb1-specific antagonist SR141716 (Figure 7).
Effect of FCS on migration of Cb2-EGFP–expressing 32D/G-CSF-R cells.
32D/G-CSF-R/Cb2-EGFP cells were exposed to RPMI medium containing or not containing serum during a transwell assay. A quantity of 100 nM of Cb1- or Cb2-specific antagonist was placed in the upper well. The y-axis indicates the percentage of migration from an input of 2 × 105 cells.
Effect of FCS on migration of Cb2-EGFP–expressing 32D/G-CSF-R cells.
32D/G-CSF-R/Cb2-EGFP cells were exposed to RPMI medium containing or not containing serum during a transwell assay. A quantity of 100 nM of Cb1- or Cb2-specific antagonist was placed in the upper well. The y-axis indicates the percentage of migration from an input of 2 × 105 cells.
Transwell experiments using conditioned medium obtained from serum-free cultures of the different 32D/G-CSF-R clones showed no migration of Cb2-EGFP–expressing cells (data not shown). These data indicate that the block of neutrophilic differentiation of 32D/G-CSF-R/Cb2-EGFP cells was caused by a potent Cb2 agonist, which was present in the serum used for these cultures.
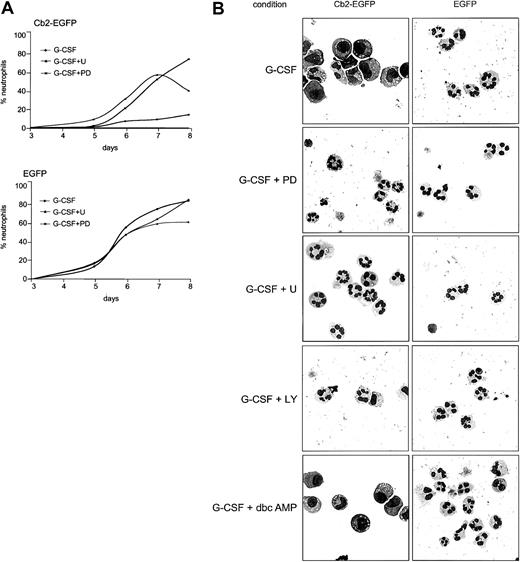
Inhibitors of the MEK/ERK and PI3-kinase pathways restore neutrophilic differentiation of Cb2-EGFP–expressing 32D/G-CSF-R cells
To determine which signal transduction pathway(s) may be involved in the Cb2 receptor–mediated block of neutrophilic differentiation we investigated the effect of distinct small molecules capable of interfering with specific signaling routes. Interestingly, addition of the MEK inhibitors, 10 μM U0126, or 25 μM PD98059, fully restore the G-CSF–induced neutrophilic differentiation of Cb2-EGFP–expressing 32D/G-CSF-R cells (Figure 8).
Effect of signal transduction pathway inhibitors on the G-CSF differentiation response of 32D/G-CSF cells.
(A) Cb2-EGFP–expressing cells and EGFP control cells were cultured for 9 days in the presence of G-CSF plus U0126 (U) or PD98059 (PD). At the y-axis the percentage of neutrophils is shown, and at the x-axis the day of culture is indicated. (B) Morphologic analysis of a Cb2-EGFP–expressing clone and an EGFP control clone cultured in the presence of G-CSF plus PD98059, U0126, LY-294002, or dbcAMP. Pictures were taken at day 8 of culture except for incubations with U0126 (day 6). Original magnifications × 63.
Effect of signal transduction pathway inhibitors on the G-CSF differentiation response of 32D/G-CSF cells.
(A) Cb2-EGFP–expressing cells and EGFP control cells were cultured for 9 days in the presence of G-CSF plus U0126 (U) or PD98059 (PD). At the y-axis the percentage of neutrophils is shown, and at the x-axis the day of culture is indicated. (B) Morphologic analysis of a Cb2-EGFP–expressing clone and an EGFP control clone cultured in the presence of G-CSF plus PD98059, U0126, LY-294002, or dbcAMP. Pictures were taken at day 8 of culture except for incubations with U0126 (day 6). Original magnifications × 63.
Furthermore, the PI3-kinase inhibitor LY-294002 (5 μM) was capable of partially recovering maturation of these cells when cultured with G-CSF (Figure 8B). On the other hand, SB203580 (5 μM), an inhibitor of p38/MAPK, had no promoting effect on neutrophilic differentiation (data not shown). We also investigated whether Cb2 interferes with differentiation via a protein kinase A (PKA)–dependent signaling mechanism. Addition of dbcAMP (100 μM) to the G-CSF cultures did not influence maturation of 32D/G-CSF-R/Cb2-EGFP clones (Figure 8B). The data suggest that the block of neutrophilic differentiation in myeloid precursor cells caused by the Cb2 receptor may involve activation of both the MEK/ERK and PI3-K pathways.
Discussion
We previously described that the gene encoding for the peripheral cannabinoid receptor Cb2 is located in a common virus integration site(Evi11) in murine myeloid leukemia, indicating that aberrant expression of this GPCR may be a critical event in leukemia development.1 2 However, by which mechanism this GPCR may be involved in leukemic transformation has remained elusive. The objective of the present study was to determine the effect of Cb2 overexpression in myeloid precursor cells. We demonstrate here that overexpression of Cb2-EGFP completely suppresses G-CSF–induced neutrophilic differentiation of 32D/G-CSF-R cells. The addition of the Cb2-specific antagonist fully recovered neutrophilic differentiation of these cells, indicating that the block of differentiation is a Cb2 receptor–specific effect. The data demonstrate that stimulation of Cb2 receptors when overexpressed on myeloid precursors turns on signals that interfere with normal neutrophilic differentiation.
A major characteristic of myeloid leukemia is a differentiation arrest of the granulocytic lineage.10 G-CSF regulates proliferation, survival, and differentiation of myeloid progenitor cells.23,24 Multiple gene products or their mutants have been implicated in impairment of G-CSF–regulated neutrophilic development. Alterations in the G-CSF-R, signaling molecules (eg, MEK, STAT1/3, SHP-2, Grb2, shc), or transcription factors (eg, Evi1, C/EBPα, Hoxa9, Hoxb8, Meis) have been reported to alter neutrophilic differentiation.19,21,25-31 Here, we describe the first example of a GPCR that interferes with G-CSF–induced neutrophilic differentiation. Several GPCRs have been implicated in transformation when overexpressed in the NIH 3T3 fibroblasts model, for example, the MAS-oncogene,32 thrombin receptor,33 and serotonin 1c receptors.34Introduction of Cb2 cDNA into NIH 3T3 cells did not result in oncogenic transformation (R.D. et al; unpublished observation, May 1997), suggesting another mechanism of transformation by this GPCR. Overexpression of the other oncogenic GPCRs in 32D/G-CSF-R cells may reveal whether the Cb2 receptor indeed uniquely mediates a block of neutrophilic maturation or whether a more general GPCR-related process causes tranformation of myeloid precursor cells.
G proteins consist of 3 heterologous subunits (α, β, and γ) which are stably associated to guanosine diphosphate (GDP) only during the inactive state. Upon ligand activation of the receptor, GDP is exchanged for guanosine triphosphate (GTP) and the trimer dissociates into a Gα-GTP subunit and a Gβγ dimer, both capable of activating intracellular signaling pathways.35-37 The Cb2 receptor belongs to the family of GPCRs that couple to Gαi/o βγ proteins. Following receptor activation, the Gαi/osubunit inhibits adenylyl cyclase (AC) activity resulting in a decreased level of intracellular cAMP.38-40 Interference of this particular intracellular process by addition of dibutyryl cyclic AMP to the G-CSF cultures did not restore granulocytic differentiation of Cb2-EGFP–expressing 32D/G-CSF-R cells. Thus, down-regulation of cAMP levels following Cb2 activation appears not responsible for the block of neutrophilic differentiation and suggests involvement of another signaling pathway. The Gβγ dimer has been shown to mediate mitogenic-mediated protein kinase (MAPK) activation,41-44 thereby influencing cell proliferation and differentiation. We observed restoration of neutrophilic development by addition of U0126 or PD98059 inhibitors to the G-CSF cultures, suggesting that overactivation of the MEK/ERK pathway following Cb2 stimulation is a major cause of the block of granulocytic differentiation. Furthermore, at day 7 of culture in the presence of G-CSF, Cb2-EGFP–expressing cells still incorporated tritiated thymidine, whereas control cells did not. These observations are in agreement with previous studies showing that activation of MEK/ERK is a critical event in G-CSF–induced proliferation and is not required for differentiation.26 45
The MEK/ERK pathway has been implicated in signaling by multiple GPCRs, including SDF-1,46 somatostatin,47 and opioid receptors.48 Expression of each of these receptors has been demonstrated on hematopoietic precursor cells,49-51but under these physiologic conditions interference with neutrophilic differentiation has not been reported. GPCR overexpression and subsequent uncontrolled MEK/ERK activation may be required for myeloid transformation. Multiple GPCR transfectants of 32D/G-CSF-R should be generated to answer the question of whether sustained MEK/ERK activation by GPCR stimulation is sufficient to cause a block of neutrophilic differentiation.
Several GPCRs can activate MAPK by signaling via PI3-K.52-54 In the presence of LY-294002, granulocytic development in response to G-CSF is partially recovered as well, suggesting the involvement of PI3-K in the block of neutrophilic differentiation evoked by activation of the peripheral cannabinoid receptor. PI3-K has been related with mitogenic signaling through a variety of growth factors,55 including G-CSF.56 Activation of PI3-K via G-CSF-R has been shown to correlate with enhanced proliferation, suggesting that activation of PI3-K by the Cb2 receptor will keep the Cb2-EGFP–expressing cells with a higher growth potential. This would be in agreement with our findings presented in Figure 6. It is possible that activation of multiple signaling pathways, including PI3-K and MEK/ERK, are required to interfere with G-CSF–induced neutrophilic differentiation. Because PI3-K may be involved in cell survival,57 58 we tested whether Cb2-expressing 32D/G-CSF-R cells, cultured in the presence of the endocannabinoid 2-AG but in the absence of cytokines, showed altered survival or proliferation. Although PI3-K signaling may be involved in the Cb2-induced block of neutrophilic differentiation, we did not observe any effect of Cb2 receptor activation on survival or proliferation under the conditions tested.
Several cannabinoid ligands of different origins have been proposed as the true ligands for the peripheral cannabinoid receptor.59-62 We recently compared the capability of these different ligands to induce migration of Cb2-expressing cells. Using transwell assays we demonstrated that 2-AG is the most potent inducer of migration.9 Since 2-AG or other cannabinoid ligands were not added to the cultures presented here, we hypothesized that a Cb2 ligand was present in the serum containing medium or produced by the cells themselves. We demonstrate in Figure 7 that significant levels of Cb2 ligands were present in the serum. It is unclear whether culture medium contains 2-AG or another yet-to-be-identified stimulus for Cb2. The fact that specific Cb2 agonist(s) are present in the serum used in our studies suggests that Cb2 ligands may be present in vivo in sufficient quantities. This finding may allow studies to investigate the effect of Cb2 overexpression in bone marrow progenitor cells in vivo. Introduction of the Cb2 gene into primitive progenitor cells followed by transplantation into sublethally irradiated mice should reveal whether high Cb2 expression causes a myeloproliferative disorder or myeloid leukemia.
Our observations raise the question of whether the human CB2 receptor or other GPCRs may be abnormally expressed in certain cases of human AML. High-throughput AML patient screens using cDNA array technology are currently in progress, which may identify such cases. Exposure of those leukemia samples to GPCR antagonists, specific MEK/ERK inhibitors, or combinations thereoff may unravel the importance of those receptors and downstream signaling routes in myeloid differentiation abnormalities and possibly open new ways for treatment of AML.
We thank Prof Dr I. P. Touw (Erasmus University Rotterdam) for donation of the 32D/G-CSF-R cell line. We thank Karola van Rooyen for preparation of the figures and Dr P. Casellas (Sanofi Recherche, Montpellier, France) for donation of the Cb1- and Cb2-specific antagonists, SR141716 and SR144528. We thank C. M. Dicke for technical assistance.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-07-2034.
Supported by the Dutch Cancer Foundation Koningin Wilhelmina Fonds, The Netherlands Organization for Scientific Research NWO, and the Royal Dutch Academy of Sciences KNAW.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ruud Delwel, Institute for Hematology, Erasmus University Rotterdam, Dr Molewaterplein 50, 3015GE Rotterdam, The Netherlands; e-mail: delwel@hema.fgg.eur.nl.

![Fig. 6. G-CSF and IL-3 proliferative response of Cb2-EGFP– and EGFP-expressing 32D/G-CSF-R clones. / (A) Different proliferation curves of 8 Cb2-EGFP–expressing clones and 4 EGFP control clones stimulated with G-CSF. The y-axis indicates the number of cells × 105/mL. (B) [3H]-TdR incorporation of Cb2-EGFP–expressing cells or EGFP control cells at day 7 of culture in G-CSF (G) with or without the Cb2-specific antagonist (C2). The y-axis represents [3H]-TdR incorporation in counts per minute. (C) Proliferation curves of 8 Cb2-EGFP– and 4 EGFP-expressing clones stimulated with IL-3 during 9 days of culture. The y-axis indicates the number of cells × 105/mL.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/4/10.1182_blood-2002-07-2034/3/m_h80433808006.jpeg?Expires=1766136090&Signature=K10ttzJcmn~5n~W2~lvA9bnNNcZRthEpA2xAffqXwyftGJYenSNfaGtkiS1x8ioKkTrcbZUfH-bono2LHkLluv8vboD1onKclEFejbsnKrICt8Q5vWhXpQexH5SffAm2FGAkdnRer0V7UU0HqUCBdLFseO4BxiE8-RoYfwkwVyh0yr7OS5wtFzq4qUCVaH3c3I5CPEKJSNbiT224-PGU5TaV5r1KJhRcssZuBpmV08VGw~11HbrLcbE5tn~Bq~fbKdhC32~~qTUkukegq6cTLnown8nliM0y6u6~fa9OMNNEuQecN29dqR4pmhYKTmK86trRXbemU5swYLdarS~Qlw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 6. G-CSF and IL-3 proliferative response of Cb2-EGFP– and EGFP-expressing 32D/G-CSF-R clones. / (A) Different proliferation curves of 8 Cb2-EGFP–expressing clones and 4 EGFP control clones stimulated with G-CSF. The y-axis indicates the number of cells × 105/mL. (B) [3H]-TdR incorporation of Cb2-EGFP–expressing cells or EGFP control cells at day 7 of culture in G-CSF (G) with or without the Cb2-specific antagonist (C2). The y-axis represents [3H]-TdR incorporation in counts per minute. (C) Proliferation curves of 8 Cb2-EGFP– and 4 EGFP-expressing clones stimulated with IL-3 during 9 days of culture. The y-axis indicates the number of cells × 105/mL.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/4/10.1182_blood-2002-07-2034/3/m_h80433808006.jpeg?Expires=1766189549&Signature=aiAUl-lW~sr7o9SEKBHQKBG022~uDiT~9htBHYfL2zy0UMG4wNn5bgp1oU6kejs~KILXotHFbLZPHWePoOjF-gFtxI0SKkK-mZxa5oC2eHda08PbgTmcxF5V6sGhhTHNY~pPTDt-cRc9J0lrSceW~~tA-4hYfCpQfOkQzT1H4VpFKRGUDroDyaaL35ieyY0btS9141vSgbUnEZbgGjcp89GGGGVGRvcOAUHMA8lDoZ8tFTsq6lgxqRruXr80YUb5ZFvc6ShU-wIdIfEzHQblIVV2diCtl-ekqfDKjlpbhZmLMrqheWi~XfmULSZaefhKkpN7saLHRwKWHCwbeqEYjw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

