Abstract
Adult T-cell leukemia (ATL) is a severe chemotherapy-resistant malignancy associated with prolonged infection by the human T cell-lymphotropic virus 1 (HTLV-1) retrovirus. Although the Tax viral transactivator is clearly an oncogene, the role of its continuous expression in the maintenance of the transformed phenotype is controversial. Because arsenic trioxide (As) and interferon α (IFN) synergize to induce cell cycle arrest and apoptosis of ATL cells both ex vivo and in vitro, we investigated the effects of As alone and As/IFN combination on gene networks in HTLV-1–infected leukemic cells. The As/IFN combination reduced Tax expression and, accordingly, reversed the Tax-induced constitutive nuclear factor κB (NF-κB) activation. Using DNA microarray analyses, we demonstrated that As rapidly and selectively blocks the transcription of NF-κB–dependent genes in HTLV-1–infected cells only. Reversal of NF-κB activation by As alone resulted from dramatic stabilization of IκB-α and IκB-ϵ, independently of IκB kinase (IKK) activity modulation or Tax degradation. In contrast, only the As/IFN combination induced late and massive down-regulation of cell cycle–regulated genes, concomitantly with Tax degradation by the proteasome and cell death induction, indicating the importance of continuous Tax expression for ATL cell survival. These 2 successive events likely account for the potent and specific effects of the As/IFN combination in ATL.
Introduction
The retrovirus human T cell-lymphotropic virus 1 (HTLV-1) is the causative agent of adult T-cell leukemia/lymphoma (ATL), an aggressive malignancy of CD4+ T lymphocytes (for a review, see Bazarbachi and Hermine1 ). Leukemia develops after a very long latency period and is preceded by oligoclonal expansions of HTLV-1–infected activated T cells.2 These clonal expansions result from the expression of the viral transactivator protein Tax, which activates various cellular genes and creates an autocrine loop involving interleukin-2 (IL-2) and its cognate receptor.3 Tax alters many transcriptional pathways: it activates CREB/ATF, AP-1, and nuclear factor-κB (NF-κB), represses p53, and interferes with several cell cycle regulators including cyclins and cdk inhibitors (for a review, see Yoshida4 ). The role of continuous Tax expression in maintaining the phenotype of established ATL is controversial, because Tax is undetectable in circulating ATL cells (for a review, see Asquith et al5 ). Tax constitutes a major target of cytotoxic lymphocytes and Tax-expressing cells are continuously eliminated from the circulation.6 Nevertheless, fresh ATL cells display exactly the same morphologic and biochemical phenotype as Tax-expressing cells.
NF-κB plays a central role in the regulation of immune and inflammatory responses. It consists of homodimeric and heterodimeric complexes of members of the Rel protein family. In unstimulated cells, NF-κB is found in an inactive cytosolic form, complexed with an inhibitory subunit known as IκB (IκB-α, IκB-β, or IκB-ϵ). The IκB protein phosphorylation is mediated by the IκB kinase (IKK) complexes (for a review, see Ghosh and Karin7 ). Phosphorylated IκBs are then ubiquitinated and degraded by the 26S proteasome. Consequently, NF-κB proteins translocate to the nucleus, bind specific promoters, and activate gene transcription. Tax is a powerful activator of the NF-κB pathway, partly through persistent stimulation of IKK activity.8
Because ATL is completely resistant to chemotherapy, it carries a very poor prognosis (for a review, see Bazarbachi and Hermine1 ). High response rates were achieved with the combination of the antiretroviral nucleotide analog zidovudine and interferon-α (IFN).9, 10, 11 However, most patients eventually have a relapse, which emphasizes the need for new therapeutic approaches. Arsenic trioxide (As) was shown to synergize with IFN to induce G1 arrest and apoptosis in ATL.12 This combination down-regulates Tax expression and consequently reverses the constitutive activation of NF-κB.13
In this study, we show that 2 distinct events are specifically triggered by As/IFN combination in ATL-derived cells: a rapid shut-off of the NF-κB pathway induced by As-triggered, IKK-independent, stabilization of IκBs, and a delayed As/IFN-triggered shut-off of cell cycle–associated genes, secondary to Tax degradation by the proteasome. These results provide a mechanistic explanation for the specificity of the As/IFN combination in ATL, demonstrate that NF-κB shut-off is insufficient to trigger apoptosis, and indicate that Tax is involved in maintenance of the leukemic phenotype.
Materials and methods
Cells, drugs, and antibodies
The ATL-derived HTLV-1–infected CD4+ T-cell lines HuT-102, MT-2, C8166, and C91-PL, and the HTLV-1– CD4+ T-cell lines CEM, Jurkat, and MOLT-4 were grown as previously described.13 Peripheral blood mono-nuclear cells (PBMCs) were collected from a healthy HTLV-1– donor after obtaining his informed consent, centrifuged over Ficoll-Hypaque (Lymphoprep, Nyegaard, Norway) and seeded at 1 × 106 cells/mL in RPMI 1640 medium containing 10% fetal calf serum (FCS; Gibco, Invitrogen, Paisley, United Kingdom), antibiotics, 100 U/mL recombinant human IL-2 (Boehringer, Mannheim, Germany), and 10 μg/mL phytohemagglutinin A (PHA; Gibco). Cell growth was assessed with a CellTiter nonradioactive cell proliferation assay kit (Promega, Madison, WI).
Recombinant IFN-α-2b (Hoffman-La Roche, Basel, Switzerland) and arsenic trioxide (As) (Sigma Chemical, St Louis, MO) were prepared as previously described12 and used at 1000 U/mL and 1 μM, respectively. The proteasome inhibitor MG-132 (zLLL) was obtained from Biomol Research Laboratories (Plymouth Meeting, PA). Stock solutions of MG132 (52.6 mM) were prepared in dimethyl sulfoxide (DMSO) and applied in less than 0.1% of the media volume. Antibodies: IκB-α(c-20), IκB-β(c-19), IκB-ϵ (M-364), P-IκB, IKK-α, IKK-β, and IKK-γ were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antiglyceraldehydephosphodehydrogenase (GAPDH) was obtained from Biogenesis (Stinford Fload, United Kingdom). Mouse monoclonal anti–Tax 168-A51 was obtained from the National Institutes of Health AIDS Research and Reagent Program. Rabbit polyclonal antiactin was obtained from Sigma.
Western blot analysis
Western analyses were performed as previously described.13 Although we started with identical total amounts of proteins, MG-132–treated samples exhibited accumulation of high-molecular-weight polyubiquitinated proteins, resulting in underestimation of the housekeeping actin and GAPDH proteins. Therefore in MG-132–treated samples, we increased the amounts of protein so as to reach equal expression of actin or GADPH in MG-132–treated and untreated samples.
Quantitative RT-PCR and Northern blot analyses
Total RNA was extracted using the TRIzol LS Reagent (Gibco BRL, Gaithersburg, MD) as recommended by the manufacturer. One microgram total RNA was used for cDNA reverse transcription (RT) using random primer p(dN)6 (Roche Biodiagnostics, Basel, Switzerland) in a final volume of 20 μL. The reaction was incubated at 37°C for 60 minutes, heated to 65°C for 10 minutes, and cooled to 4°C until further use. Then, 5 μL of the cDNA reaction was used for the respective amplification of IL-6 and tumor necrosis factor α (TNF-α) cDNA using gene-specific primers: IL-6 U (CCAGAGCTGTGCAGATGAGTACA), IL-6 R (CGCAGAATGAGATGA GTTGTC), TNF-α S (CCTGTAGCCCATGT TGTAGCAA), and TNF-α A (AAGAGGACCTGGGAGTAGATGA) by real-time polymerase chain reaction (PCR) on the light cycler (Roche Biodiagnostics). For IL-6 and TNF-α product detection, IL-6 gene-specific fluorescent probes IL-06 FL (AGATGCAATAACCACCCCTGACCCAA X) and IL-06 LC (LC Red640-CACAAATGCCAGCCTGCTGACGAA p) and TNF FL (GCATTGGCCCGGCGGTTC X) and TNF LC (LC Red640-CCACTGGAGCTGCCCCTCAGCT p), respectively, were used. Probes and primers were designed by TIB MOLBIOL (Berlin, Germany). The reaction mix contained 0.5 μM of each forward and reverse primer specific to each reaction, 0.3 μM of each probe, 2.25 mM MgCl2,1 × Fast Start DNA master mix (from DNA master hybridization probes Kit, Roche Biodiagnostics), and enough water to make up to 20 μL. PCR amplification consisted of 35 cycles having each an annealing step of 5 seconds at 95°C, elongation at 55°C for 10 seconds, and denaturation at 72°C for 26 seconds. Fluorescence was measured at the end of the annealing step at the F2 (640 nm)/F1 (530 nm) channel ratio and analyzed using the light cycler software. Glucose-6-phosphate dehydrogenase (G6PD) was amplified using the same cDNA that was used for the cytokine detections. Products of this reaction served as both a control for RT-PCR performance and as reference for relative quantification.
Northern blot analysis was performed as previously described.13 A total of 15 μg RNA was separated on a 1% agarose gel and transferred to nitrocellulose membrane. The filter was hybridized with the IκB-α cDNA probe (gift from Sylvie Memet, Pasteur Institute, Paris, France). Equal loading was assessed by propidium iodide staining of the 28S and 18S rRNA.
Transfection assays
HeLa cells (0.9 × 106) were seeded in 60-mm culture dishes and transiently transfected with 300 ng pf the indicated plasmid using Lipofectamine Plus (Gibco). We used the pSG5-Tax expression vector and the pSG5-RAR-α expression vector served as control. Eight hours after transfection, cells were trypsinized and divided into 2 wells: one control and one treated for 36 hours with 1 μM As and 1000 U/mL IFN. Cells were then washed with phosphate-buffered saline (PBS) in the presence of protease inhibitors (Boehringer Mannheim) and solubilized in Laemmli buffer. Cycloheximide (Sigma) chases were performed as previously described.14
In vitro kinase activity
Forty-eight hours after As, IFN, or As/IFN treatment, cells were resuspended in lysis buffer (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.6, 250 mM NaCl, 1% NP-40 (IGEPAL), 20 mM β-glycerophosphate, 1 mM EDTA [ethylenediaminetetraacetic acid], and 20 mM para-nitrophenylphosphate [PNPP]) supplemented with protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol [DTT], and 0.1 mM sodium orthovanadate; Sigma) added just before use. After incubation for 20 minutes on ice, the lysates were cleared by centrifugation and the supernatant fraction was analyzed for its protein concentration using the DC protein assay (Biorad Laboratories, Hercules, CA). Equal amounts of the lysates were immunoprecipitated with the anti–IKK-α antibody H-744 (which cross reacts with IKK-β). After addition of protein A-agarose beads (Boehringer Mannheim), the immunocomplexes were washed 3 times in lysis buffer and equilibrated in kinase buffer without adenosine triphosphate (ATP; 20 mM HEPES, pH 7.6, 20 mM MgCl2, 20 mM β-glycerophosphate, 1 mM EDTA, and 2 mM DTT). Kinase activity was assayed using 5 μCi (0.185 MBq) γ-32P ATP (Amersham Biosciences, Buckinghamshire, United Kingdom) and 1 μg recombinant glutathione S-transferase (GST)–IκB (Santa Cruz Biotechnology) as substrate, in 30 μL kinase buffer at 30°C and incubated for 30 minutes. The reaction was stopped by adding 2 × sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and boiling for 5 minutes. Products were separated by SDS-PAGE, electrophoretically transferred to nitrocellulose membranes, and analyzed by autoradiography. The membranes were then probed with anti–IKK-α antibody to determine the amount of immunoprecipitated kinases.
Microarray procedures
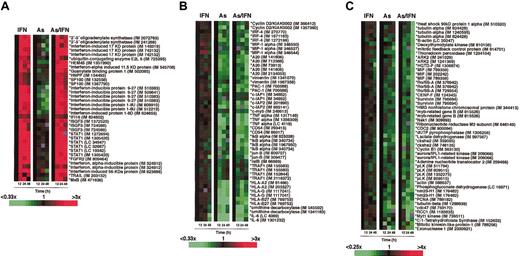
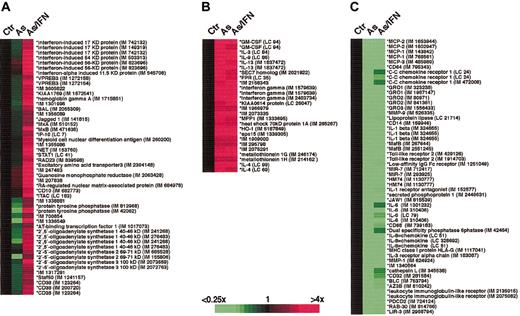
Lymphochip cDNA microarrays containing 12 196 or 16 128 array elements were used to quantify gene expression. The Lymphochip consists of genes whose products are preferentially expressed in lymphoid cells or known to play a role in cancer biology or immune function. Fluorescent cDNA probes were prepared from poly-(A)+ mRNA isolated from HuT-102 cells or from PBMCs by various treatments (Fast Track, Invitrogen, Paisley, United Kingdom). Each of the cDNA samples was hybridized against a common reference RNA pool,15 which allowed comparison of all 15 samples with each other. Microarrays were analyzed on a GenePix scanner (Axon Instruments, Union City, CA), and data files were entered into a custom-made database maintained at the National Institutes of Health (http://nciarray.nci.nih.gov). For analysis, each of the 3 conditions (IFN alone, As alone, and As/IFN) was compared to its control at 12, 24, and 48 hours. The data display showed changes in gene expression over time for each condition in relation to the 12-hour control, which was transformed as well so that all measurements for a given gene started from 0 (= black square in Figures 1,Figure 2 and 3). In Figures 1 and 3, each row represents the hybridization results for a single array element for all the experiments, and each column represents the array results for selected genes in one experiment. The relative level of gene expression is depicted according to the color scale shown at the bottom of these figures. Gray squares indicate missing or excluded data. Some of the genes displayed were represented by 2 or more different cDNA clones, thus providing internal controls for the reproducibility of gene expression quantitation.
As and As/IFN treatment affect 2 distinct gene networks in HTLV-1+cells. Microarray analysis of the gene expression profile of HuT-102 cells in response to treatment with IFN, As, and combined treatment with As/IFN at 12, 24, and 48 hours. The gene expression profile is presented as a matrix in which the rows represent individual array clones and the columns, individual mRNA samples from each of the 12 experiments. The relative level of gene expression is depicted according to the color scale shown. Gray squares indicate missing or excluded data. The results displayed represent the changes in gene expression over time in relation to the 12-hour control. Asterisks denote a sequence verified clone, IM indicates an IMAGE consortium clone identification number, and LC a Lymphochip clone identification number. (A) Selected genes up-regulated by IFN and As/IFN, but not by As alone. (B) Down-regulation of a selected number of NF-κB–dependent genes by As and As/IFN treatment at 12, 24, and 48 hours (details described in “Materials and methods”). (C) Cell cycle arrest by combined treatment with As/IFN. Proliferation signature genes (see “Materials and methods”) exhibit massive down-regulation at 48 hours after combined treatment with As/IFN.
As and As/IFN treatment affect 2 distinct gene networks in HTLV-1+cells. Microarray analysis of the gene expression profile of HuT-102 cells in response to treatment with IFN, As, and combined treatment with As/IFN at 12, 24, and 48 hours. The gene expression profile is presented as a matrix in which the rows represent individual array clones and the columns, individual mRNA samples from each of the 12 experiments. The relative level of gene expression is depicted according to the color scale shown. Gray squares indicate missing or excluded data. The results displayed represent the changes in gene expression over time in relation to the 12-hour control. Asterisks denote a sequence verified clone, IM indicates an IMAGE consortium clone identification number, and LC a Lymphochip clone identification number. (A) Selected genes up-regulated by IFN and As/IFN, but not by As alone. (B) Down-regulation of a selected number of NF-κB–dependent genes by As and As/IFN treatment at 12, 24, and 48 hours (details described in “Materials and methods”). (C) Cell cycle arrest by combined treatment with As/IFN. Proliferation signature genes (see “Materials and methods”) exhibit massive down-regulation at 48 hours after combined treatment with As/IFN.
Effect of As and As/IFN on gene expression in activated PBMCs. Microarray analysis of the gene expression profile of activated PBMCs in response to treatment with As and combined As/IFN treatment for 48 hours as compared to control (ctr). (A) IFN-responsive genes. (B) Genes induced by As. (C) Genes repressed by As (for details, see “Materials and methods”).
Effect of As and As/IFN on gene expression in activated PBMCs. Microarray analysis of the gene expression profile of activated PBMCs in response to treatment with As and combined As/IFN treatment for 48 hours as compared to control (ctr). (A) IFN-responsive genes. (B) Genes induced by As. (C) Genes repressed by As (for details, see “Materials and methods”).
Results
As and As/IFN treatment affect 2 distinct gene networks
Using Lymphochip DNA microarrays, we explored the global changes induced by As and IFN treatments. The gene expression profiles of untreated HuT-102 cells and of cells treated with IFN alone, As alone, or As/IFN were studied at 12, 24, and 48 hours. Less than 1% of genes were affected hence demonstrating the specificity of As and As/IFN effects. These treatment-responsive genes comprised 4 cluster groups: (1) A set of well-known IFN-induced genes rapidly up-regulated by both IFN and IFN/As treatments, but not by As alone (Figure 1A). In this set, induction was already detectable at 12 hours, was sustained up to 48 hours, and was not modified by concomitant exposure to As. (2) A small cluster of genes comprising ferritin and metallothionein genes (not shown) rapidly up-regulated by As alone and whose induction persisted for up to 48 hours. As expected, IFN had no effect on this cluster, either alone or combined with As. (3) Strikingly, arsenic sharply down-regulated the expression of a third set of genes, known to be NF-κB targets,16 which was unaffected by IFN alone (Figure 1B). This down-regulation was rapid (complete at 12 hours) and, importantly, was not affected by concomitant exposure to IFN (Figure 1B). (4) The fourth set of genes was down-regulated slightly by As alone, but was virtually eliminated after 48 hours of exposure to As/IFN (Figure 1C). All these genes belong to the proliferation signature set that we identified in a previous investigation.17
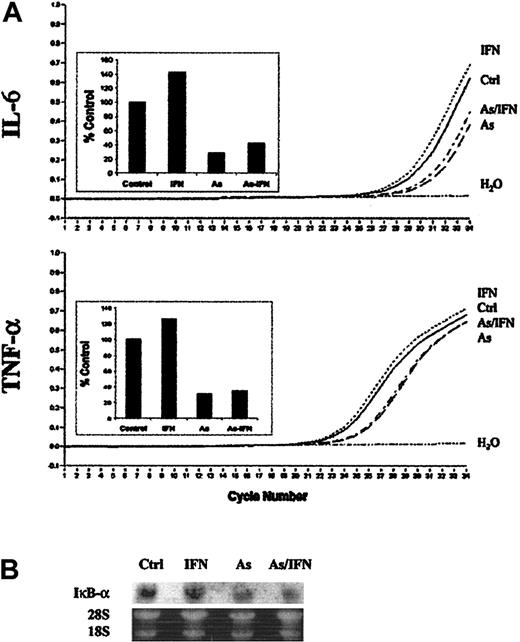
To confirm these results, the mRNA level of 2 NF-κB–dependent genes, IL-6 and TNF-α, in HuT-102 cells was analyzed by quantitative RT-PCR after treatment with IFN, As, and their combination for 12 hours (Figure 2A). IFN alone induced a slight increase in the mRNA level of both IL-6 (142% of control) and TNF-α (125% of control). As expected, As alone induced a dramatic decrease in the mRNA level of both IL-6 (28% of control) and TNF-α (30% of control). Interestingly, the effects of As/IFN combined treatment on the mRNA level of IL-6 (42% of control) and TNF-α (34% of control) were similar to those obtained with As alone. Hence, quantitative RT-PCR analysis clearly confirms the DNA microarray results showing that As rapidly and selectively blocks the transcription of NF-κB–dependent genes in HTLV-1–infected cells. Moreover, Northern blot analysis of IκB-α mRNA also showed a significant down-regulation of the transcription of this NF-κB target gene (Figure 2B).
As and As/IFN treatments down-regulate the transcription of IκB-α, IL-6, and TNF-α genes in HTLV-1+ cells. (A) Relative quantification of IL-6 and TNF-α mRNA in untreated HuT-102 cells (ctrl) or in response to treatment with IFN, As, and combined treatment with As/IFN at 12 hours. Curves represent fluorescence versus RT-PCR cycle number. Amplification plot of the negative control (H2O) is also included. Inset histograms show the mRNA expression levels as a percentage of the untreated control cells after normalization to G6PD as described in “Materials and methods.” (B) Northern blot analysis of IκB-α mRNA expression in untreated HuT-102 cells (ctrl) or in response to treatment with IFN, As, and combined treatment with As/IFN at 48 hours. Loading was assessed by propidium iodide staining of the 28S and 18S rRNA.
As and As/IFN treatments down-regulate the transcription of IκB-α, IL-6, and TNF-α genes in HTLV-1+ cells. (A) Relative quantification of IL-6 and TNF-α mRNA in untreated HuT-102 cells (ctrl) or in response to treatment with IFN, As, and combined treatment with As/IFN at 12 hours. Curves represent fluorescence versus RT-PCR cycle number. Amplification plot of the negative control (H2O) is also included. Inset histograms show the mRNA expression levels as a percentage of the untreated control cells after normalization to G6PD as described in “Materials and methods.” (B) Northern blot analysis of IκB-α mRNA expression in untreated HuT-102 cells (ctrl) or in response to treatment with IFN, As, and combined treatment with As/IFN at 48 hours. Loading was assessed by propidium iodide staining of the 28S and 18S rRNA.
In view of the specific targeting of Tax by the As/IFN combination,13 we performed a set of control arrays with normal PHA/IL-2–stimulated PBMCs (Figure 3). After 48 hours of exposure to As or As/IFN, only As/IFN blocked the growth of resting T cells (36% of control), but activated T cells were sensitive to both As alone (48% of control) and the As/IFN combination (32% of control). Analyses of PHA/IL-2–activated T cells treated for 48 hours with either As or As/IFN showed: (1) up-regulation of IFN targets by As/IFN, but not by As alone (Figure 3A); (2) induction of several cytokine genes (IL-3, -4, -9, -13, granulocytemacrophage colony-stimulating factor [GM-CSF], and IFN-γ) by As, alone or combined with IFN (Figure 3B); and (3) repression by As or As/IFN of a heterogeneous group of genes comprising cytokines, chemokines, and their receptors (Figure 3C). Most importantly, neither prototypical NF-κB targets (eg, IκB-α and TNF-α), nor proliferation-associated genes were modulated by As or As/IFN. These array experiments demonstrated that, in ATL-derived cells only, As has the immediate effect of shutting-off the NF-κB pathway and that only combined As/IFN has a major late effect on proliferation signature genes.
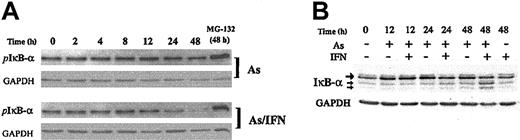
IκB-α is stabilized by As in an IKK-independent manner
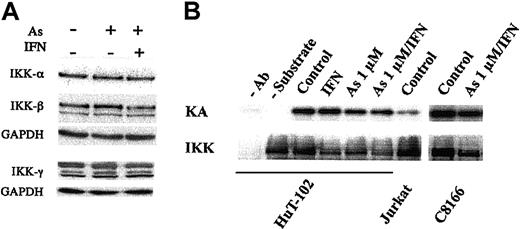
Previous authors reported that very high doses of As inhibit IKK activity, resulting in stabilization of IκB-α,18,19 in agreement with our previous observations.13 We therefore examined the effects of As, IFN, and As/IFN on IKK protein levels and kinase activity in ATL-derived cells. Western blot analysis showed that IKK-α, IKK-β, and IKK-γ protein levels in HuT-102 cells (Figure 4A) and MT-2 cells (data not shown) were not affected by pharmacologic doses of As (1 μM) or As/IFN. Kinase assays using IκB-α as substrate confirmed that IKK activity is constitutively activated in the HTLV-1–transformed HuT-102, C8166, and MT2 cells but not in the HTLV-1– Jurkat and CEM cells (Figure 4B and not shown). Importantly, IKK activity was not affected by As, IFN, or As/IFN at the doses used here (Figure 4B and not shown). Accordingly, the use of an antibody recognizing the phosphorylated form of IκB-α indicated that phosphorylation of IκB-α was not affected by As, IFN, or As/IFN, except at very late time points (48 hours with As/IFN; Figure 5A). Nevertheless, despite its ongoing phosphorylation by IKKs, IκB-α is clearly stabilized by As or As/IFN (Figure 5B). Transcription of the IκB-α gene, a well-characterized NF-κB target, was reduced by exposure to As (Figures 1B and 2A). Hence, as its gene transcription was reduced but its protein phosphorylation remained unchanged, the enhanced level of IκB-α on As exposure was probably the result of a reduction in its ongoing proteolysis by the 26S proteasome. In line with this, an increase of more than 20-fold in IκB-α expression was noted in MG132-treated ATL-derived cells, thus confirming the well-known rapid proteolysis of phosphorylated I-κB in these cells (not shown). Two protein species with a lower molecular weight20 were always observed after 48 hours of As/IFN treatment (small arrows in Figure 5B). Their formation was completely dependent on IFN signaling, because it was blocked by the addition of protein synthesis inhibitors (not shown). The delayed decrease in phospho-IκB-α levels on As/IFN treatment (Figure 5 and Mahieux et al21 ) was probably due to this proteolysis.
IKK protein level and kinase activity are not affected by As/IFN in HTLV-1+ cells. (A) Effects of As, IFN, and combined As/IFN on IKK-α, IKK-β, and IKK-γ protein levels in HuT-102 cells after 48 hours of exposure to these drugs. Equal protein loading was assessed by hybridization with anti-GAPDH antibodies. (B) Effect of As, IFN, and combined As/IFN on IKK activity in HTLV-1+ cells. Equal amounts of lysates from HuT-102, Jurkat, and C-8166 cells were immunoprecipitated with anti–IKK-α antibody. Kinase activity (KA) was determined as described in “Materials and methods” using (GST)-IκB-α as substrate. The membranes were subsequently probed with anti–IKK-α antibody to determine the amounts of immunoprecipitated kinases (IKK). Negative control reactions were performed without anti–IKK-α antibody (–Ab) or without (GST)-IκB-α (–substrate).
IKK protein level and kinase activity are not affected by As/IFN in HTLV-1+ cells. (A) Effects of As, IFN, and combined As/IFN on IKK-α, IKK-β, and IKK-γ protein levels in HuT-102 cells after 48 hours of exposure to these drugs. Equal protein loading was assessed by hybridization with anti-GAPDH antibodies. (B) Effect of As, IFN, and combined As/IFN on IKK activity in HTLV-1+ cells. Equal amounts of lysates from HuT-102, Jurkat, and C-8166 cells were immunoprecipitated with anti–IKK-α antibody. Kinase activity (KA) was determined as described in “Materials and methods” using (GST)-IκB-α as substrate. The membranes were subsequently probed with anti–IKK-α antibody to determine the amounts of immunoprecipitated kinases (IKK). Negative control reactions were performed without anti–IKK-α antibody (–Ab) or without (GST)-IκB-α (–substrate).
IκB-α is stabilized by As in an IKK-independent manner. Effects of As (1 μM), IFN (1000 IU/mL), combined As/IFN, or MG132 (5 μM) on phospho-IκB-α (A) and IκB-α (B) protein levels in HuT-102 cells at different time points. For IκB-α expression, the upper band (large arrow) represents the 36-kDa major IκB-α protein, whereas the lower bands of 34 and 32 kDa (small arrows) presumably represent N-terminal proteolytic forms of IκB-α up-regulated by As/IFN treatment.
IκB-α is stabilized by As in an IKK-independent manner. Effects of As (1 μM), IFN (1000 IU/mL), combined As/IFN, or MG132 (5 μM) on phospho-IκB-α (A) and IκB-α (B) protein levels in HuT-102 cells at different time points. For IκB-α expression, the upper band (large arrow) represents the 36-kDa major IκB-α protein, whereas the lower bands of 34 and 32 kDa (small arrows) presumably represent N-terminal proteolytic forms of IκB-α up-regulated by As/IFN treatment.
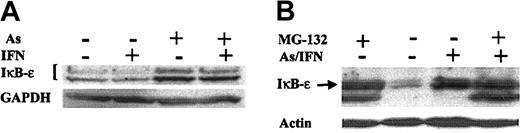
IκB-ϵ degradation by the proteasome is blocked by As exposure
IκB-ϵ is a specific regulator of NF-κB activity through direct binding to RelA-containing NF-κB complexes.22, 23, 24 The IκB-ϵ protein level was much lower in 4 HTLV-1–transformed cell lines than in 2 HTLV-1– cell lines (not shown). MG-132 stabilized IκB-ϵ dramatically in the HTLV-1–transformed cells only (Figure 6 and not shown), suggesting that, as in the case of IκB-αβ, Tax-activated, IKK-dependent, IκB-ϵ phosphorylation triggers IκB-ϵ proteasomal degradation. Strikingly, like IκB-α, IκB-ϵ protein was stabilized markedly by As in HTLV-1–infected HuT-102 and MT-2 cells but not in HTLV-1– cells (Figure 6 and not shown). Addition of IFN did not enhance IκB-ϵ stabilization. Note that MG-132 and As/IFN similarly stabilized IκB-ϵ and did not have additive effects (Figure 6B), suggesting that As inhibits the proteasomal degradation of IκB-ϵ. Importantly, the magnitude of stabilization by As was much greater for IκB-ϵ than for IκB-α (Figures 5B and 6), possibly suggesting that IκB-ϵ plays a dominant role in As-induced RelA cytoplasmic sequestration and consequently in shutting off the NF-κB pathway.
IκB-ϵ degradation by the proteasome is blocked by As exposure in HTLV-1+ cells. Effects of As, IFN, As/IFN combination, or MG132 on IκB-ϵ protein levels in HuT-102 cells after 48 hours of exposure to these drugs. Equal protein loading was assessed by hybridization with antiactin or anti-GAPDH antibodies.
IκB-ϵ degradation by the proteasome is blocked by As exposure in HTLV-1+ cells. Effects of As, IFN, As/IFN combination, or MG132 on IκB-ϵ protein levels in HuT-102 cells after 48 hours of exposure to these drugs. Equal protein loading was assessed by hybridization with antiactin or anti-GAPDH antibodies.
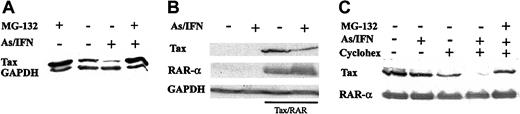
The As/IFN combination degrades Tax through the proteasome
Treatment of ATL-derived cells by As/IFN markedly reduced Tax protein expression. As expected, cycloheximide, which blocks protein synthesis and hence IFN action, and DTT, which interferes with the redox balance and As action,25 both completely abolished Tax down-regulation by As/IFN (not shown). To assess the role of the proteasome in Tax degradation, we used an inhibitor, the peptide aldehyde z-LLL or MG132. Because the effect of As/IFN on Tax level was only detectable after 48 hours, prolonged exposure to MG-132 was required with a maximal possible dose of 5 μM. Under these conditions, significant stabilization of Tax was observed in all the ATL-derived cells tested (Figure 7A and not shown), thus indicating basal proteasome-mediated Tax degradation. Strikingly, MG132 completely abolished the effects of As/IFN on the Tax level in both HuT-102 and MT2 cells (Figure 7A and not shown); this implied that degradation, rather than decreased synthesis, is involved in the down-regulation of Tax. To confirm the As/IFN-induced Tax degradation, HeLa cells were transiently cotransfected with pSG5-Tax and pSG5-RAR-α expression vectors, and the level of these proteins was measured after As/IFN treatment of the transfected cells. A fast decrease in Tax expression was again noted, but the expression of RAR-α was unaffected (Figure 7B). As expected, As/IFN induced large differences in the rates of Tax degradation under conditions of cycloheximide chase (Figure 7C). These difference rates were reversed by MG-132 exposure. Overall, in cells that do not undergo IFN/As-mediated apoptosis, this combination induces Tax degradation by the proteasome.
The As/IFN combination degrades Tax through the proteasome. (A) Effect of As/IFN or MG132 (5 μM) on the Tax protein level in HuT-102 cells after 48 hours of exposure to these drugs. Equal protein loading was assessed using anti-GAPDH antibodies. (B) Effect of As/IFN on Tax and RAR-α protein levels in HeLa cells cotransfected with PSG-Tax and PSG-RAR-α (underlined wells). Equal protein loading was assessed using anti-GAPDH antibodies. (C) Cycloheximide chase in HeLa cells cotransfected with PSG-Tax and PSG-RAR-α. Cells were exposed to As/IFN for 24 hours. When indicated, cycloheximide (50 μg/mL) or MG132 (50 μM) or both were added during the last 6 hours only.
The As/IFN combination degrades Tax through the proteasome. (A) Effect of As/IFN or MG132 (5 μM) on the Tax protein level in HuT-102 cells after 48 hours of exposure to these drugs. Equal protein loading was assessed using anti-GAPDH antibodies. (B) Effect of As/IFN on Tax and RAR-α protein levels in HeLa cells cotransfected with PSG-Tax and PSG-RAR-α (underlined wells). Equal protein loading was assessed using anti-GAPDH antibodies. (C) Cycloheximide chase in HeLa cells cotransfected with PSG-Tax and PSG-RAR-α. Cells were exposed to As/IFN for 24 hours. When indicated, cycloheximide (50 μg/mL) or MG132 (50 μM) or both were added during the last 6 hours only.
Discussion
Distinct gene networks are repressed by As and As/IFN in ATL-derived cells
Clustering analyses demonstrated the existence of 4 distinct gene clusters regulated by As and IFN in HTLV-1–transformed cells. The As-up cluster only comprised a few genes that are not specific to ATL-derived cells and are involved in detoxification. As has been shown to induce Jun-kinase (JNK) activity and massive activation of AP-1–dependent genes26 (H.d.T. unpublished data, June 2002). The constitutive activation of the JNK pathway by Tax in HTLV-1–transformed cells27 likely accounts for the smaller number of genes induced by As in ATL-derived cells compared to activated PBMCs. In the latter, most of the As-induced genes are known AP-1 targets involved in myeloid or lymphoid maturation. IFN rapidly up-regulated its target genes, in an As-independent manner, both in HTLV-1–transformed and normal activated PBMCs. The third cluster, consisting of NF-κB target genes, was rapidly down-regulated by As in an IFN-independent manner and was specific to ATL-derived cells. A last set of genes, known to reflect cell cycle progression,17 was slightly down-regulated by As alone, and dramatically repressed by the As/IFN combination at 48 hours only, in ATL-derived cells alone, in agreement with our previous demonstration of complete ATL cell growth arrest on exposure to As/IFN.12
These results indicated that 2 distinct gene networks are specifically modulated in ATL-derived cells: one is characterized by fast As-induced, extinction of NF-κB target genes, and the other by delayed As/IFN-triggered cell cycle arrest. The former depends on IκB-α and IκB-ϵ stabilization, whereas the latter likely results from Tax degradation. Because NF-κB constitutes a well-known survival pathway in ATL cells, its inactivation likely contributes to the induction of apoptosis. Nevertheless, our results also show that NF-κB shut-off alone is insufficient for apoptosis induction. Addition of IFN to As is required to precipitate cell death, by inducing the degradation of Tax, and presumably reversing its oncogenic effects on multiple cellular targets.
Molecular basis of NF-κB repression by As
The results of previous studies suggested that As represses the NF-κB pathway by inhibiting IKK activity.18,19 Much higher As concentrations were used in these studies than here, and we failed to demonstrate any inhibition of IKK activity or phospho-IκB levels by As. Up-regulation of IκB-α protein by As was not due to enhanced transcription because As down-regulated IκB-α mRNA. In HTLV-1–transformed cells, IκB-α was rapidly degraded by the 26S proteasome. Enhanced expression of this inhibitor likely results from an As-induced decrease in its catabolism. Another inhibitor, IκB-ϵ, was strongly stabilized on As exposure. The significant proteasomal degradation of IκB-ϵ in untreated ATL-derived cells probably contributes to the constitutive activation of NF-κB. Our results are consistent with the hypothesis that As blocks IκB-ϵ degradation. IκB-ϵ is a specific regulator of the genes controlled by RelA/RelA and RelA/c-Rel dimers,22,23 which have major antiapoptotic properties.28 Because its stabilization by As was much greater than that of IκB-α, IκB-ϵ likely plays a major role in As-induced RelA cytoplasmic sequestration, NF-κB deactivation, and ATL cell death.
The IFN/As combination degrades Tax
We showed here that the down-regulation of the viral oncoprotein Tax by As/IFN treatment is mediated by the proteasome. Tax protein was previously shown to bind 2 subunits of the 19S proteasome.29, 30, 31 Such binding may either participate in the postactivation degradation of Tax-bound transcription factors or play a direct role in transcriptional activation per se.32,33 Why As triggers Tax degradation by the proteasome is not yet understood. As in the case of many proteins, proteasome-mediated degradation of Tax may involve posttranslational modifications such as phosphorylation, ubiquitination, sumolation, or direct As binding. That IFN greatly enhances As-induced Tax degradation may be due to the fact that IFN modulates the subunit composition of both the catalytic core of the 20S proteasome and its 11S regulatory cap.34,35 Similarly, PML, another IFN-induced gene, was shown to recruit the 11S proteasome and to be degraded in the presence of As,36, 37, 38 thus providing another example of a gene whose As-induced degradation is enhanced by IFN. Our demonstration that Tax is continuously degraded by the proteasome may account for the subsequent presentation of Tax peptides on major histocompatibility complex (MHC) class I molecules39 and for the high frequency of circulating Tax-specific cytotoxic T lymphocytes found in most individuals infected with HTLV-1 (for a review, see Bangham40 ). Because Tax is an essential activator of the viral gene expression that is absolutely indispensable to replication, the As/IFN combination may be clinically useful not only in ATL, but also in chronic carriers with high viral loads or during initial infection. In that sense, the preliminary results of a phase 2 clinical trial in relapsed/refractory patients with ATL, previously treated with an IFN-containing regimen, demonstrate that As has striking antileukemic effects. More specifically, 4 of the 7 patients treated with As/IFN responded, 1 experienced complete remission (O.H. et al, unpublished data, January 2002). Such specific As-induced Tax degradation is highly reminiscent of the As-induced promyelocytic leukemia-retinoic acid receptor (PML-RAR) degradation observed in acute PML,37,41 and strengthens the case for oncogene-targeted cancer therapy. Conversely, the efficacy of a Tax-targeted treatment strongly favors the concept that continuous Tax expression has a role in maintaining the ATL leukemic phenotype.
Prepublished online as Blood First Edition Paper, January 30, 2003; DOI 10.1182/blood-2002-09-2986.
Supported by the American University of Beirut Medical Practice Plan and University Research Board, the Lebanese National Research Council, the CNRS, ARECA, HERN, the Programme Franco-Libanais “CEDRE,” the Diana Tamari Sabbagh Research Fund, and the Eli-Lilly International Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Kamal Badr and Dr Ghassan Dbaibo for critical reading of this manuscript. The expert assistance from the personnel of the Core Laboratory Facilities of the American University of Beirut is greatly appreciated.