Most lymphomas that involve the central nervous system are B-cell neoplasms that express the cell surface molecule CD20. After intravenous administration, rituximab can be reproducibly measured in the cerebrospinal fluid (CSF) in patients with primary central nervous system lymphoma; however, the CSF levels of rituximab are approximately 0.1% of serum levels associated with therapeutic activity in patients with systemic non-Hodgkin lymphoma. Because lymphomatous meningitis is a frequent complication of non-Hodgkin lymphoma, we have conducted an analysis of the safety and pharmacokinetics of direct intrathecal administration of rituximab using cynomolgus monkeys. No significant acute or delayed toxicity, neurologic or otherwise, was detected. Pharmacokinetic analysis suggests that drug clearance from the CSF is biphasic, with a terminal half-life of 4.96 hours. A phase 1 study to investigate the safety and pharmacokinetics of intrathecal rituximab in patients with recurrent lymphomatous meningitis will be implemented based on these findings.
Introduction
Central nervous system (CNS) involvement is associated with an adverse outcome in patients with non-Hodgkin lymphoma (NHL).1 Most NHLs that involve the CNS are B-cell neoplasms that express CD20.2 Dissemination within the leptomeninges represents a common pathway of progression in systemic NHL and primary CNS lymphoma and usually heralds neurologic deterioration and a fatal outcome.3
Rituximab monoclonal antibody therapy is an effective treatment for B-cell NHL.4-7 To date, preclinical and clinical practice involving rituximab has been limited to intravenous administration. This agent binds specifically to the antigen CD20, which is expressed on more than 90% of B-cell NHL and primary CNS lymphoma but is not expressed by normal neurons or glia in the brain.
A number of preclinical models have demonstrated that therapeutic antibodies administered into the cerebrospinal fluid (CSF) are able to concentrate in and eradicate tumors within the craniospinal axis with minimal toxicity.8,9 The primate model of drug delivery into the CSF represents a valid experimental model for the prediction of CSF drug pharmacokinetics in humans.10-13 This is the first preclinical analysis of the safety and pharmacokinetics of rituximab administration within the craniospinal axis.
Study design
All experimental procedures have been reviewed and approved by the Committee on Human Research and by the Committee on Animal Research, University of California, San Francisco.
Four female cynomolgus monkeys, aged 16 to 18 years, weighing 3 to 5 kg, were obtained from Biosurg (Winters, CA). A total of 7 experiments were performed with one monkey in each experiment. Data are reported from the 4 experiments that involved suboccipital administration of rituximab. Three experiments used an Ommaya reservoir (Integra NeuroCare, Plainsboro, NJ) in the right lateral ventricle. Although intra-Ommaya administration of rituximab resulted in high CSF concentrations, the size of the reservoir adversely affected normal CSF flow; therefore, these experiments did not provide data suitable for pharmacokinetic analysis. Two of the reported experiments used the same monkey, and the other 2 experiments used separate animals.
Animals were fasted overnight before anesthesia for rituximab intrathecal administration and CSF and serum collections. Animals were anesthetized with intramuscular midazolam plus ketamine and then intubated and maintained under anesthesia with isoflurane. Intravenous vancomycin and atropine were administered. Continuous intravenous fluid was provided during anesthesia. No intubation was required for cisternal puncture for CSF and serum collections on days subsequent to rituximab intrathecal administration.
Suboccipital administration of antibody was performed as described after an equivalent volume of CSF was removed.12 Rituximab (10 mg/mL) was administered as a single dose over 1 minute in a total volume of 0.2 to 0.5 mL, yielding 2 to 5 mg. CSF samples (less than 0.5 mL) were obtained by repeat suboccipital puncture and analyzed for chemistry, cell count, and rituximab concentration.
Pharmacokinetics
Rituximab concentrations were determined by enzyme-linked immunosorbent assay (ELISA). Compartmental pharmacokinetic analysis of rituximab concentrations in the CSF was performed by fitting a biexponential equation representing a 2-compartment biologic model to the CSF concentrations. A nonlinear regression software was used for this purpose (WinNonlin-Pro version 3.0, Pharsight, Mountain View, CA).
Evaluation of toxicity
CSF cell count, differential, total protein, and glucose were measured at baseline, 48 hours, and at 1 week. Animals were closely monitored immediately following drug administration and daily for up to 8 months after treatment. Neuropathologic analysis was performed on each animal. Good laboratory practices were maintained.
Results and discussion
After intravenous administration in patients with CNS lymphoma, rituximab is reproducibly detected in the CSF at concentrations that are at most 0.1% that of matched serum14 (Table1). It is probable that the blood-brain barrier limits the potential efficacy of intravenous rituximab in the prophylaxis or treatment of CNS lymphoma or lymphomatous meningitis.
To improve the therapeutic index of rituximab in CNS lymphoma, it is critical that higher concentrations of this antibody be delivered into the intracranial compartment. For this reason there has been significant interest in intrathecal administration of rituximab in the treatment of patients with lymphomatous meningitis.
The observed CSF and serum profiles of rituximab concentrations after intrathecal injection (Figure 1; Table2) are consistent with the existence of a space within the CSF-brain compartment that is separate from the central space following intrathecal administration. This is supported both by the fit of a 2-compartment model to the CSF concentration data and by the nonparallel profiles of rituximab in CSF and in serum, which indicate that there is a delayed transfer of rituximab from the CSF to the serum. This delay may indicate a distinct distribution space within the CSF prior to distribution to the systemic circulation. A rapid direct transfer from a single space within the CSF to serum would have resulted in parallel rituximab profiles in the 2 biologic fluids. It is possible that following intrathecal administration rituximab distributes within the extracellular space of brain parenchyma and then undergoes a transfer from the CSF through the arachnoid granulations or the Virchow-Robin spaces into the blood. A capacity-limited saturable transfer process from the CSF to the blood may also explain this observation.
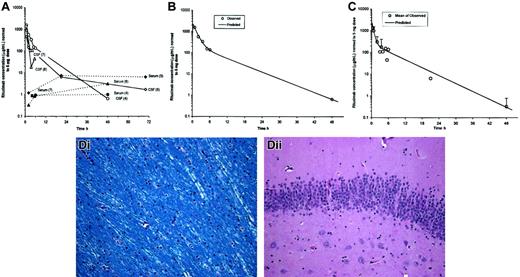
Pharmacokinetic and histopathologic analysis of intrathecal rituximab infusion.
(A) CSF and serum profiles in cynomolgus monkeys injected with 3 to 5 mg rituximab via 1-minute intrathecal infusion. Observed CSF and serum concentrations were normalized to a 5-mg dose. Experiment numbers are shown in parentheses. Measured CSF concentrations of rituximab were maximal within 2 hours of intrathecal administration and ranged from 460 to 1550 μg/mL. CSF concentrations were approaching minimal detectable levels between 24 to 70 hours after administration in those animals with later collection time points. (B) Fit of 2-compartment intravenous infusion model to CSF concentrations from experiment no. 4 (1 monkey). Observed concentrations were normalized to a dose of 5 mg prior to fitting a 2-compartment model. (C) Fit of 2-compartment intravenous infusion model to CSF mean concentrations averaged across all experiments. Observed concentrations were normalized to a dose of 5 mg and then averaged across all experiments prior to fitting a 2-compartment model. (D) Gross and histologic analyses using hematoxylin and eosin and Luxol fast blue stains were performed between 1 and 8 months after either 1 or 2 intrathecal rituximab administrations. There was no evidence of neuronal loss, demyelination, ventriculitis, ependymitis, or vasculitis that was attributable to rituximab administration. (Di) No evidence of demyelination of white matter tracts (Luxol fast blue stain). (Dii) No evidence of neuronal loss in C1A cells of hippocampus (hematoxylin and eosin stain). Original magnifications × 200.
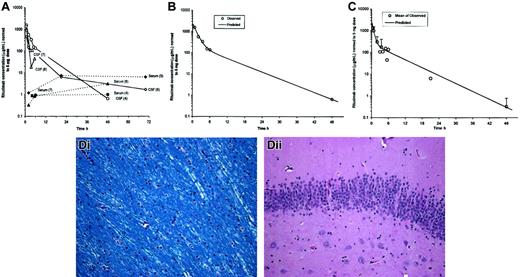
Pharmacokinetic and histopathologic analysis of intrathecal rituximab infusion.
(A) CSF and serum profiles in cynomolgus monkeys injected with 3 to 5 mg rituximab via 1-minute intrathecal infusion. Observed CSF and serum concentrations were normalized to a 5-mg dose. Experiment numbers are shown in parentheses. Measured CSF concentrations of rituximab were maximal within 2 hours of intrathecal administration and ranged from 460 to 1550 μg/mL. CSF concentrations were approaching minimal detectable levels between 24 to 70 hours after administration in those animals with later collection time points. (B) Fit of 2-compartment intravenous infusion model to CSF concentrations from experiment no. 4 (1 monkey). Observed concentrations were normalized to a dose of 5 mg prior to fitting a 2-compartment model. (C) Fit of 2-compartment intravenous infusion model to CSF mean concentrations averaged across all experiments. Observed concentrations were normalized to a dose of 5 mg and then averaged across all experiments prior to fitting a 2-compartment model. (D) Gross and histologic analyses using hematoxylin and eosin and Luxol fast blue stains were performed between 1 and 8 months after either 1 or 2 intrathecal rituximab administrations. There was no evidence of neuronal loss, demyelination, ventriculitis, ependymitis, or vasculitis that was attributable to rituximab administration. (Di) No evidence of demyelination of white matter tracts (Luxol fast blue stain). (Dii) No evidence of neuronal loss in C1A cells of hippocampus (hematoxylin and eosin stain). Original magnifications × 200.
A 2-compartment model was fit to data from one full CSF profile (Figure1B) and to the mean CSF profile across all 4 experiments (Figure 1C).
Based upon the mean CSF profile, half-lives for the initial distribution/elimination and terminal elimination phase were 0.414 hours and 4.96 hours, respectively. Predicted maximal CSF concentration (Cmax at time zero) was 2250 μg/mL. The CSF distribution volume for rituximab was estimated to be 6.82 mL at distribution equilibrium (Vss; Table 2). It was not possible to compute pharmacokinetic (PK) parameters for serum concentrations, because rituximab levels were generally still plateaued at the end of the sampling period.
Intrathecal rituximab administration was well tolerated in cynomolgus monkeys. There was no clinical evidence of neurotoxicity after 7 intrathecal administrations in a total of 4 animals (3 animals received intrathecal rituximab twice) (Figure 1D).
The administration of rituximab into the CSF in patients with lymphomatous meningitis may provide an opportunity to deduce its mechanism of action. Blockade of CD20 may elicit B-cell lysis by several mechanisms, including induction of apoptosis and complement-mediated or antibody-mediated cellular cytotoxicity. Under normal conditions and in the presence of intracranial tumors, CSF levels of complement proteins C3 and C4 are approximately 100- to 200-fold lower than serum levels.15 We were unable to detect complement activation or C3 antigen in the CSF of cynomolgus monkeys before and after the addition of rituximab.
The emergence of monoclonal antibody therapy in the treatment of leptomeningeal cancer may provide a potentially important addition to the limited armamentarium currently available for this devastating problem. Our pharmacokinetic data suggest that the intrathecal administration of a monoclonal antibody results in an initial high concentration before distribution into the CSF-brain compartment. There was no evidence for CNS toxicity in this study. This is the first analysis of the safety and pharmacokinetics of intrathecal administration of an FDA-approved monoclonal antibody targeted to treat cancer. These data may constitute a basis for the evaluation of other emerging antibodies that target tumors that commonly exhibit leptomeningeal involvement. We are currently using this information to initiate the first of these investigations: a phase 1 study of intrathecal rituximab in the treatment of recurrent lymphomatous meningitis.
The authors thank Dr Ronald Levy of Stanford University School of Medicine. We are also indebted to Laurie Brignolo, Peter Thorsen, Larry Carbone, Daniel Lau, Ron Bayreuther, Cheyne Stokes, Linda Brovarney, Michelle Aird, and Marsha Potolo for assistance with the animal studies.
Prepublished online as Blood First Edition Paper, September 5, 2002; DOI 10.1182/blood-2002-06-1636.
Supported by grants from the California Cancer Research Program, IDEC Pharmaceuticals/Genentech, and the UCSF Mt Zion Cancer Center.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
James L. Rubenstein, University of California, San Francisco, Division of Hematology/Oncology M1282, Box 1270, San Francisco, CA 94143; e-mail:jamesr@medicine.ucsf.edu.