Abstract
Leukoreduced blood products are reportedly comparable to cytomegalovirus (CMV)–seronegative products for the prevention of transfusion-transmitted CMV (TT-CMV) infection after stem cell (SC) transplantation. To determine if the incidence of TT-CMV was affected by the increasing use of leukoreduced blood products, we followed a prospective cohort of 807 CMV-seronegative SC transplant (SCT) recipients who underwent weekly surveillance using the pp65 antigenemia assay. The incidence of TT-CMV for 2 time periods was recorded: Period 1 (5/94-11/96), when only CMV-seronegative and/or filtered blood products were provided, and period 2 (12/96-2/00), when leukocyte-reduced platelets obtained by apheresis without filtration were also used. The incidence of TT-CMV was higher during period 2 (18/447, 4%) than period 1 (6/360, 1.7%) (P < .05); this was correlated with higher utilization of both filtered and apheresed products from CMV-positive donors in period 2. Multivariable analysis identified filtered red blood cell (RBC) units (but not apheresis platelet products) from CMV-positive donors as the primary predictor of TT-CMV: each additional filtered RBC unit was associated with a 32% increase in the odds for TT-CMV (95% confidence interval [CI]: 8%-61%, P = .006). Pre-emptive therapy with ganciclovir after detection of antigenemia prevented all but one case of CMV disease prior to day 100. CMV-seronegative products may thus be superior to leukoreduced products (particularly filtered RBCs) for the prevention of TT-CMV. In an era of “universal leukoreduction,” the abandonment of CMV-seronegative inventories appears premature, particularly among populations at high risk of CMV disease that do not receive active surveillance.
Introduction
Transfusion-transmitted cytomegalovirus infection (TT-CMV) is associated with considerable morbidity and mortality in at-risk populations, which include CMV-seronegative neonates, patients with AIDS, and stem cell transplant (SCT) recipients. The provision of CMV-seronegative blood product support to these individuals became the standard of care in the late 1980s after studies showed this strategy significantly reduced the rate of TT-CMV.1-3 The maintenance of CMV-seropositive and -seronegative “dual inventories” is expensive, however, and some communities with high CMV seroprevalence have found it difficult to maintain adequate supplies of CMV-seronegative products. Thus, alternate methods for the provision of “CMV-safe” blood products have been pursued.
Studies have demonstrated that CMV is latent in cells of the monocyte/macrophage lineage and that these cells can support CMV replication.4,5 Third-generation filters effectively remove approximately 3-log10 of the contaminating leukocytes in blood products,6 thus theoretically reducing the probability of TT-CMV. In a landmark study of 502 seronegative SCT recipients which tested this hypothesis, the incidence of TT-CMV when filtered blood products were used for blood product support (2.4%) was not significantly different from the observed incidence when only seronegative blood products were used (1.4%) (P = .5).7 Others have noted, however, that even though the study was not sufficiently powered to detect clinically significant differences in efficacy, it still documented a significantly higher rate of CMV disease in the filtered arm (2.4%) than in the seronegative arm (0%).8
Despite the controversy, filtered products have been increasingly used at oncology and SCT centers9-13 in addition to neonatal units.14 Due to ongoing concerns of TT-CMV associated with the use of leukoreduced products, we initiated a prospective surveillance system in 1994 to capture the occurrence of TT-CMV in a larger population of seronegative SCT recipients; these patients were also treated with pre-emptive antiviral therapy with the goal of preventing CMV-related complications. Since that time, new techniques for leukoreduction have been introduced as well. The collection of single donor apheresis products using certain cell separators achieves similar levels of leukoreduction when compared with filtration; as such, these products were also introduced into clinical practice despite an absence of studies that document their clinical efficacy as “CMV safe.”
We recently noted an increase in the incidence of TT-CMV that appeared to be correlated with the utilization of apheresis platelet products for blood product support. As such, we sought to determine if the incidence of TT-CMV had changed over time with changes in blood bank practices. In addition, we examined the impact of pre-emptive antiviral therapy on the occurrence of CMV disease and CMV-related death in this population, which still may occur in up to 1% to 3% of these SCT recipients in the absence of such therapy.7
Patients and methods
Study design
All CMV-seronegative patients undergoing autologous SC transplantation or receiving allografts from CMV-seronegative donors between May 1, 1994 and February 28, 2000 were prospectively monitored for TT-CMV as described in the next section. Serologic testing of patients and their donors was performed prior to transplantation using enzyme immunoadsorbent assays (EIA) (CMV STAT; Whittaker Bioproducts, Walkersville, MD, and Gull Laboratories, Salt Lake City, UT); retrospective (confirmatory) serologic testing for patients with documented primary CMV infection were performed using the Biotest enzyme-linked immunosorbent assay (ELISA) (Denville, NJ). Conditioning for SC transplantation15-17 and prophylaxis/treatment18 19 of graft-versus-host disease (GVHD) was performed as previously described. The study was approved by the institutional review board at the Fred Hutchinson Cancer Research Center.
CMV virologic monitoring and pre-emptive therapy
All patients throughout the period of study received weekly monitoring using the pp65 antigenemia assay as described previously.20 For allogeneic SCT recipients, pre-emptive ganciclovir therapy was administered for any positive test21; autologous SCT recipients were treated only for pp65 antigenemia levels more than or equal to 5 cells per slide.22
Blood bank practices during period of study and quality control of blood components
From May 1994 until November 1996 (defined in the study as period 1), blood products (red blood cells [RBCs] or platelets) for these patients were provided from CMV-seronegative donors or were leukocyte reduced at the Puget Sound Blood Center by filtration. If seronegative components were requested, an effort was made to provide them; if not available, then leukocyte-reduced products from CMV-positive donors were provided. Platelet concentrates (either pooled or apheresis) were filtered after storage at the blood bank using the Pall LRF 10 (Pall Biomedical Products, East Hills, NY), whereas RBC units were filtered before storage using the Pall BPF4/Leukotrap filter.
From December 1996 until February 2000 (period 2), blood products were provided as done during period 1, except that plateletpheresis components were leukocyte reduced as an integral part of the centrifugal apheresis process itself (LR apheresis platelets), rather than by subsequent filtration. Platelet apheresis was performed using the COBE Spectra LRS or LRS TURBO (Gambro BCT, Lakewood, CO).
Quality control procedures to evaluate the quantity of residual postleukoreduction white blood cells (WBCs) were in place throughout the study period. Approximately 15 U filtered RBCs, 4 U filtered pooled platelets, and 20 U platelets obtained by apheresis were tested on a monthly basis. The failure rate (postleukoreduction WBC count > 5 × 106)23 was extremely low for all components tested: filtered RBCs, 2/849 U (0.2%, mean 6.0 × 106 WBC/U among “filter failures”); LR apheresis platelets, 3/746 U (0.4%, mean 15.1 × 106WBC/U among “apheresis failures”); and filtered pooled platelets, 0/81 U (0%). Products identified as leukoreduction failures during quality control evaluation were not administered as “CMV-safe” components.
Evaluation of CMV infection and disease
CMV infection was defined as the identification of CMV by culture or antigen detection from any clinical specimen. CMV disease was defined according to consensus guidelines.24 Both CMV infection and CMV disease were analyzed separately as early TT-CMV (occurring before day 100 after transplantation, during antigenemia monitoring) and late TT-CMV (occurring after day 100, after routine monitoring had been discontinued). CMV-related mortality was defined as death occurring within 6 weeks of the diagnosis of CMV disease or CMV disease discovered at autopsy.
Data sources
Clinical and laboratory data were extracted from a prospectively entered, integrated database that is complete for the first 100 days after transplantation. Patient clinical records after discharge are maintained in a long-term, follow-up database, which includes prospectively entered data from a one-year follow-up examination and review, yearly questionnaires to local physicians and patients, and copies of hospital discharge summaries, death certificates, and autopsy reports.
Data regarding blood products received by each patient during the first 100 days after SC transplantation were retrieved from the computerized database at the Puget Sound Blood Center. Blood products were characterized by type (RBC, pooled platelet, or apheresis platelet) and CMV serostatus of the donor or pool (positive, negative, or not tested). Units that were not tested were considered to be CMV positive; these accounted for less than 0.1% of the products provided.
Statistical analysis
Univariate comparisons were performed using the chi-square or Student t test, as appropriate. Logistic regression was used to examine the association of various factors with the probability of TT-CMV. For blood products, evaluation was centered upon the number of each product type administered and the CMV serostatus of the unit or pool; the number of products of each type that patients received per day was then normalized for the number of days of patient follow-up. Follow-up time was set as the time to death or CMV infection; time denominator was set as 100 for those who survived to 100 days without evidence of TT-CMV. Variables were first analyzed for their association with the probability of TT-CMV in univariate models and later in multivariable models; thus, each blood product type was evaluated in conjunction with overall blood product support. All P values from the regression models are 2-sided and were calculated from the Wald test.
Results
Occurrence of TT-CMV
Primary CMV infection occurred in 24 of 807 (3.0%) of these CMV-seronegative transplant recipients, with median onset 35 days after transplantation (range, 12-97 days). To rule out serologic misclassification of either SCT recipient or donor, banked pretransplantation sera from 23/24 patients and their donors were obtained and retested using a second highly sensitive ELISA for CMV immunoglobulin G (IgG) and IgM. None of the stem cell donors were positive, and only 3 stem cell recipients had equivocal serologies (ie, positive and negative CMV IgG titers on repeated testing). No SCT recipient, however, had CMV IgM detected before transplantation, and all were entirely asymptomatic entering transplantation, making primary infection around the time of transplantation unlikely. All 3 individuals with equivocal serologies had also recently received pooled intravenous immunoglobulin (IVIG) for their underlying diseases, which was deemed to be the likely cause of the equivocal titers; these patients were thus included in subsequent analyses.
Incidence of TT-CMV according to blood bank policy
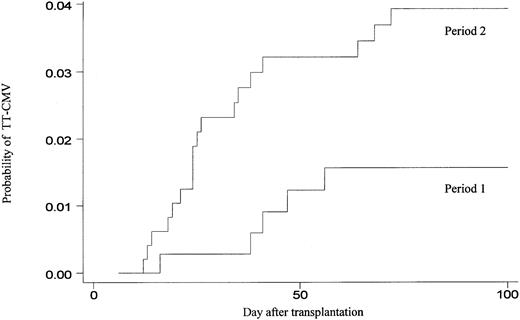
The occurrence of early TT-CMV was first compared between period 1 (when only seronegative or filtered products were used for blood product support) and period 2 (when platelet products that were leukocyte reduced via apheresis without further filtration were used as well). The probability of early TT-CMV during period 2 (18/447, 4.0%) was significantly higher than during period 1 (6/360, 1.7%) (P = .05) (Figure 1).
Cumulative incidence of TT-CMV among 807 transplant recipients, according to transfusion strategy.
During period 1, 360 patients were supported with only CMV-seronegative or filtered blood products from CMV-positive donors, whereas during period 2, 447 patients were also supported with platelets obtained via apheresis from CMV-positive donors without further filtration.
Cumulative incidence of TT-CMV among 807 transplant recipients, according to transfusion strategy.
During period 1, 360 patients were supported with only CMV-seronegative or filtered blood products from CMV-positive donors, whereas during period 2, 447 patients were also supported with platelets obtained via apheresis from CMV-positive donors without further filtration.
The mean number of total blood products, red blood cell units, and platelet units administered per patient between period 1 and period 2 were similar (Table 3). Patients received significantly more apheresis platelets during period 2 than period 1, but also received significantly more filtered products in the latter period. Notably, the overall use of leukoreduced blood of any type was low in both time periods. During period 1, 0.7% of RBC products and 1.2% of platelet products were filtered from CMV-positive donors. During period 2, the proportion of RBC products that were filtered from CMV-positive donors increased to 1.5%; leukoreduced platelet products from CMV-positive donors accounted for 15.8% (filtered pooled platelets, 1.8%; apheresis platelets, 14%) of total platelet support. The remaining RBC and platelet products were derived from CMV-seronegative donors.
Blood product support and risk for TT-CMV
Patients with TT-CMV received significantly more total blood product support than those who did not develop TT-CMV (P = .04) (Table 4). Patients who developed TT-CMV also received significantly more filtered RBCs from CMV-positive donors (P = .002) and more leukoreduced apheresis platelets (P = .002) from CMV-positive donors.
Risk factors for TT-CMV: univariate and multivariable analysis
Univariate and multivariable analyses for risk factors associated with TT-CMV were performed for the 447 patients who underwent transplantation during period 2, since patients during the initial period did not receive leukoreduced apheresis platelets. In univariate analysis, the receipt of each additional CMV-seronegative unit or leukoreduced apheresis platelet product (but not filtered platelet products) was associated with a significant increase in the risk for TT-CMV (Table 5). Despite the low proportion of filtered RBC products provided during this period (1.5% of overall RBC support), a strong trend between the receipt of these products and the occurrence of TT-CMV was noted (P = .06). Patients who received any leukoreduced product had a higher incidence of TT-CMV (14/235, 5.6%) than those who received only seronegative products (4/194, 2.0%) (P = .05). However, those who received any leukoreduced product had significantly higher blood product requirements than those who received only seronegative components (mean 35.1 U vs 12.4 U, P < .001).
In a multivariable analysis that controlled for each blood product received, the receipt of each additional filtered RBC product from a seropositive donor was associated with a 32% increase in the odds for TT-CMV (95% CI 8%-61%, P = .006) (Table 5). The receipt of CMV-seronegative, leukoreduced apheresis platelet products or filtered platelets was not associated with TT-CMV once overall blood product support was considered. A separate multivariable analysis of the 360 patients who underwent transplantation during period 1 showed similar results (data not shown).
An analysis of factors other than blood product support showed that recipients of allogeneic transplants were no more likely to develop TT-CMV than their autologous counterparts (Table 5). Other factors, including patient age, underlying disease, stem cell source, presence of acute graft-versus-host disease, or season of transplantation25 were not associated with TT-CMV.
Outcomes of TT-CMV with pre-emptive ganciclovir therapy
In 24 of 807 patients (3.0%), pp65 antigenemia developed and patients were treated according to protocol; no cases of CMV disease occurred among these 24 patients within the first 100 days of transplantation. Of the 6 autologous transplant recipients who developed antigenemia, 3 received ganciclovir; the remaining 3 were not treated due to antigen levels less than 5. Of the 18 recipients of allogeneic transplants who developed antigenemia and received pre-emptive ganciclovir, 4 (22%) developed neutropenia after 5 to 7 weeks of therapy, which resolved with cessation of therapy and/or use of granulocyte colony-stimulating factor (G-CSF). One allogeneic recipient developed biopsy-proven CMV enteritis at day 35 after transplantation; he had persistently negative CMV antigenemia assays, was treated with ganciclovir until day 100 after transplantation, and had an uneventful recovery. Thus, the overall incidence of CMV disease in the cohort was 1 of 807 (0.1%), and no cases of CMV-related death occurred in the first 100 days after transplantation. When compared with the randomized controlled trial previously conducted at our center,7 monitoring and pre-emptive therapy significantly reduced the incidence of CMV disease (1/807 vs 6/502,P = .01) and CMV-related death (0/807 vs 5/502,P = .005) during the first 100 days after transplantation in this highly susceptible cohort of patients.
After the first 100 days (when patients commonly return to their referring physicians), patients were not routinely monitored for CMV infection. Survey of long-term follow-up records indicated an additional 7 cases (0.8%) of CMV infection and 6 additional cases of late CMV disease among patients without primary infection during the first 100 days (CMV enteritis on day 104, day 220, and day 422; fatal CMV pneumonia on day 149 and day 205, and nonfatal CMV pneumonia on day 697). All 4 of the allogeneic transplant recipients who developed late CMV disease had ongoing transfusion requirements in addition to chronic GVHD treated with high-dose steroids; one of 2 autologous transplant recipients with late CMV disease had pseudo-GVHD and was receiving high-dose prednisone. Thus, these data illustrate that CMV-negative SCT recipients remain at risk for primary infection, CMV disease and CMV-related death if not consistently monitored for pp65 antigenemia.
Discussion
We have demonstrated that blood bank practices may significantly influence the risk of TT-CMV after stem cell transplantation. Although the most notable change in practice during the study was the use of leukoreduced apheresis platelets from CMV-positive donors, these products did not appear to increase the risk for TT-CMV when the use of other blood products was considered. Rather, a multivariable model showed that the receipt of each unit of filtered RBCs from CMV-positive donors (a practice that increased over time as well) was correlated with a 32% increase in risk for TT-CMV after controlling for other blood products received. The use of virologic monitoring and pre-emptive therapy prevented most cases of CMV disease and all cases of CMV-related death early after transplantation, but patients remained at risk for CMV infection and disease long after discharge from the transplant center.
Although many groups have published nonrandomized studies that show low rates of TT-CMV among patients supported with leukoreduced blood products, our study is different in several regards. First, our cohort was significantly larger than other published reports (range, 29-60 patients).9-13 Larger populations are critical for studies of relatively rare outcomes, and also allowed us to perform multivariable analysis of risk factors for TT-CMV. Most importantly, all patients in our cohort were prospectively monitored with the pp65 antigenemia assay, and all bronchoalveolar lavage, biopsy, and autopsy specimens were rigorously assessed for the presence of CMV, ensuring the capture of both primary CMV infection and CMV disease. It is notable that other, smaller studies that have employed screening for primary CMV infection among seronegative SCT recipients who receive “CMV-safe” blood product support have documented infection rates ranging from 6.7% to over 50%, depending upon whether pp65 antigenemia or polymerase chain reaction (PCR) for CMV DNA is utilized.26-28 The higher sensitivity of PCR for CMV DNA may lead to lower positive predictive values for CMV disease in seronegative patients; thus, antigenemia-based surveillance may be preferable for this patient population.26
Importantly, our results are also consistent with the results of the previously reported randomized trial that compared filtered and seronegative blood product support, where a trend toward a higher rate of TT-CMV in the filtered arm (2.4%) than in the seronegative arm (1.4%) was demonstrated.7 That this difference was not “statistically significant” may be related to statistical power, rather than the true equivalence of the approaches. Indeed, the investigators chose to set the quantitative boundary for equivalence quite high; assuming an incidence of 1% in the seronegative arm, the authors deemed an incidence of up to 6% in the filtered arm as “statistically equivalent.” Although criteria do not exist for determining what is reasonable in terms of “clinically significant” proportional differences in efficacy, the 500% difference used in the power calculations for this study (or even the 71% difference that was observed) is far greater than a 20% cutoff for “clinically significant differences” that has been suggested in the literature.29
The quantity of residual WBCs (which may carry the latent CMV genome) after leukoreduction may explain the association of filtered RBC products from CMV-positive donors with the occurrence of TT-CMV. Prior to leukoreduction, packed RBC products contain 10 times the number of contaminating WBCs (approximately 1-5 × 109)30 than do platelet concentrates (approximately 108).31 Thus, the quantity of contaminating WBCs in filtered RBCs is higher (105-106) than in filtered platelet concentrates (104-105) or in platelet products obtained via apheresis (104-105).32 However, analysis of the quantity of residual leukocytes (and the subtypes that are preferentially removed) is difficult and requires inferences regarding the site of viral latency. Thus, a recent study assessed the effect of leukoreduction in reducing the quantity of CMV DNA present in blood products obtained from healthy CMV-seropositive patients who were known to have reactivated CMV. Notably, the proportion of leukoreduced products with more than 50 CMV genomic equivalents appeared to be higher for filtered RBCs (6/32) than for filtered platelet products (1/32) or platelet products obtained via apheresis (4/32).25 While a “minimal infectious dose” for CMV in blood products has not been established, these data suggest that filtered RBC products from CMV-positive donors may carry more CMV than do other leukoreduced products.
Although filtered RBC products were associated with an increased risk for TT-CMV in our study, it is important to note that the exclusive use of seronegative products does not prevent all cases of “primary CMV infection.” Indeed, a 2% incidence of CMV infection was noted among patients who received only CMV-seronegative products in this study. CMV serology is not 100% sensitive for the detection of true latent infection, and as such misclassification of CMV serostatus can occur at the level of the blood donor, stem cell recipient, or stem cell donor. Misclassification of the stem cell recipient was proposed as the reason for the increased rate of CMV disease in the filtered arm of the randomized trial7; our data indicate that this may only be a partial explanation for the incidence of primary infection seen in that study. Plasma viremia among immunocompetent, CMV-seropositive blood donors may occur more often than previously recognized, and may explain the occurrence of “leukoreduction failures.”25Because only a single product may be required to transmit CMV to the immunocompromised seronegative host, the probability that such an “infectious product” is administered increases with higher use of that product. We believe that any blood product has the potential to transmit CMV; our data suggest that the odds of TT-CMV associated with each additional filtered RBC product may be higher than that associated with other “CMV-safe” products.
The impact of TT-CMV on patient outcomes was reduced by the use of surveillance for pp65 antigenemia and pre-emptive ganciclovir therapy. Given the results of the randomized trial7 and the high correlation between primary CMV infection and disease,1 it is reasonable to project that pre-emptive therapy prevented one to 2 cases of disease and one death due to CMV per 100 patients. Once monitoring was discontinued at 100 days after transplantation, however, CMV infection and disease emerged. On the basis of these data, we have initiated prolonged monitoring and therapy for seronegative transplant recipients on chronic immunosuppression and with ongoing transfusion requirements. Many centers have been hesitant to screen seronegative SCT recipients for CMV infection given cost considerations and the perceived low incidence of the event. We believe that the high cost associated with the treatment of CMV disease (and the immeasurable cost of preventable death) likely outweighs the cost of monitoring for this population, albeit cost-benefit analyses are needed.
Finally, our study may have important implications for “unmonitored” populations, such as CMV-seronegative pregnant women, preterm infants, or patients with AIDS, where primary CMV infection may go undetected but may be equally as deadly. In the future, blood products may be treated with technologies that can inactivate viruses and other blood-borne pathogens.33 For now, seronegative components may be preferable to filtered components for these high-risk individuals. Given the movement toward “universal leukoreduction” of blood products for other indications34 and the perception that leukoreduced products are comparable with seronegative products for the prevention of TT-CMV, some have suggested that the maintenance of CMV-seropositive and -seronegative “dual inventories” is no longer necessary.35 36 Given the devastating consequences of intrauterine or neonatal CMV infection, we believe that this position merits reconsideration.
Although our study is the largest yet performed to address this issue, several limitations are worthy of discussion. Residual confounding due to unidentified factors could conceivably be present, for example, since randomized allocation to different blood product strategies was not used in this cohort study. In addition, attributing the source of primary CMV infection to blood products is assumed in this setting, but is difficult to prove conclusively. “Nosocomial transmission” from healthcare workers or visitors (ie, shedding toddlers) is possible, but has never before been documented among transplant recipients; because these events would be random (if they occurred), they would be unlikely to bias our analysis. Repeat testing of both stem cell recipients and their donors suggested that most (if not all) were indeed CMV naive entering transplantation; thus, the subsequent detection of CMV antigenemia in this setting is usually considered diagnostic of primary infection (rather than reactivation from latency). While additional testing for CMV IgG or IgM in these patients could be considered for confirmation, these assays are inaccurate in the SCT recipient due to abrogated B-cell immunity (leading to poor sensitivity) and the frequent receipt of pooled immunoglobulins (resulting in poor specificity).
Thus, primary CMV infection in these multiply transfused patients has historically been attributed to blood products. Definitive linkage to individual transfusions, however, is technologically and logistically difficult given currently available specimens and technologies. Even if aliquots from all 19 000 blood products administered to our patients were available, testing of these specimens using highly sensitive PCR assays for CMV DNA would be unlikely to reveal which products were linked with transmission. This is because CMV is virtually undetectable in the immunocompetent blood donor due to the rarity of the genome in circulating plasma or WBCs; as a result, detection of CMV via PCR in the immunocompetent host using current technologies may be just as likely to represent a false-positive as a true positive result.33 Even if DNA were detected, currently available strain typing techniques have not to date allowed efficient tracking of viral DNA from the donor into the recipient. The development of these tools is an active area of laboratory research at our program; the present study provides a strong rationale to pursue this area of research in the laboratory.
We conclude that leukoreduced and seronegative blood products may not be equivalent as “CMV-safe” components for the support of CMV-seronegative, susceptible individuals. While a multivariate analysis primarily implicates filtered RBCs in the rising incidence of TT-CMV in our SCT population, this analysis should be viewed with caution given the dramatic increase in the use of apheresis platelet products during the latter period of our study. Rather, we believe that our analysis should spur further investigation of the relative safety of these components. Regardless of transfusion strategy, prospective surveillance of high-risk populations accompanied by pre-emptive therapy allows one to identify potential failures of that strategy, while at the same time preventing serious complications for individual patients. Nevertheless, we believe that the abandonment of “dual inventories” in the approaching era of universal leukoreduction appears premature. Indeed, the same conclusion was reached at a recent consensus conference that examined this issue in Canada32; despite the implementation of universal leukoreduction in that country, the use of CMV-seronegative products was maintained for these high-risk populations.
The authors wish to thank Chris Davis and Adrienne Devine for assistance in data extraction.
Prepublished online as Blood First Edition Paper, January 16, 2003; DOI 10.1182/blood- 2002-10-3143.
Supported by National Institutes of Health (NIH) grant K23 AI01839 and the Infectious Disease Society Fellowship in Medical Mycology and Opportunistic Pathogens (W.G.N.), NIH grant CA 18029 (M.B., L.C.), and NIH grant CA 15704 (L.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
W. Garrett Nichols, Program in Infectious Diseases, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave North, D3-100, PO Box 19024, Seattle, WA 98109-1024; e-mail:gnichols@fhcrc.org.