Abstract
As many as 10% of infants with Down syndrome (DS) present with transient myeloproliferative disorder (TMD) at or shortly after birth. TMD is characterized by an abundance of blasts within the peripheral blood and liver, and notably undergoes spontaneous remission in the majority of cases. TMD may be a precursor to acute megakaryoblastic leukemia (AMKL), with an estimated 30% of TMD patients developing AMKL within 3 years. We recently reported that mutations in the transcription factor GATA1 are associated with DS-AMKL. To determine whether the acquisition of GATA1 mutations is a late event restricted to acute leukemia, we analyzed GATA1 in DNA from TMD patients. Here we report that GATA1 is mutated in the TMD blasts from every infant examined. These results demonstrate that GATA1 is likely to play a critical role in the etiology of TMD, and mutagenesis of GATA1 represents a very early event in DS myeloid leukemogenesis.
Introduction
Children with Down syndrome (DS) have a 10- to 20-fold increased risk of developing leukemia, in particular acute megakaryoblastic leukemia (AMKL).1 Children with DS are also predisposed to a related myeloid disorder, termed transient myeloproliferative disorder (TMD).2 As many as 10% of infants with DS develop TMD, in which immature megakaryoblasts accumulate in the peripheral blood and liver. TMD spontaneously resolves in most cases, without therapeutic intervention. However, severe and sometimes fatal forms of TMD do occur, with hepatic fibrosis and liver dysfunction. On the basis of the liver infiltration and the spontaneous remission, it has been speculated that TMD may arise from fetal liver hematopoietic progenitors.2 Of note, approximately 30% of infants with DS and TMD develop AMKL within 3 years. TMD blasts are morphologically indistinguishable from those observed in AMKL, contributing to the hypothesis that the second disease is derived from the first.1,3 It is likely that AMKL results from the acquisition of additional genetic mutations following remission of TMD.
We recently reported that mutations in the essential X-linked hematopoietic transcription factor gene GATA1 are tightly associated with AMKL in Down syndrome.4 We detected mutations in GATA1 in 6 out of 6 DS-AMKL samples, but did not find mutations in GATA1 in leukemic cells of patients with DS who had other types of acute leukemia, or in other patients with AMKL who did not have DS. Furthermore, we did not detect GATA1 mutations in DNAs from more than 75 other patients with acute leukemia or from 21 healthy individuals. Finally, we established that these mutations are somatically acquired, as remission samples from patients did not harbor GATA1 mutations. On the basis of these observations, we hypothesized that disruption of normal GATA-1 function is an essential step in the initiation or progression of megakaryoblastic leukemia in DS.
To determine whether GATA1 mutations represent a late event that contributes to the acute phase of DS myeloid leukemia, we assayed DNA samples from the peripheral blood of infants with TMD for GATA1 mutations. Here we report that GATA1 is mutated in every case of TMD examined. These findings demonstrate that the development of a GATA1 mutation is an early event in DS myeloid leukemogenesis and contributes to both TMD and AMKL.
Study design
Patient samples
Cryopreserved peripheral blood samples from infants with TMD, who were enrolled on the prospective Children's Oncology Group (COG) trial, A2971, for children with TMD or AML, were provided by the Children's Oncology Group. All clinical samples were obtained with informed consent and used with approval from the University of Chicago Institutional Review Board. DNA was extracted by means of standard methods.
SSCP and sequence analysis
DNA samples were screened for mutations in GATA1 by means of the single-strand polymorphism assay (SSCP) as previously described,4 as well as by direct sequencing of polymerase chain reaction (PCR)–amplified DNA. SSCP assays were performed on exons 2 and 3 of GATA1. Primer sequences are available upon request.
Results and discussion
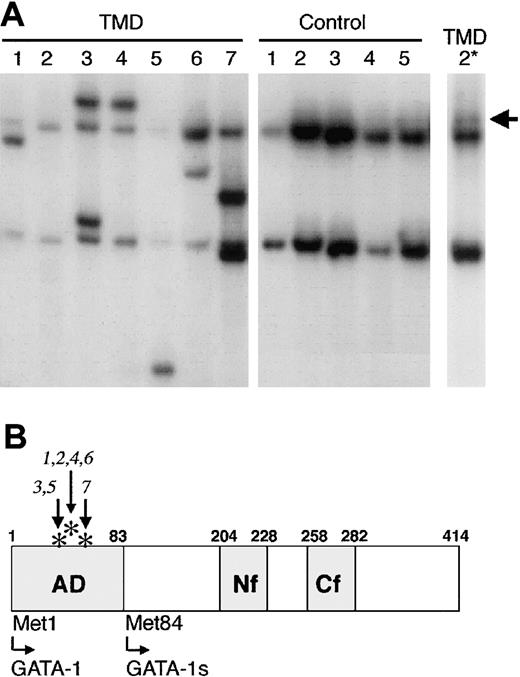
DNAs extracted from peripheral blood of 7 infants with TMD were assayed for the presence of GATA1 mutations by SSCP. All 7 patient samples displayed abnormal migrating PCR products, indicative of a mutation in GATA1 (Figure 1A, TMD samples 1-7). As previously demonstrated, DNAs from healthy individuals generated a single SSCP product (Figure 1A, controls 1-5). Sequencing of the excised SSCP products, as well as direct sequencing of GATA1 in the samples, confirmed that each sample harbored a functional alteration in GATA1. In all cases, the mutations occurred within exon 2, which encodes the N-terminal transactivation domain (Figure 1B). Each of the mutations is a small insertion or deletion that alters the reading frame of GATA-1 and introduces a premature stop codon (Table 1). These mutations are similar to those detected in DS-AMKL.4
Mutation of GATA1 in TMD. (A) SSCP analysis of the second exon of GATA1 revealed the presence of mutated conformational GATA1 alleles in DNA from each of 7 TMD patients. In contrast, GATA1 mutations were not detected in DNAs from 5 healthy individuals. A longer exposure of the SSCP gel revealed a mutant allele in DNA from TMD-2 (arrow, TMD-2* lane). (B) Schematic illustration of the functional domains of GATA-1. AD indicates activation domain; Nf, N terminal zinc finger; Cf, C-terminal zinc finger. The asterisks indicate the positions of the mutations in the 7 individuals with TMD (numbered 1 through 7). Met84 is an alternative translation initiation codon, which leads to the production of GATA-1s.
Mutation of GATA1 in TMD. (A) SSCP analysis of the second exon of GATA1 revealed the presence of mutated conformational GATA1 alleles in DNA from each of 7 TMD patients. In contrast, GATA1 mutations were not detected in DNAs from 5 healthy individuals. A longer exposure of the SSCP gel revealed a mutant allele in DNA from TMD-2 (arrow, TMD-2* lane). (B) Schematic illustration of the functional domains of GATA-1. AD indicates activation domain; Nf, N terminal zinc finger; Cf, C-terminal zinc finger. The asterisks indicate the positions of the mutations in the 7 individuals with TMD (numbered 1 through 7). Met84 is an alternative translation initiation codon, which leads to the production of GATA-1s.
We previously demonstrated that a short isoform of GATA-1, named GATA1s, is produced in the leukemic blasts of an individual with DS-AMKL and in the cell line CMK, which was derived from the malignant cells of a child with Down syndrome and AMKL.4 GATA-1s is initiated at Met84, which lies in exon 3, downstream of each of the patient mutations (Figure 1B). GATA-1s lacks the N-terminal transactivation domain and, consequently, exhibits a much lower transcriptional activation potential than wild-type GATA-1 (Wechsler et al4 ). While it has not been established whether GATA-1s is an oncogenic factor in these myeloid disorders, the finding that all GATA1 mutations identified to date in both DS-AMKL and TMD can potentially produce GATA-1s strongly supports the hypothesis that the short isoform of GATA-1 has an active oncogenic role.
The observation that GATA1 is mutated in the abnormal cells of every TMD patient examined indicates that the gene is affected in as many as 10% of infants with Down syndrome. Furthermore, the finding that the TMD blasts from patient TMD-7, which were harvested from peripheral blood the day of birth, indicates that the acquisition of GATA1 mutations can occur in utero. It remains to be determined whether this high rate of mutation is a consequence of Down syndrome or representative of an extreme growth advantage of megakaryoblasts that harbor trisomy 21 and a truncating GATA1 mutation. While we are unable to confirm that these GATA1 mutations are not germ line, owing to the unavailability of DNA from nondiseased tissue, it is very unlikely that these GATA1 mutations are constitutional. First, the GATA1 mutations in DS-AMKL are somatically acquired.4 Second, the known rare inherited mutations within GATA1 result in chronic anemia (or thalassemia) and thrombocytopenia,5, 6, 7, 8 a feature that is not observed in individuals with Down syndrome. Finally, GATA1 mutations were not previously identified in DNA samples from 21 healthy individuals, 75 patients with AML unrelated to DS-AMKL, or a patient with DS who had acute lymphocytic leukemia (ALL).4
Mutagenesis of GATA1, in conjunction with trisomy 21, may be sufficient to promote the transient expansion of immature megakaryoblasts seen in TMD. However, the acquisition of additional mutations or chromosomal alterations are likely to be necessary for leukemic transformation. For example, alterations in TP53 may be involved in the evolution of this malignancy, as one report described TP53 mutations in 2 of 3 patients with DS-AMKL but no mutations in 7 patients with TMD.9 A detailed study of the status of other genes that are commonly mutated in AML, such as FLT3 and RAS,10 is warranted. Furthermore, comparing the type of GATA1 mutations found in the AMKL blasts with the TMD blasts from the same individual will provide key insights into the relationship between TMD and AMKL.
Prepublished online as Blood First Edition Paper, January 30, 2003; DOI 10.1182/blood-2002-12-3904.
Supported in part by a Junior Faculty Award from the American Society of Hematology (J.D.C) and by the Aplastic Anemia and MDS International Foundation (M.E.G.). J.D.C is a recipient of a Career Development Award from the Burroughs Wellcome Foundation. In this study, the authors used samples from study COG A2971, under the National Cancer Institute (NCI) grant nos. 13539 and 30960.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
This research was facilitated by a collaboration with the Children's Oncology Group. The authors thank the members of the COG Myeloid Biology Subcommittee, Dr Irwin Bernstein, and Dr Todd Alonzo, for providing samples from the COG A2971 study. Additional thanks to Dr Michelle Le Beau for helpful advice and critical reading of the manuscript.