Abstract
Mitogen-activated protein (Map) kinases are widely expressed serine-threonine kinases that mediate important regulatory signals in the cell. Three major groups of Map kinases exist: the p38 Map kinase family, the extracellular signal-regulated kinase (Erk) family, and the c-Jun NH2-terminal kinase (JNK) family. The members of the different Map kinase groups participate in the generation of various cellular responses, including gene transcription, induction of cell death or maintenance of cell survival, malignant transformation, and regulation of cell-cycle progression. Depending on the specific family isoform involved and the cellular context, Map kinase pathways can mediate signals that either promote or suppress the growth of malignant hematopoietic cells. Over the last few years, extensive work by several groups has established that Map kinase pathways play critical roles in the pathogenesis of various hematologic malignancies, providing new molecular targets for future therapeutic approaches. In this review, the involvement of various Map kinase pathways in the pathophysiology of hematologic malignances is summarized and the clinical implications of the recent advances in the field are discussed.
Introduction
The different members of the superfamily of mitogen-activated protein (Map) kinases participate in signaling cascades conserved through evolution, which regulate important biologic activities. Three major groups of Map kinases (MAPKs) exist: the p38 Map kinase family, the extracellular signal-regulated kinase (Erk) family, and the c-Jun NH2-terminal kinase (JNK) kinase family1-12 (Table 1). The p38 Map kinase family is composed of 4 different isoforms (p38α, p38β, p38γ, and p38δ) that share significant structural homology,1,4,7,10,13-24 while the JNK kinase group includes 3 members, JNK1, JNK2, and JNK3.2-5,25-28 The Erk family of kinases includes Erk1 and Erk22,29,30 (Table 1). Erk1 and Erk2 are structurally and functionally similar kinases,2,25,26 while Erk331 is a Map kinase that shares significant homology with Erk2 but also has distinct functions that differentiate it from the 2 classic Erk kinases (Erk1 and Erk2).2 Furthermore, a subfamily of Erk3-related Map kinase genes, composed of 2 functional genes, MAPK6 and MAPK4, and several pseudogenes, has been recently identified.32 Similarly, 2 other recently identified kinases, Erk5/BMK1 and Erk7, share some structural homology with the classic Erk kinases, but appear to also have distinct upstream and downstream effectors, and they are not classified in the classic Erk kinase group2,33-35 (Table 1). Finally, a more recently cloned kinase, Erk8,36 shares substantial homology with Erk7, but appears to have some differences in its substrate specificity (Table 1).
Map kinase signaling cascades are activated by a variety of different cellular stimuli and mediate diverse responses. Accumulating evidence indicates that an important function of Map kinases is the generation of signals of critical value to the control of normal and malignant hematopoiesis by cytokines and growth factors. The Erk pathway is activated in response to several cytokines and growth factors, and primarily mediates mitogenic and antiapoptotic signals.1-3,10,12 Members of the p38 family of Map kinases are primarily activated by stress stimuli, but are also activated during engagement of various cytokine receptors by their ligands.2-4,7,37 The function of the p38 kinases is required for the generation of various activities, including regulation of apoptosis and cell-cycle arrest, induction of cell differentiation, as well as cytokine production and inflammation.2-4,7,38 The JNK pathway is also activated in response to stress and growth factors, and, similarly, mediates signals that regulate apoptosis, cytokine production, and cell-cycle progression.2-5,10
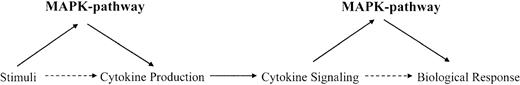
In general, Map kinase pathways are activated by various stimuli to regulate, among other things, production of various cytokines and growth factors. Subsequently, they participate in signaling pathways activated by such cytokines or growth factors to mediate generation of specific biologic responses. Thus, for a given cytokine-dependent response, the function of Map kinases may be critical at 2 steps. The first step involves the biosynthesis and production of the cytokine in response to a stimulus, while the second step involves participation of Map kinases in signaling cascades activated by the cytokine to induce its biologic effects on target cells (Figure 1).
Dual roles for Map kinase pathways in the induction of cytokine responses. Regulation of cytokine production and participation in cytokine-dependent signaling cascades.
Dual roles for Map kinase pathways in the induction of cytokine responses. Regulation of cytokine production and participation in cytokine-dependent signaling cascades.
Regulation of activation of Map kinase signaling cascades
In order for the different Map kinases to be activated by various stimuli, there is a requirement for dual phosphorylation on threonine (Thr) and tyrosine (Tyr) residues present in specific motifs (ThrXaaTyr) for each kinase group.1-12 Such dual phosphorylation motifs are located in the activation loops of the different Map kinases. The distinct motifs for the different groups include: Thr-Gly-Tyr for the p38 kinases, Thr-Pro-Tyr for JNK kinases, and Thr-Glu-Tyr for the classic Erk kinases (Erk1 and Erk2) and for Erk5.1-12 The phosphorylation of the different members of the Map kinase groups is regulated by upstream dual-specificity kinases, which are capable of phosphorylating Map kinases on both serine/threonine, as well as on tyrosine residues.1-12 These kinases are called Map kinase kinases (MAPKKs or Meks) and exhibit relative specificity for the substrate Map kinase proteins that they phosphorylate. Mkk1 and Mkk2 phosphorylate Erk kinases in the Thr-Glu-Tyr motif; Mkk3, Mkk4, and Mkk6 phosphorylate p38 kinases in the Thr-Gly-Tyr motif, while Mkk4 and Mkk7 regulate dual phosphorylation of JNK kinases in the Thr-Pro-Tyr motif (Table 2). Another member of the MAPKK family of kinases is Mkk5, which selectively phosphorylates the Thr-Glu-Tyr motif in Erk5/BMK-1.1-12,38 Interestingly, MAPKK proteins also exhibit relative specificity for the different isoforms that they target within each group of Map kinases. For instance, among the different p38 subtypes, Mkk6 functions as a common activator for p38α, p38β, and p38γ, while Mkk3 activates p38α and p38γ, but not p38β.39
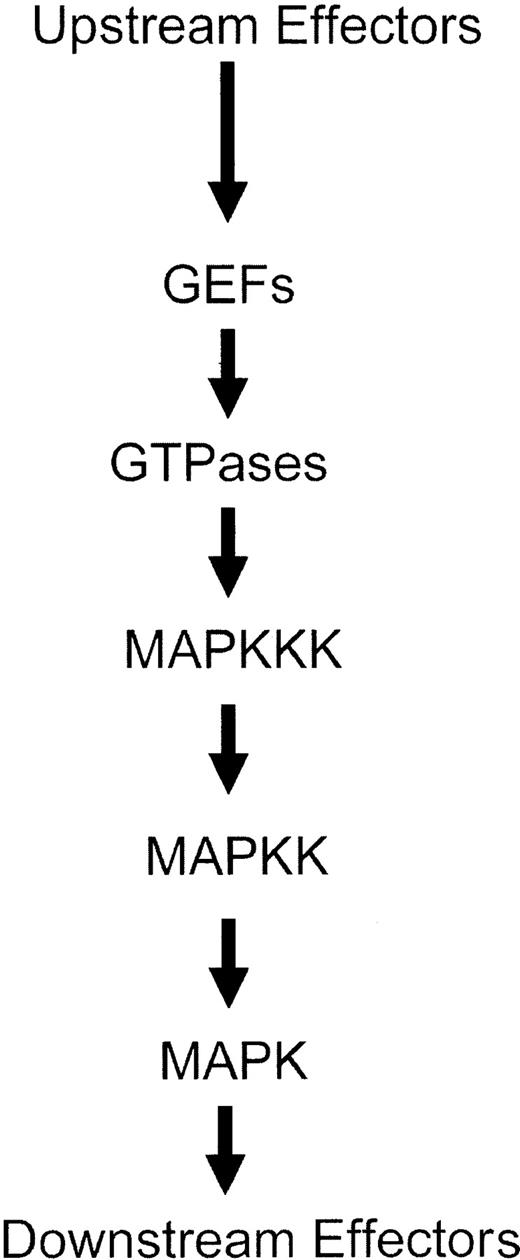
The activation of MAPK kinases (MAPKKs) is regulated by other upstream serine-threonine kinases, called MAPKK kinases (MAPKKKs), which phosphorylate the MAPK kinases (MAPKKs) on specific serine residues.1-12 Thus, a series of phosphorylation events result in consecutive activation of serine-threonine and dual specificity kinases, ultimately leading to induction of the kinase domains of Map kinases and engagement of downstream pathways1-12 (Figure 2). Several serine-threonine kinases have been implicated in acting as MAPKKKs for the 3 different groups of Map kinase pathways. In the case of the Erk pathway, the family of Raf kinases, as well as Mos and Tpl2, act as MAPKKKs.1-4,40-42 On the other hand, the different MAPKKs that regulate activation of the JNK and p38 kinases include mixed-lineage kinases (Mlk1, Mlk2, Mlk3, Dlk, and Lzk), Mekk kinases (Mekk1, Mekk2, Mekk3, and Mekk4), Tak1, Ask1, Ask2, and Tpl-2.1-7,42-60 It should be noted that, although transfection assays had demonstrated that Tpl-2 is a component of the JNK pathway, knock-out studies have indicated that it is a regulator of the Erk pathway.61
Schematic generic overview of the sequence of events leading to activation of Map kinase pathways.
Schematic generic overview of the sequence of events leading to activation of Map kinase pathways.
Activation of the MAPKKKs or MAPKKs occurs downstream from small G-proteins, whose function is regulated by guanine exchange factor (GEF) proteins (Figure 1). The small G-proteins that regulate activation of various Map kinase family members include Ras for the Erk pathway1-3,10,12,62 and members of the Rho family of proteins (Rac1, Cdc42, RhoA, and RhoB) for the p38 and JNK pathways.4-10,63-68 Thus, initial activation of GEFs leads to activation of GTPases and downstream initiation of distinct kinase cascades that regulate activation of different Map kinases (Figure 2). A summary of the distinct pathways that are activated downstream of various GTPases, ultimately leading to MAPK activation, is shown in Figure 3.
MAPKKK and MAPKK proteins that regulate activation of the Erk, p38, and JNK Map kinase pathways.
MAPKKK and MAPKK proteins that regulate activation of the Erk, p38, and JNK Map kinase pathways.
Cellular responses mediated by Map kinases
The activation of different Map kinase signaling cascades by various stimuli is required for induction of various important cellular biologic responses, including phosphorylation of transcription factors and transcriptional regulation, nuclear chromatin remodeling and immediate gene induction, cytokine production, as well as regulation of apoptosis and cell-cycle progression. Such biologic activities vary with the specific family of Map kinases activated and the distinct stimulus inducing such activation. In general, the Ras/Erk pathway mediates primarily cell growth and survival signals, but under certain circumstances also promotes induction of cell differentiation.1-3,10-12 On the other hand, the stress-activated p38 and JNK kinase pathways mediate primarily proapoptotic and growth inhibitory signals, as well as proinflammatory responses.1-12 However, activation of the p38 pathway may also induce antiapoptotic, proliferative, and cell survival signals under certain conditions, depending on the tissue and specific isoform involved.5
Thus, multiple and divergent cellular functions are controlled by the activation of various components of Map kinase signaling cascades in response to a large number of stimuli. This raises questions regarding how the specificity of Map kinase–regulated responses occurs. An important issue revolves around the identity of cellular mechanisms that are in place to prevent cross-talk between the various cascades and to maintain the veracity and specificity of different signals generated by distinct Map kinase groups.69 Extensive studies over the last few years have identified mechanisms that may account for Map kinase specificity.69-71 It is likely that the events that define such specificity involve physical interactions of Map kinases with other proteins.69-71 It has been established that Map kinases utilize for their interactions with other proteins the common docking site and the Glu-Asp (ED) site, while the proteins that interact with Map kinases have in their structure D-domains or Phe-Xaa-Phe-Pro (FXFP) domains.69-71 Recently, the resolution of the crystal structure of p38 Map kinase bound to the docking domains of MEF2A and Mkk3b72 defined the nature of the interaction between p38 and its substrates and provided a model on the structural basis of substrate specificity that may apply to the other Map kinase groups as well.69 Thus, it appears that despite the extensive Map kinase networks and the multiple Map kinase members involved, specificity for Map kinase responses relies upon distinct interactions of different effector proteins with specific domains within the structure of Map kinases.
Map kinases and cytokines that regulate hematopoiesis
It is well established that various cytokines and growth factors that regulate normal hematopoietic cell proliferation and differentiation activate Map kinase signaling pathways to generate their effects. Among the different hematopoietic growth factors, erythropoietin has been shown to activate, in responsive cell lines or primary hematopoietic progenitors, members of all different Map kinase groups, including Erk, p38, and JNK.73-80 Similarly, another cytokine whose function is required for normal erythropoiesis, stem cell factor (SCF), is capable of inducing activation of all different Map kinase groups under certain conditions.77,79,81 In addition, interleukin-3 (IL-3), granulocyte colony-stimulating factor (G-CSF), macrophage CSF (M-CSF), granulocyte-macrophage CSF (GM-CSF), and thrombopoietin, also activate Map kinase pathways.73,75,81-87 Thus, it appears that essentially all known hematopoietic growth factors utilize Map kinase pathways for the generation of signals that potentially regulate normal hematopoiesis. In certain cases, the activation of a specific Map kinase pathway may be synergistically induced by a combination of growth factors to promote hematopoietic cell survival and growth. For instance, erythropoietin and stem cell factor synergistically activate Erk1/2 in purified human erythroid progenitors, and such synergistic activation of the classic Erk pathway appears to correlate with induction of cell growth.79 On the other hand, certain Map kinase pathways also play critical roles in production of hematopoietic growth factors. As an example, targeted disruption of the p38 α gene in mice generally leads to embryonic lethality between days 11.5 and 12.5, but those mice who survive past this stage have normal morphology except that they are anemic because of diminished erythropoietin gene expression.88 Thus, the p38α Map kinase appears to be critical for developmental erythropoiesis via regulation of erythropoietin expression.88 On the other hand, activation of p38 is necessary for erythropoietin-dependent differentiation of erythroid cells.78 Such a dual function of the p38 pathway in erythropoiesis suggests the existence of Map kinase–regulated circuits, in which the function of a given Map kinase may control developmental expression of a certain hormone or growth factor and, at the same time, regulate its signaling capacity (Figure 1). However, it should be emphasized that defective erythropoiesis is not the primary consequence of targeted disruption of the p38α gene in mice, and the major phenotype appears to be embryonic lethality related to placental developmental defects.89-91
In addition to their activation by hematopoietic growth factors, Map kinases are activated by cytokines that negatively regulate normal human hematopoiesis. Among the several cytokines that have been shown to suppress hematopoiesis in vitro and in vivo, the type I interferons (IFNα, IFNβ, and IFNω)92,93 are probably the most extensively studied. It has been known for many years that these cytokines are potent inhibitors of the growth of hematopoietic progenitors of all different lineages, including erythroid progenitors (burst-forming unit erythroid [BFU-E], colony-forming unit erythroid [CFU-E]), myeloid progenitors (CFU granulocyte-macrophage [CFU-GM]), megakaryocytic progenitors (CFU megakaryocyte [CFU-MK]) and mixed lineage progenitors (CFU granulocyte erythroid macrophage mixed [CFU-GEMM]).94-100 Both the Erk1/2101,102 and p38 pathways103,104 are activated in response to treatment of human cell lines with type I interferons IFNα or IFNβ. Moreover, the p38 Map kinase cascade plays a critical role in type I interferon signaling, as it is required for regulation of type I IFN–dependent gene transcription, without modifying activation of signal transducer and activator of transcription (Stat) proteins.103,104 Recent studies have shown that IFNα and IFNβ induce activation of the α and β isoforms of the p38 Map kinase and its downstream effector Map kinase activated protein kinase-2 (MapKapK-2) in primary human erythroid progenitors.105 Furthermore, treatment of normal bone marrow cells with the p38 pharmacologic inhibitors SB203580 and SB202190 reverses the suppressive effects of type I IFNs on normal human hematopoiesis.105 On the other hand the Mek kinase inhibitor PD98059, which blocks activation of Erk kinases but not p38, had no effects on such hematopoietic suppression.105 Thus, the p38 Map kinase pathway is required for the generation of the suppressive effects of type I interferons on normal human hematopoiesis, while the Erk pathway plays no role in the induction of such effects.105
The requirement of the p38 pathway for the generation of the antiproliferative effects of type I interferons ignited further studies, aimed to evaluate the role of this pathway in the generation of the antiproliferative effects of other well known myelosuppressive cytokines. Such studies demonstrated that, in addition to type I interferons, p38α and p38β are also activated in primitive human hematopoietic progenitors in response to transforming growth factor β (TGFβ),105 tumor necrosis factor α (TNFα),106 as well as type II interferon (IFNγ)106 treatment. Importantly, pharmacologic inhibitors of the p38 Map kinase reversed the inhibitory effects of all these different myelosuppressive cytokines on normal human hematopoiesis in vitro.105,106 The results of these studies strongly suggested that the p38 Map kinase pathway acts as a common signaling mediator for growth inhibitory signals generated by different myelosuppressive cytokines (Figure 4). Such a role for p38 in normal hematopoiesis may have implications in the pathogenesis of certain bone marrow failure syndromes, in which suppression of normal hematopoiesis results from overproduction of myelosuppressive cytokines. Such a hypothesis was recently directly tested in studies using bone marrows from patients with idiopathic aplastic anemia, an acquired bone marrow failure syndrome caused by cytokine overproduction by activated immune cells.107,108 Addition of pharmacologic inhibitors of p38 in aplastic anemia bone marrows enhanced erythroid (BFU-E) and myeloid (CFU-GM) hematopoietic colony formation in vitro,106 raising the possibility that drugs that block activation of the p38 pathway may prove useful in the future treatment of aplastic anemia and other bone marrow failure syndromes.106
Myelosuppressive cytokines that utilize the p38 Map kinase pathway to suppress the growth of human primitive hematopoietic progenitors.
Myelosuppressive cytokines that utilize the p38 Map kinase pathway to suppress the growth of human primitive hematopoietic progenitors.
In general, the available evidence to date suggests that different Map kinase pathways play important roles in signaling for hematopoietic growth factors as well as for myelosuppressive cytokines. Such engagement of Map kinases in signaling for various cytokines appears to exhibit regulatory effects on normal human bone marrow hematopoiesis and has prompted extensive studies to define whether Map kinases participate in the regulation of the abnormal hyperproliferative signals seen in malignant hematopoiesis, as discussed below.
Map kinases in human leukemias
The Erk pathway in leukemias
The best characterized Map kinase signaling cascade in acute and chronic human leukemias is the Raf/Mek/Erk signaling cascade.12 As indicated earlier in this review, the activation of Erk kinases is regulated via protein signaling cascades downstream of Ras, involving sequential engagement of Raf→Mkk1/2(Mek1/2)→Erk1/2 (Figure 3). It is of interest that a target protein for the Erk pathway is the acute myelogenous leukemia gene product (AML1, also called CBFA2 or PEPB2 alpha B), a transcription factor with transforming capacity that is involved in myeloid hematopoietic differentiation.109 AML1 is phosphorylated by Erk in 2 serine sites within its proline-, serine-, and threonine-rich regions, and such phosphorylation is required for its transforming activity.109
There is direct evidence that the Raf/Mek/Erk pathway promotes growth and prevents apoptosis of hematopoietic cells. Expression of a constitutively active mutant form of Mek1 can abrogate the requirement of human and murine hematopoietic cell lines to hematopoietic growth factors,109 while such an abrogation of cytokine-dependence is associated with enhanced Map kinase phosphorylation/activation.110 Similarly, expression of a constitutively active form of Raf kinase in hematopoietic cell lines results in Mek1 and Erk1/Erk2 activation and growth factor independence.111 Furthermore, concomitant expression of Bcl-2 and a conditionally active form of the Mek1 protein result in a synergistic induction of cytokine independence and protection of cells from apoptosis, strongly suggesting that the Mek/Erk pathway and Bcl-2 act synergistically to induce leukemogenesis.112
Recent studies have shown that Erk1/2 and their upstream effectors Mek1/2 are constitutively activated in primary human acute myelogenous leukemia (AML) cells113-115 and that such a constitutive activation correlates with down-regulated expression of the PAC1 phosphatase.114 Furthermore, the Erk protein was found to be overexpressed in the majority of acute leukemia cases studied,114 suggesting that both overexpression and abnormal constitutive activation of Erk kinases are contributing to abnormal cell growth in acute leukemias. Similarly, another study demonstrated activation of the Mek/Erk pathway and its downstream targets, the CREB-1, ATF-1, and c-Myc transcriptional activators, in 9 of 14 acute myelogenous leukemia (AML) cell lines and 2 of 5 chronic myelogenous leukemia (CML) cell lines studied.116 Others, however, failed to demonstrate a dependency of myeloid leukemia growth to Erk activation in certain myeloid leukemia cell lines examined.117
Independently of results observed with cell lines, which may reflect adaptive changes resulting from long-term culture of the leukemia cells, it is clearly established that in the majority of primary acute leukemia cases, the Erk pathway is constitutively activated and mediates mitogenic signals.113-115,118 In a large study, constitutive MAPK kinase phosphorylation was detected in 138 (74%) of 186 freshly isolated leukemic blasts from AML patients.115 Treatment of leukemia blasts with the Mek inhibitors PD98059 or PD184352 inhibited growth and induced apoptosis, while it also sensitized the cells to chemotherapy (Ara-C)–induced cytotoxicity.115 Other studies have also demonstrated synergistic effects of chemotherapeutic agents and drugs that inhibit Map kinase activation in inducing growth suppression and apoptosis of acute leukemia cells.119,120 Interestingly, such potentiating effects of Mek inhibition on chemotherapy-induced cytotoxicity appear to be sequence-dependent, with Mek kinase inhibitors being effective only when administered after the chemotherapeutic agents.115,120 The demonstration of such synergistic effects of Mek kinase inhibitors with chemotherapeutic agents115,120,121 and radiotherapy121 has provided important models for the future clinical development of such inhibitors for the treatment of acute leukemias and other hematologic malignancies as well.
N-Ras or K-Ras mutations, which lead to constitutive activation of this GTPase and downstream activation of the Raf/Mek/Erk pathway, occur in a significant number of acute myeloid and acute lymphoblastic leukemias (ALLs) (20%-30%).122-124 However, the constitutive activation of the Mek/Erk pathway in acute leukemias does not appear to correlate with the presence of N-Ras mutations,125 suggesting that, in addition to Ras, other yet unidentified small G-proteins may also be abnormally activated and contribute to the activation of the Erk pathway in leukemic cells. Alternatively, in the absence of Ras mutations, autocrine or paracrine growth factor/cytokine pathways may be regulating activation on normal Ras and downstream engagement of the Mek/Erk cascade in acute myeloid leukemia cells. Interestingly, in a recent phase 1 clinical trial of the R11577 farnesyltransferase inhibitor in adults with poor-risk acute leukemias it was found that in approximately 36% of pretreatment patient bone marrows the phosphorylated/activated form of Erk was detectable.126 No N-Ras mutations were found in any of the 35 patients enrolled in that study, while in half of the cases in which Erk was phosphorylated/activated, such phosphorylation disappeared after one cycle of treatment.126
Altogether, these studies have established that the Raf/Erk kinase pathway plays a role in the pathophysiology of acute leukemias. However, other Erk-independent mitogenic mechanisms are apparently also involved, as constitutive activation of Mek1/2 and/or Erk1/2 is not detectable in all cases of acute leukemia. Independently of the roles that other signaling pathways may play in the pathophysiology of acute leukemias, pharmacologic targeting of the Erk pathway may be an attractive clinical-translational approach. Also, as there is recent evidence demonstrating synergistic effects of Mek/Erk pharmacologic inhibitors with Bcl-2 inhibitors in AML cells,127 efforts to develop clinical trials combining such inhibitors for the treatment of acute leukemias may be warranted.
Chronic myelogenous leukemia (CML) results from oncogenic transformation of hematopoietic stem cells by the product of the bcr-abl oncogene, which is generated by the reciprocal translocation between chromosomes 9 and 22, resulting in the fusion of the bcr gene to the c-abl gene.128-132 The function of the Erk signaling cascade has been implicated in transformation by the BCR-ABL proto-oncogene,131-133 while inhibition of activation of this cascade in BCR-ABL–expressing cell lines results in cell death and correlates with the induction of apoptosis by various agents, including STI571.134-136 Furthermore, Mek/Erk pharmacologic inhibitors appear to exhibit synergistic effects with STI571 in the induction of apoptosis of BCR-ABL–expressing cells.136 Thus, it is possible that combined administration of Mek inhibitors and STI571 may prove useful in the development of future treatment approaches for CML patients and/or Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL) patients. However, it should be pointed out that in contrast to acute leukemia, in which constitutive activation of Mek/Erk is seen in the majority of primary leukemic blasts, such an activation was not observed in 14 primary CML cases reported in one study.113 Thus, more extensive studies to better understand the role of the Erk pathway in the pathogenesis and growth of CML cells may be required prior to the development of clinical trials using Mek inhibitors for the treatment of chronic myelogenous leukemia.
Other studies have examined the role that the Erk pathway plays in the maintenance of survival of malignant lymphocytes from patients with chronic lymphocytic leukemia (CLL).137,138 In CLL cells, there is no constitutive activation of Erk1 or Erk2, but such activation can be induced by phorbol-ester treatment of the cells137 or by engagement of the B-cell antigen receptor.139 However, pharmacologic inhibition of the Erk pathway in malignant lymphocytes does not induce apoptosis.137,138 Thus, Erk kinases do not appear to play a role in maintaining cell survival in CLL cells, and the defective apoptosis seen in these cells appears to be primarily mediated by activation of the phosphatidylinositol 3′-kinase (PI-3′K) and protein kinase C δ (PKC-δ).137,138
A leukemia in whose pathophysiology the Ras/Raf/Mek/Erk pathway appears to play a key role is natural killer (NK) large granular lymphocyte leukemia. In a recent study, it was found that constitutively active Erk was detectable in the peripheral blood of 11 of 11 patients with NK cell leukemia studied.140 Of these patients, 2 had the aggressive form of the disease, while 9 had chronic leukemia. Treatment of the cells with Mek inhibitors (PD98059 and U0126) or overexpression of a dominant-negative form of Mek-1 resulted in apoptosis.140 The constitutive activation of Mek-Erk in these cells was apparently downstream of Ras, as treatment of the cells with a farnesyl transferase inhibitor or ectopic expression of a dominant-negative form of Ras inhibited Erk phosphorylation and induced cell death.140 Thus, this relatively rare form of leukemia may be another disease in which Mek inhibitors, alone or in combination with other agents, may prove to be clinically efficacious.
The p38 and JNK pathways in leukemias
Although the role of Erk kinases in mediating mitogenic and antiapoptotic signals in acute leukemia cells is well defined, the roles that members of the p38 and JNK kinase groups play in regulation of growth of acute leukemia blasts are not well established. A recent report demonstrated a relationship between constitutive activity of JNK in leukemic blasts and treatment failure in acute myelogenous leukemia.141 In that study, a biochemical analysis of 67 primary adult acute myelogenous leukemia samples demonstrated a correlation between JNK expression/activation in the leukemic cells and hyperleukocytosis at presentation of disease.141 Importantly, a correlation between JNK kinase activity and increased multidrug resistance–associated protein efflux was observed.141 This raises the intriguing possibility that targeting JNK and/or its substrate, c-jun, may be a novel clinical approach to overcome multidrug resistance in AML. However, the development of such approaches is unlikely to be straightforward, as the JNK pathway is also activated by various chemotherapeutic agents, and its function may also be required for the induction of apoptosis by certain agents that are used in the treatment of AML.142-144 As there are 3 different known JNK isoforms, it is possible that the JNK isoforms that mediate chemotherapy-dependent apoptosis are different from the ones involved in the promotion of multidrug resistance in AML cells. Thus, studies to precisely identify the isoforms involved in each of the 2 JNK-dependent responses in AML cells may provide valuable information for the design of new isoform-specific inhibitors with clinical translational potential.
There are no reports directly demonstrating a role for constitutive activation of the p38 Map kinase pathway in the pathophysiology of acute leukemias. A recent study showed that p38 and its downstream effector MapKapK-2 are activated during treatment of the NB-4 acute promyelocytic leukemia (APL) cell line with all-trans retinoic acid (ATRA).145 Interestingly, pharmacologic inhibition of the p38 Map kinase using the SB203580 or SB202190 inhibitors was found to strongly enhance all-trans retinoic acid–dependent induction of APL cell differentiation and all-trans retinoic acid–dependent growth inhibition.145 On the other hand, the Mek inhibitor PD98059 was found to block the induction of differentiation of NB-4 cells145 or HL-60 cells146,147 in response to all-trans retinoic acid, consistent with a positive role for the Mek/Erk pathway in the induction of differentiation of acute promyelocytic leukemia cells. These findings indicate that the p38 Map kinase pathway plays a negative role in the induction of all-trans retinoic acid responses in acute promyelocytic leukemia and raise the possibility that combined use of all-trans retinoic acid with pharmacologic inhibitors of p38 may prove more effective than ATRA alone in inducing differentiation of APL blasts in vivo. Similar mechanisms of synergy with pharmacologic inhibitors of p38 may also be applicable for other drugs used in the treatment of acute promyelocytic leukemia. Another agent, which is approved for the treatment of ATRA refractory acute promyelocytic leukemia, is arsenic trioxide (AS2O3).148 In a similar manner to what was observed with all-trans retinoic acid, treatment of NB-4 APL cells with arsenic trioxide also resulted in activation of the p38/MapKapK-2 pathway, while pharmacologic inhibition of p38 further enhanced arsenic trioxide–induced apoptosis and growth inhibition of APL cells.149 It is of interest that the activation of p38 and its upstream regulator Rac1 by arsenic trioxide is also inducible in an NB-4 variant cell line that is resistant to the effects of all-trans retinoic acid.149 This indicates that different upstream regulatory mechanisms mediate activation of p38 in response to ATRA or AS2O3.149 However, in both cases pharmacologic inhibition of p38 promotes the induction of antileukemic responses, suggesting common downstream regulatory mechanisms.145,149
Other studies have shown that, under certain circumstances, the p38 pathway can cooperate with the Erk pathway to mediate cytokine-induced proliferation of AML cells.150 In particular, it was shown that both p38 and Erk are required for optimal proliferation of the OCI-AML5 cell line in response to G-CSF and Flt3-ligand.150 Thus, depending on the specific circumstances, the p38 pathway can either antagonize the Erk pathway or work synergistically with it to promote growth factor–dependent proliferation of AML blasts.
The functional roles that the p38 and JNK Map kinase pathways may play in the pathogenesis and pathophysiology of chronic leukemias have also been extensively studied. It is of particular interest that the RhoGEF domain of Bcr has the capacity to activate p38 and that the function of p38 is required for Bcr-regulated activation of nuclear factor–κB (NF-κB).151 Thus, in addition to transformation via the tyrosine kinase activity of BCR-ABL, the RhoGEF domain of Bcr may be contributing to transformation, and p38 activity may mediate such effects.151
Other studies have shown that the function of the p38 pathway is essential for the suppression of growth of chronic myelogenous leukemia cells by interferon-α (IFNα).152 It has been demonstrated that IFNα selectively activates p38 in a type I IFN–sensitive BCR-ABL–expressing cell line (KT-1), but not in the BCR-ABL–expressing K562 cell line, which is refractory to the antiproliferative effects of IFNα.152 Concomitant treatment of KT-1 cells with the p38-specific inhibitor SB203580 or the SB202190 inhibitor reverses the growth inhibitory effects of IFNα on these cells, while the Mek inhibitor PD98059 has no effects.152 Most importantly, IFNα treatment of isolated peripheral blood granulocytes from CML patients induces phosphorylation/activation of p38 in vitro and addition of pharmacologic inhibitors of p38 in CML bone marrow cultures reverses the suppressive effects of IFNα on leukemic CFU-GM progenitor colony formation.152 Thus, activation of the p38 signaling cascade appears to be essential for the generation of the antileukemic effects of IFNα in chronic myelogenous leukemia cells in vitro and possibly in vivo. It should also be pointed out that differentiation of BCR-ABL–expressing K562 cells during treatment with butyrate results in phosphorylation/activation of p38 and down-regulation of Erk kinase activity.153 Such phosphorylation of p38 appears to be essential for the differentiation of such cells, as it is blocked by pharmacologic inhibition of p38 kinase activity.153 On the other hand, inhibition of Erk activity using the U0126 selective Mek kinase inhibitor enhances such differentiation, demonstrating that Erk and p38 exhibit opposing functions in the induction of differentiation of BCR-ABL–expressing cells.153
BCR-ABL has been shown to activate the JNK kinase pathway,154 while expression of the c-jun gene in BCR-ABL–transformed cells correlates with JNK kinase activity.155 Dominant-negative mutants of c-Jun impair the transforming activity of BCR-ABL, indicating a requirement for the JNK pathway in BCR-ABL–mediated transformation of hematopoietic cells.155 The JNK-mediated transforming activity of BCR-ABL has also been shown to be inhibited by a JNK cytoplasmic inhibitor, the JNK interacting protein-1 (JIP-1).156 A more recent study has demonstrated that disruption of the JNK ortholog Mapk8 (JNK1) in mice results in defective transformation of pre-B cells by BCR-ABL, in vitro and in vivo.157 This study also established that failure of BCR-ABL–transformed cells to survive in the absence of JNK is because of decreased expression of Bcl2, as the effect of JNK deficiency could be reversed by transgenic Bcl2 expression.157 Thus, the JNK pathway plays an important role in BCR-ABL–mediated transformation via regulation of c-jun activity, indicating that selective inhibitors of this pathway may be of clinical relevance in the treatment of chronic myelogenous leukemia.
The roles of the JNK and p38 pathways in the pathophysiology of other leukemias are not well known. There is some evidence implicating constitutive JNK activation in the pathogenesis of human lymphotropic virus (HTLV-1) tumorigenesis and indirectly implying a role for this pathway in the pathogenesis of adult T-cell leukemia.158 Consistent with this, other studies have shown that a MAPKK kinase, Mlk-3, is involved in Tax-mediated NF-κB activation.155 The Tax protein of HTLV-1 is an oncoprotein that transactivates various genes, which play key roles in HTLV-1 replication and pathogenesis.159 The fact that Mlk-3 regulates Tax-mediated activation of the transcription factor NFκB further supports a putative involvement of the JNK pathway in the pathogenesis of adult T-cell leukemia and raises the possibility that the JNK kinase may be an appropriate molecular therapeutic target.
Efforts have also been made to address the potential roles of the JNK and p38 pathways in the pathophysiology of chronic lymphocytic leukemia (CLL). It has been demonstrated that, in chronic lymphocytic leukemia cells, cross-linking of the B-cell antigen receptor induces activation of Erk, but not JNK or p38,139 suggesting that these kinases do not play important roles in CLL cell proliferation.139 Consistent with this, other studies have shown that pharmacologic inhibition of the p38 pathway does not induce apoptosis of CLL cells.138 Interestingly, a recent study demonstrated that the induction of apoptosis of CLL cells by the chimeric anti-CD20 antibody rituximab is p38-dependent.160 p38 and its downstream effector, MapKapK-2, were found to be activated during culture of isolated CLL cells with anti-CD20, while treatment with the p38 pharmacologic inhibitor SB203580 resulted in induction of apoptosis of the malignant lymphocytes.160 Cross-linking of rituximab to CLL cells also induced strong phosphorylation of Erk and JNK kinases. However, the Mek inhibitor U0126 had no inhibitory effects on anti-CD20–induced apoptosis despite the fact that it blocked Erk activation, strongly suggesting that Erk is not required for its antileukemic activity.160 Thus, the p38 pathway appears to play an important and specific role in the generation of the antileukemic effects of rituximab in chronic lymphocytic leukemia.
Map kinases in lymphomas
The difficulty in working with primary tumor samples from patients suffering from lymphomas has been a limiting factor in efforts to uncover the roles that Map kinases may play in the pathogenesis and pathophysiology of these malignancies. So far, most of the evidence on the putative roles that Map kinases may play in the pathogenesis of lymphomas is based on work with lymphoma-derived cell lines. Tumor necrosis factor α (TNFα) has been shown to induce autocrine regulation of the growth of several lymphoma or leukemia cell lines, alone or in combination with other growth factors.161-163 It has also been previously established that the p38 pathway is required for the regulation of TNFα production.4 Furthermore, the downstream effector of p38, Map kinase–activated protein kinase-2 (MapKapK-2), mediates such regulatory effects of the p38 pathway on TNFα production. This has been shown in studies using mice with targeted disruption of the MapKapK-2 gene, which have shown that p38/MapKapK-2 is required for TNFα protein biosynthesis in response to lipopolysaccharide (LPS), without affecting TNFα gene mRNA transcription.164 Recently, it was demonstrated that the TNFα-inducible proliferation of certain non-Hodgkin lymphoma (NHL) and leukemia cell lines is dependent on activation of the p38 Map kinase in response to engagement of the TNFα receptor in malignant cells.164 Thus, p38 may both regulate TNFα production and also, upon its activation by TNFα, mediate signals that regulate growth of lymphoma/leukemia cells. However, it is difficult to extrapolate from these studies using cell lines whether such effects also occur in primary lymphoma cells. Other studies have implicated the Raf-1/Mek/Erk and PI-3′ kinase pathways in the suppression of Fas-induced apoptosis in lymphoma cells, as evidenced by experiments using the PD98059 Mek inhibitor and the LY294002 PI-3′ kinase inhibitor.165 It has been shown that both of these pharmacologic inhibitors lead to accumulation of Erk in the cytosol of lymphoma cells, suggesting a cross-talk between the 2 pathways.165 Interestingly, such an induction of Fas-dependent apoptosis was selectively seen in lymphoma cells, but not normal thymocytes, that express low Raf-1 levels,161 suggesting divergent Map kinase–dependent regulatory effects on apoptosis in malignant lymphoma cells versus normal cells.
The p38 Map kinase pathway has been implicated in the regulation of interleukin-10 (IL-10) production in Burkitt lymphoma cell lines.166 This cytokine normally regulates growth and differentiation of B cells.167 It has been demonstrated that the Epstein-Barr virus latent membrane protein 1 (LMP1) induces expression of IL-10 in Burkitt lymphoma cell lines in a p38 Map kinase–dependent manner.166 Such an effect was selectively seen in Burkitt cell lines, but not in several other non-Hodgkin lymphoma (NHL) and Hodgkin disease–derived cell lines that were analyzed.166 A recent report has also demonstrated that Epstein-Barr virus latent membrane protein 2A (LMP2A) activates both Erk and JNK kinases, and that LMP2A-inducible phosphorylation of c-Jun is Erk kinase–dependent.168 Altogether, these studies suggest that Map kinase pathways play roles in the pathogenesis of Epstein-Barr virus–related lymphomas and raise the prospect that selective pharmacologic inhibitors of Map kinases may find clinical applications in the treatment of these lymphoma types in the future.
Map kinase pathways may also play roles in growth factor loops that promote cell proliferation of the malignant cells in Hodgkin disease. There is now strong evidence implicating aberrant expression of c-Jun and JunB, which are downstream effectors of Map kinase pathways, in the proliferation of malignant Hodgkin lymphoma cells.169 In one study, constitutively activated AP-1 and significant overexpression of c-Jun and JunB were found in all primary tumor cells from patients with Hodgkin disease, while a similar activation of AP-1 was detected in anaplastic large-cell lymphoma cases, but not other types of lymphoma.169 Interestingly, activated AP-1 was found to support the growth of Hodgkin lymphoma cells, but in anaplastic large-cell lymphoma cells it was mediating antiapoptotic effects.169 Finally, other more recent studies have shown that all different Map kinase groups, Erk, p38, and JNK, are activated in Hodgkin disease cell lines in response to receptor activator of NF-κB ligand (RANKL),170 a factor that is involved in the regulation of cytokine/chemokine secretion in Reed-Sternberg cells via autocrine mechanisms.171 Although there is no direct evidence to date implicating such activation of Map kinases in the growth of Reed-Sternberg cells, it is possible that they are involved in such activities, and future studies in that direction may uncover interesting and potentially important information.
Map kinases in multiple myeloma
There is accumulating evidence that several hematopoietic growth factors are present in the bone marrow microenvironment and regulate survival and proliferation of the malignant plasma cells in multiple myeloma.172 The most important myeloma growth factor is interleukin-6 (IL-6), a cytokine that is produced by myeloma cells in an autocrine or paracrine manner and promotes their survival in vitro and in vivo.173-181 In addition, insulin-like growth factor 1 (IGF-1),182-184 granulocytic colony-stimulating factor (G-CSF),172-175 and interleukin-10172-175 also act as growth factors for primary multiple myeloma cells and/or certain cell lines in vitro. Thus, various signaling networks are activated by different cytokines, primarily interleukin-6, to regulate the growth of malignant myeloma cells. Because of this, the pathways activated by such cytokines are of critical importance in the pathogenesis of the disease.
Interleukin-6 activates multiple signaling cascades, including the Jak-Stat, the Ras/Raf/Mek/Erk, and the PI-3′ kinase/Akt pathways.172-175 As interleukin-6 is a critical factor for the growth of multiple myeloma cells, it is not surprising that the function of the Raf/Mek/Erk cascade mediates signals that promote malignant myeloma cell proliferation.185 It has been demonstrated that treatment of dependent myeloma cell lines with interleukin-6 induces tyrosine phosphorylation of Shc and its association with the Ras-guanine exchange factor Sos-1, resulting in downstream activation of the Mek/Erk pathway.185 Importantly, using an antisense approach, it was established that Erk activation is essential for interleukin-6–dependent myeloma cell proliferation, while the phosphorylation of Stat1 and Stat3 are unrelated to multiple myeloma cell growth.185 Thus, the Raf/Mek/Erk pathway is required for the proliferation of malignant plasma cells in cases in which such cells depend for their growth on interleukin-6.
As Ras is a well-known upstream effector of Raf, it is also likely that the Raf/Mek/Erk pathway contributes to the malignant phenotype in patients with multiple myeloma whose malignant cells express Ras mutations, leading to constitutive Ras activation. Such mutations usually occur in patients with advanced stage disease and involve N-Ras and K-Ras mutations.172,186,187 However, there is also evidence that, in at least one interleukin-6–independent multiple myeloma cell line with constitutively activated K-Ras, the proliferation of cells was not blocked by the pharmacologic inhibition of the Mek/Erk pathway with PD98059.188 Thus, although mutations that activate Ras may mediate proliferative signals via Mek/Erk activation in multiple myeloma cells, it is possible that other Ras-dependent, Mek/Erk-independent pathways are activated in certain cases and contribute to the malignant phenotype.
There is accumulating evidence that in addition to interleukin-6, other growth factors also play important roles in the growth of malignant myeloma cells. One such factor is insulin-like growth factor 1 (IGF-1). Recent studies have demonstrated that IGF-1 activates the PI-3K/Akt/FKHRL-1 and Mek/Erk pathways in multiple myeloma cell lines.189-191 The IGF-1–induced multiple myeloma cell proliferation is dependent on the activation of PI-3′ kinase–dependent pathways, as treatment of cells with the PI-3′ kinase inhibitor LY294002 abolishes the IGF-1–dependent proliferative response.189 On the other hand, the Mek inhibitor PD98059 had no significant effects on the IGF-1–dependent promotion of cell growth of malignant myeloma cells in one study.189 In studies using growth factor–independent cell lines, it was also demonstrated that the growth of multiple myeloma cells is dependent on PI-3′ kinase activation, as reflected by the inhibition of growth of such cells by the PI-3′ kinase inhibitors LY294002 and wortmannin.190 In these studies, Erk inhibition with the PD98059 inhibitor had minimal effects on myeloma cell growth. Based on these facts, a proposed model for the functional contribution of the different signaling pathways in malignant myeloma cell proliferation is that the Mek/Erk pathway is required for IL-6–dependent myeloma cell proliferation. However, this pathway plays a minimal or no role in IGF-1–dependent cell proliferation, in which case the responses are primarily PI-3′ kinase/Akt-dependent (Figure 5). It is of interest that some cross-talk between the IGF-1–inducible PI-3′ kinase pathway and the Mek/Erk pathway in myeloma cells exists, as evidenced by the inhibition of Mek1/2 and Erk phosphorylation by LY294002.189 On the other hand such a cross-talk was not seen in other studies using cell lines in which constitutive activation of PI-3′K and Map kinase pathways was found,187 suggesting that this cross-talk selectively occurs during IGF-1–dependent activation of these pathways. It should also be pointed out that interleukin-6 activates PI-3′ kinase and AKT, and the function of PI-3′ kinase is required for multiple myeloma cell proliferation but not inhibition of IL-6–dependent Erk activation,191 indicating that there is no cross-talk between the PI-3K/AKT and Mek/Erk pathways in response to IL-6 induction189 (Figure 5).
Schematic overview of the activation of different Map kinase pathways to regulate malignant plasma cell proliferation and/or inhibit apoptosis of multiple myeloma cells. * indicates constitutively activated.
Schematic overview of the activation of different Map kinase pathways to regulate malignant plasma cell proliferation and/or inhibit apoptosis of multiple myeloma cells. * indicates constitutively activated.
It is well established that vascular endothelial growth factor (VEGF) promotes angiogenesis in various models.192 It has been previously demonstrated that VEGF is produced in the bone marrows of severe combined immunodeficiency (SCID)–hu mice with multiple myeloma to promote angiogenesis.193 This finding has strongly suggested that this protein may play an important role in the pathogenesis of multiple myeloma. This has prompted studies to understand the mechanisms of signal transduction of VEGF and the biologic relevance of such signals in multiple myeloma. Recently, it was demonstrated that VEGF activates the Raf/Mek/Erk signaling cascade in a myeloma cell line, as well as in malignant cells from patients with multiple myeloma.194 Such VEGF activation of the Erk pathway was PKC-independent and was essential for VEGF-induced myeloma cell proliferation.194 VEGF was also found to induce migration of myeloma cells in an Erk-independent manner, while such a migration was blocked by treatment with a pharmacologic inhibitor of PKC.194 Thus, in addition to mediating IL-6–dependent cell-proliferative signals, the Mek/Erk pathway promotes VEGF-dependent proliferation, but not migration, of multiple myeloma cells.
Although there is no evidence that the p38 pathway directly promotes the growth of multiple myeloma cells in response to growth factors, there is evidence that it may be doing so indirectly. A recent study demonstrated that a specific p38 Map kinase inhibitor (VX-745) inhibits IL-6 and VEGF secretion in bone marrow stromal cells.195 Furthermore, it was shown that p38 inhibition blocks proliferation of multiple myeloma cells.195 Such an effect appears to be the result of inhibition of IL-6 secretion induced by adherence of malignant plasmacytes to bone marrow stromal cells, which is a major mechanism by which these cells develop resistance to chemotherapy.195 These results strongly suggest that the p38 Map kinase pathway is required for paracrine secretion of IL-6 in multiple myeloma bone marrows and raise the possibility that targeting the p38 Map kinase pathway may have therapeutic implications in the treatment of multiple myeloma.195
Map kinase pathways also play important roles in the regulation of apoptosis in malignant myeloma cells. Previous studies have demonstrated that ionizing radiation induces apoptosis of multiple myeloma cells, which is associated with activation of the JNK kinase signaling pathway.196 On the other hand, corticosteroid (dexamethasone)–induced apoptosis has been found to be JNK-independent, and is associated with a decrease in Erk kinase and p70 S6 kinase activities.196 It has also been demonstrated that, in response to ionizing irradiation, the JNK kinase associates with the retinoblastoma protein (Rb) and induces its phosphorylation, providing a mechanism by which apoptosis occurs in a JNK-dependent manner in myeloma cells.197
In addition to promoting cell proliferation, interleukin-6 inhibits Fas-induced apoptosis of multiple myeloma cells.198,199 Such effects of interleukin-6 appear to result from inhibition of activation of the JNK kinase pathway.198,199 Although the precise mechanisms by which interleukin-6 blocks activation of JNK remain to be established, these studies have provided evidence for an additional important mechanism that contributes to the malignant phenotype in multiple myeloma. It is likely that, in addition to inhibition of JNK activation, induction of the PI-3′ kinase/Akt and Mek/Erk pathways by interleukin-6 and/or insulin-like growth factor 1 also contributes to the generation of an antiapoptotic state in vitro188-190 and possibly in vivo, providing multiple potential therapeutic targets for this disease.
Various new pharmacologic agents are currently under clinical development for the treatment of multiple myeloma,200 including agents that either block growth-promoting signaling cascades or trigger apoptotic signals in malignant plasma cells. Among the agents shown to induce apoptosis of myeloma cells is the farnesyl transferase inhibitor R115777.201 Although the precise mechanisms by which such effects are induced remain to be defined, this drug blocks the interleukin-6–dependent phosphorylation of Stat3 and Erk1/2 in multiple myeloma cells, suggesting that it may work, in part, via interruption of IL-6–regulated pathways.201 A very promising agent in the treatment of multiple myeloma is the proteasome inhibitor PS-341.202-205 This agent has entered clinical trials and has already been shown to exhibit significant clinical activity in the treatment of patients with refractory multiple myeloma.205-207 PS-341 induces apoptosis and inhibits the growth of drug-resistant multiple myeloma cells, as well as the binding of multiple myeloma cells to the bone marrow microenvironment.202-204 Treatment of target cells with PS-341 induces several biochemical changes, including activation of the JNK pathway204 and inhibition of Erk1/Erk2 kinase–dependent signaling.202 The precise contributions of different Map kinase pathways to the effects of PS-341 remain to be determined, but it is likely that stimulation of JNK activity and Mek/Erk inhibition contribute to PS-341–induced apoptosis.
The demonstration that Map kinase pathways play important roles in the growth of myeloma cells has provided important insights that may lead to future efforts to combine Map kinase inhibitors with other novel therapeutic agents that inhibit myeloma cell growth. A recent study demonstrated that combined treatment of myeloma cells with the Mek1/2 inhibitor PD184352 and UCN-01, a drug that inhibits Chk1 and abrogates the G2M checkpoint in the cell cycle, results in synergistic induction of mitochondrial damage and apoptosis. Such a combined use of these inhibitors also resulted in inhibition of growth of drug-resistant myeloma cells through an interleukin-6–independent mechanism.208 These findings strongly suggest that simultaneous disruption of the Mek/Erk pathway and the cell cycle may be a more effective approach to target myeloma cells than Mek inhibitors alone, and they have provided an important model for the potential combined use of these inhibitors in clinical trials.208
Conclusions and future directions
Dramatic advances have occurred over the last few years in the research field of Map kinases in hematologic malignancies. The realization that Map kinase–dependent signaling cascades play important roles in the regulation of apoptosis and growth of malignant hematopoietic cells has led to extensive studies, aimed to characterize the precise mechanisms that are responsible for such effects. It is now clear that the Raf/Mek/Erk pathway participates in the generation of mitogenic responses in essentially all hematologic malignancies, including acute and chronic leukemias, lymphomas, and multiple myeloma. On the other hand, the regulatory effects of the JNK and p38 Map kinase pathways vary, depending on the specific cellular type and possibly the distinct isoforms involved. Importantly, the JNK and p38 pathways appear to also mediate signals responsible for sensitivity or resistance to the effects of various pharmacologic and biologic agents currently in use for the treatment of various hematologic malignancies.
The acquired knowledge from all of these efforts has led to the development of specific pharmacologic inhibitors, some of which are now under evaluation in ongoing clinical trials. It is likely that over the next few years we will observe an exponential growth in the number of translational clinical research efforts, resulting from the rapidly accumulating new information. The remarkable scientific and clinical advances in the field of chronic myelogenous leukemia, which ultimately led to the introduction of STI571209,210 in the treatment of the disease, further increase the enthusiasm toward such efforts. The STI571 paradigm underscores the importance of understanding in detail the molecular and signaling mechanisms responsible for kinase-dependent malignant cell proliferation and has provided an important model for the development of small molecules that target other kinases involved in the pathogenesis of hematologic malignancies.
The evidence accumulated so far, on the roles that Map kinases play in hematologic malignancies, points toward certain clinical and basic research directions that may be of importance in the development of new therapeutic approaches. Clearly, further characterization of the upstream regulatory signals and the downstream effectors for the different Map kinases will help us to better understand the ways in which Map kinases generate their effects. Also, further delineation of the multiple kinases involved in the different Map kinase group cascades may provide more specific targets for translational approaches. There is also a need to better characterize the functional roles that distinct isoforms within each Map kinase group play in the pathogenesis of various hematologic malignancies. Despite the substantial structural homology among the isoforms in each group, there is evidence that distinct isotypes also exhibit different properties and in some cases may mediate opposing biologic responses. Efforts to define the precise biologic activities mediated by such distinct isotypes in different hematologic malignancies, and to design selective pharmacologic inhibitors that target their distinct kinase domains, may prove valuable in the future. In the same context, very little is known about the functional roles that Erk 3, Erk5, and Erk7 Map kinases may play in the regulation of malignant hematopoiesis. It is possible that these kinases, which do not belong in any of the classic Map kinase groups, also contribute to the pathogenesis of certain hematologic malignancies, and studies in that direction are warranted.
Independently of any new information that will arise from future basic science research efforts, there is already ample evidence to support the further development of clinical studies using pharmacologic inhibitors of known Map kinase pathways. For instance, the design of trials using Mek inhibitors, alone or in combination with cell-cycle inhibitors, for the treatment of leukemias and multiple myeloma is strongly supported from the available preclinical data. Similarly, studies of pharmacologic inhibitors of Bcl-2 in leukemias would be important and may lead to the future design of trials combining Mek inhibitors with Bcl-2 inhibitors for the treatment of refractory acute leukemias. Beyond studies with Mek kinase inhibitors, studies using drugs that block p38 Map kinases are likely to be initiated in multiple myeloma patients, based on very recent studies indicating that p38 promotes multiple myeloma cell growth via paracrine secretion of IL-6 and VEGF. It is also likely that studies of pharmacologic inhibitors of p38, in combination with all-trans retinoic acid and/or arsenic trioxide, will be developed for the treatment of acute promyelocytic leukemia, based on already described synergistic interactions. Finally, clinical trials to evaluate the combined use of Mek/Erk inhibitors with traditional chemotherapy for a variety of hematologic neoplasias would be appropriate, and their development is likely in the future.
Prepublished online as Blood First Edition Paper, March 6, 2003; DOI 10.1182/blood-2002-12-3647.