Abstract
Children with constitutional trisomy 21 (Down syndrome) have an approximately 500-fold increased risk of developing acute megakaryoblastic leukemia (AMKL), a form of acute myeloid leukemia. Unique to newborn infants with Down syndrome is a transient leukemia (TL), also referred to as transient myeloproliferative syndrome, that undergoes spontaneous remission in the majority of cases but in approximately 20% is followed by AMKL later in life. Recently mutations of the gene encoding the hematopoietic transcription factor GATA1 were shown to be specific for AMKL of Down syndrome. Here, we demonstrate that GATA1 mutations are present in blasts of TL and show the identical GATA1 mutation in sequential samples collected from a patient during TL and subsequent AMKL. These findings suggest a model of malignant transformation in Down syndrome AMKL in which GATA1 mutations are an early event and AMKL arises from latent TL clones following initial apparent remission.
Introduction
Children with Down syndrome (constitutional trisomy 21) have a 10- to 20-fold increased risk of developing acute leukemia.1, 2, 3 Particularly frequent in this group are 2 forms of megakaryoblastic leukemia. Acute megakaryoblastic leukemia (AMKL), a subtype of acute myeloid leukemia (FAB classification M74 ) occurs with an incidence estimated to be 500-fold greater than in the general pediatric population.5 Further, approximately 10% of newborn infants with Down syndrome develop transient leukemia (TL; reviewed in Zipursky;6 Gamis and Hilden;7 and Taub and Ravindranath8 ), also referred to as transient myeloproliferative disorder and transient abnormal myelopoiesis, a form of megakaryoblastic leukemia specific for Down syndrome. Both in TL and AMKL, blasts accumulate in blood and bone marrow, and megakaryocytic differentiation is abnormal.6,9 In contrast to AMKL, TL undergoes spontaneous remission within the first 3 months. In approximately one fifth of TL cases, however, AMKL develops later in life.5,10,11
The basis of this predisposition of Down syndrome individuals to megakaryoblastic leukemia remains to be determined despite increasing insight into the genes encoded on chromosome 21.12 In a current model of acute leukemia at least 2 cooperating mutations—1 impairing differentiation and another increasing cell proliferation or survival—are required for the malignant transformation of a hematopoietic precursor cell.13 The unique sequence of TL and AMKL in Down syndrome as successive stages in a process of leukemic transformation provides an opportunity to identify the nature and timing of these events.
Recently, somatic mutations of the gene encoding the hematopoietic transcription factor GATA1 were found in AMKL blasts of patients with Down syndrome, suggesting a significant role for these mutations in the development of leukemia. GATA1 has essential functions during the normal erythroid14 and megakaryocytic differentiation of hematopoietic stem cells.15 Lack of GATA1 function in hematopoietic cells had previously been shown to result in the accumulation of abnormally differentiated megakaryocytes and thrombocytopenia without leukemic transformation.16,17 Since the bone marrow in both AMKL and TL shows evidence of abnormal megakaryocytic differentiation,6,9 we hypothesized that GATA1 mutations are present in TL of Down syndrome. In this model, GATA1 mutations would act as an early pathogenic event—prior to the onset of TL—rather than account for the eventual transition of TL to AMKL. Under the hypothesis that AMKL arises from a clone of TL cells, we further predicted the identical type of GATA1 mutation to be present in blasts of TL and subsequent AMKL of the same patient. We performed a mutational analysis of GATA1 in blasts of Down syndrome TL and found evidence in support of both hypotheses.
Study design
Cell samples
The diagnosis of TL and AMKL was based on the morphologic, immunophenotypic, and in selected cases ultrastructural demonstration of megakaryoblastic features of the leukemic blasts. Samples were obtained from nonmosaic Down syndrome patients after informed consent and with the approval of the institutional review board at The Hospital for Sick Children. Samples of transient leukemia were predominantly derived from peripheral blood at the time of a high blast count. DNA was extracted by standard methods from mononuclear bone marrow and peripheral blood cells prepared by Ficoll density separation. A cell line, HSC-GRW, derived from Down syndrome AMKL blasts18 was included in the analysis. Genomic DNA samples extracted from peripheral blood lymphocytes of 20 non–Down syndrome individuals were used as controls.
Mutation analysis
Genomic DNA corresponding to exon 2 of GATA1 (genomic DNA accession no. AF196971, complementary DNA [cDNA] accession no. NM_002049) was amplified by polymerase chain reaction (PCR) using flanking oligonucleotide primers (forward primer, 5′-AAAGGAGGGAAGAGGAGCAG-3′; and reverse primer, 5′-GACCTAGCCAAGGATCTCCA-3′) following a standard protocol (35 cycles of 10 seconds at 94°C, 10 seconds at 60°C, and 60 seconds at 68°C). Amplification products were sequenced directly and after subcloning (TA vector; Invitrogen, Carlsbad, CA). Sequence comparisons and translation were performed using programs available from the National Centre for Biotechnology Information.
Results and discussion
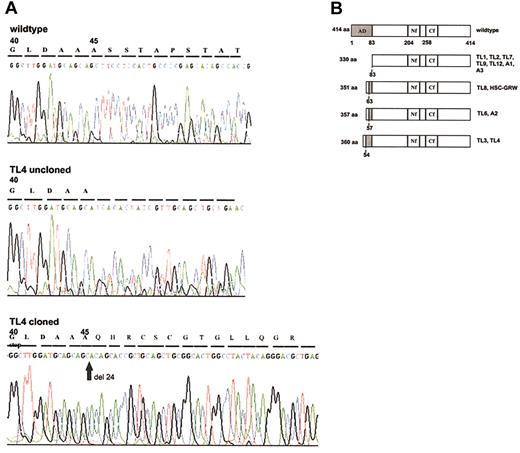
Based on the observation that mutations of GATA1 clustered within the first coding exon (exon 2) in AMKL blasts of Down syndrome,19 we used a PCR-based method to screen for mutations within the coding sequence of exon 2 of GATA1 in peripheral blood and bone marrow samples of patients with Down syndrome TL (DS-TL) and AMKL (DS-AMKL). We found in 9 of 12 patients with DS-TL and in all 3 patients with DS-AMKL, who served as positive controls, GATA1 mutations, chiefly short deletions and insertions (Table 1) that result in the introduction of a premature termination codon as previously described for DS-AMKL.19 Because of alternative downstream initiation codons, the mutant GATA1 proteins are predicted to lack all or part of the transcriptional activation domain encoded by the N-terminal 83 amino acids (aa's) (Figure 1B). In some instances the generation of a new termination codon and shift of the reading frame results in a novel peptide sequence encoded by an alternative reading frame at the N-terminus of the truncated GATA1 (Figure 1B). A deletion within exon 2 of GATA1 was also detected in a cell line established from a patient with DS-AMKL (HSC-GRW).18 In 2 cases we found in addition to insertions (cases TL6 and TL12) and a deletion (case A2) point mutations that did not alter the predicted truncation of the wild-type protein. In 3 cases of TL that require further analysis (cases TL5, TL10, and TL11) we identified single point mutations in exon 2 that predict an N-terminally truncated protein of 330 aa's as observed in cases with nucleotide insertions and deletions (Figure 1B). In contrast, normal GATA1 sequences were observed in samples of 20 non–Down syndrome individuals.
Clinical characteristics and GATA1 mutations of patients with TL and AMKL of Down syndrome
Patient identifier . | Age at diagnosis, d . | Sex . | Age at diagnosis of AMKL . | GATA1 mutation* . | Length of predicted protein, aa† . |
|---|---|---|---|---|---|
| Transient leukemia | |||||
| TL1 | 1 | F | 1 y; survived after treatment of AMKL | 226-272del47 | 330 |
| TL2 | 1 | M | Died on d 16 of CHD | 233-243del11 | 330 |
| TL3 | 1 | M | Died on d 97 of liver disease | 266-267insCAGC | 373 |
| TL4 | 1 | M | NA | 247-270del24 | 365 |
| 270-271insACAG | |||||
| TL6 | 1 | M | NA | 272A>G | 373 |
| 273-274insAGTG | |||||
| 274del1 | |||||
| 278insT | |||||
| TL7 | 5 | M | NA | 273-274insAGCACAGCCAC | 330 |
| TL8 | 52 | M | 2 y, 3 mo | 298-299insT | 372 |
| TL9 | 30 | M | Well until 3 y then lost to follow-up | 150-151del2 | 330 |
| TL12 | 4 | F | NA | 288C>T | 330 |
| 217-218insC | |||||
| AMKL | |||||
| A1 | NA | M | 19 mo | 124-125insT | 330 |
| A2 | NA | M | 8 mo | 144C>A | 363 |
| 255-280del26 | |||||
| A3 (same patient as TL1) | 1 | F | 1 y; survived after treatment of AMKL | 226-272del47 | 330 |
| Cell line | |||||
| HSC-GRW | — | — | — | 262-296del35 | 360 |
Patient identifier . | Age at diagnosis, d . | Sex . | Age at diagnosis of AMKL . | GATA1 mutation* . | Length of predicted protein, aa† . |
|---|---|---|---|---|---|
| Transient leukemia | |||||
| TL1 | 1 | F | 1 y; survived after treatment of AMKL | 226-272del47 | 330 |
| TL2 | 1 | M | Died on d 16 of CHD | 233-243del11 | 330 |
| TL3 | 1 | M | Died on d 97 of liver disease | 266-267insCAGC | 373 |
| TL4 | 1 | M | NA | 247-270del24 | 365 |
| 270-271insACAG | |||||
| TL6 | 1 | M | NA | 272A>G | 373 |
| 273-274insAGTG | |||||
| 274del1 | |||||
| 278insT | |||||
| TL7 | 5 | M | NA | 273-274insAGCACAGCCAC | 330 |
| TL8 | 52 | M | 2 y, 3 mo | 298-299insT | 372 |
| TL9 | 30 | M | Well until 3 y then lost to follow-up | 150-151del2 | 330 |
| TL12 | 4 | F | NA | 288C>T | 330 |
| 217-218insC | |||||
| AMKL | |||||
| A1 | NA | M | 19 mo | 124-125insT | 330 |
| A2 | NA | M | 8 mo | 144C>A | 363 |
| 255-280del26 | |||||
| A3 (same patient as TL1) | 1 | F | 1 y; survived after treatment of AMKL | 226-272del47 | 330 |
| Cell line | |||||
| HSC-GRW | — | — | — | 262-296del35 | 360 |
Mutations result in a shift of the reading frame; mutations result in a premature termination codon; and nucleotide numbers refer to the cDNA sequence of human GATA1 (“Study design”).
Number of amino acids (aa's) encoded by the open reading frame of the mutant GATA1 gene (Figure 1B). We are also characterizing 3 samples TL5, TL10, and TL11 with single point mutations either in exon 2 (TL5: nucleotide 161C>T; TL11: 201C>G of cDNA sequence NM_002049) or in intron 1 (TL10: 10603T> A in genomic sequence AF196971). Mutations in TL5 and TL11 predict a 330-aa truncated protein. These cases with point mutations are phenotypically indistinguishable from the remainder of the cohort. Further analysis is in progress. CHD indicates congenital heart disease; NA, not available; and —, not applicable.
GATA1 mutant proteins. (A) Direct sequence analysis of genomic DNA from sample TL4 shows a mixed signal downstream of codon 44 indicating a frame-shift mutation. Sequencing of the cloned mutated GATA1 allele demonstrates a new stop codon at position 59. (B) Bar diagram of mutant GATA1 proteins. Compared with the 414-aa wild-type GATA1, the mutant proteins in DS-TL and DS-AMKL cases contain truncations of the N-terminal activation domain (AD, shaded in dark gray). Novel peptide sequences (shaded in light gray) added to the N-terminus of truncated GATA1 are due to a shift of the reading frame. Numbers to the left of bars indicate the length of the open reading frame encoded by wild-type (414 aa) and mutant GATA1. The corresponding cases of DS-TL (coded TL) and DS-AMKL (coded A) are indicated to the right of each bar. Numbers beneath bars refer to the amino acid sequence of wild-type GATA1 and either delineate the functional domains of the wild-type protein (top bar) or the residue at which N-terminal truncation occurs in the mutant protein (dashed lines). AD indicates activation domain; Nf, N-terminal zinc finger; and Cf, C-terminal zinc finger.
GATA1 mutant proteins. (A) Direct sequence analysis of genomic DNA from sample TL4 shows a mixed signal downstream of codon 44 indicating a frame-shift mutation. Sequencing of the cloned mutated GATA1 allele demonstrates a new stop codon at position 59. (B) Bar diagram of mutant GATA1 proteins. Compared with the 414-aa wild-type GATA1, the mutant proteins in DS-TL and DS-AMKL cases contain truncations of the N-terminal activation domain (AD, shaded in dark gray). Novel peptide sequences (shaded in light gray) added to the N-terminus of truncated GATA1 are due to a shift of the reading frame. Numbers to the left of bars indicate the length of the open reading frame encoded by wild-type (414 aa) and mutant GATA1. The corresponding cases of DS-TL (coded TL) and DS-AMKL (coded A) are indicated to the right of each bar. Numbers beneath bars refer to the amino acid sequence of wild-type GATA1 and either delineate the functional domains of the wild-type protein (top bar) or the residue at which N-terminal truncation occurs in the mutant protein (dashed lines). AD indicates activation domain; Nf, N-terminal zinc finger; and Cf, C-terminal zinc finger.
The presence of GATA1 mutations in blasts of DS-TL is consistent with a model in which normal GATA1 function is lost during erythropoietic and megakaryopoietic differentiation of hematopoietic precursors with constitutional trisomy 21 by the time TL emerges. Because of the localization of GATA1 on the X chromosome the loss of normal GATA1 function may result from the mutation of a single GATA1 allele in hematopoietic precursor cells of Down syndrome.
Using the variable length of nucleotide insertions and deletions within exon 2 of GATA1 in DS-AMKL and DS-TL as a marker of individual TL clones we then compared GATA1 mutations in sequential samples collected from the same patient during TL (Table 1, case TL1), remission, and AMKL (case A3). The identical GATA1 mutation was found in blasts of TL and subsequent AMKL and diagnosed within the first week of life and at 1 year of age, respectively. In contrast, this mutation was not present after the remission of AMKL at 35 months of age (data not shown). The evolution of DS-AMKL from a population of TL blasts is suggested by the observation that chromosomal abnormalities are more frequent in AMKL blasts than in TL.20, 21, 22 Although cytogenetic abnormalities other than trisomy 21 are rare in TL, 3 cases were documented with recurrent cytogenetic abnormalities in blasts of TL and subsequent AMKL.23, 24, 25 These findings and our demonstration of the identical GATA1 mutations in TL and AMKL blasts of the same patient support a model in which AMKL arises from clones of TL blasts that persist during apparent remission.
In summary, GATA1 mutations similar in effect to those reported previously in DS-AMKL19 were also found in the majority of cases with DS-TL. This suggests a model of AMKL in Down syndrome in which GATA1 mutations represent an early pathogenic event that occurs prior to the transformation of TL to AMKL. The new insight into the role of GATA1 mutations should direct further efforts to the identification of the cooperating mutation(s) and the role of gene dosage in trisomy 21 that are able to complete the leukemic transformation in Down syndrome AMKL.
Prepublished online as Blood First Edition Paper, February 13, 2003; DOI 10.1182/blood-2003-01-0013.
Supported in part by The Hospital for Sick Children Foundation (J.K.H.), and a grant from the Canadian Institutes of Health Research (CIHR) and Genome Canada (S.W.S.). S.W.S. is a Scholar of CIHR and International Scholar of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank J. Dick and S. Morris for helpful discussions and Elizabeth Brown for expert technical assistance with the tissue bank.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal