Abstract
Antibody-reliant destruction of tumor cells by immune effector cells is mediated by antibody-dependent cellular cytotoxicity, in which Fc receptor (FcR) engagement is crucial. This study documents an important role for the β2 integrin Mac-1 (CD11b/CD18) in FcR-mediated protection against melanoma. CD11b-deficient mice, those that lack Mac-1, were less protected by melanoma-specific monoclonal antibody TA99 than wild-type (WT) mice. Significantly more lung metastases and higher tumor loads were observed in Mac-1−/− mice. Histologic analyses revealed no differences in neutrophil infiltration of lung tumors between Mac-1−/− and WT mice. Importantly, Mac-1−/−phagocytes retained the capacity to bind tumor cells, implying that Mac-1 is essential during actual FcR-mediated cytotoxicity. In summary, this study documents Mac-1 to be required for FcR-mediated antimelanoma immunity in vivo and, furthermore, supports a role for neutrophils in melanoma rejection.
Introduction
Antibody (Ab)–dependent cellular cytotoxicity (ADCC) is considered crucial for Ab-mediated tumor cell degradation. Specific Ab–Fc receptor (FcR) interactions establish close contacts between tumor targets and immune effector cells, which triggers cytotoxicity and cytokine release. Neutrophils, monocytes, macrophages, and natural killer (NK) cells can mediate ADCC via activating FcRs, which include FcγRIa (CD64), FcγRIIa (CD32), FcγRIIIa (CD16), and FcαRI (CD89) in man, and FcγRI and FcγRIII in mice.1-4 Although Abs may affect tumor growth via FcR-unrelated mechanisms (such as complement-dependent lysis, blockade of growth factor receptors, or via induction of apoptosis),5 in vivo antitumor effects of Abs have been documented to depend on immune activation through FcRs.6-8
Numerous studies in cancer immunology focused on melanoma and melanoma-specific differentiation antigens that induce immune responses.9 If tolerance is broken, melanosomal proteins can be recognized by T cells, which may provide B-cell help and participate in Ab production. Actual tumor rejection seems dependent on phagocytes, which may be activated by CD4+ or NK cells.10-12 Improved clinical outcome has, furthermore, been correlated with the presence of melanoma-specific Abs in patients.13 Ab-mediated protection in the murine B16F10 melanoma model is well established. Monoclonal antibody (mAb) TA99, specific for melanoma differentiation antigen gp75 (brownlocus protein, or TRP-1), is effective in preventing and eradicating early established metastases.11 Studies with mice deficient in the FcR γ chain, lacking expression of FcγRI and FcγRIII, revealed activating FcR to be critical in TA99-mediated tumor rejection.6 Further evidence supporting FcR dependence in Ab-mediated melanoma rejection was established by (1) the documented inability of F(ab′)2 fragments to mediate protection,12 (2) lack of Ab effects on tumor cells in the absence of effector cells,12 and (3) enhancement of antitumor immunity in FcγRII (inhibitory murine FcR) knock-out mice.7
Mac-1 (CD11b/CD18) represents the leukocyte αmβ2 integrin, which is expressed on neutrophils, monocytes, macrophages, and NK cells. Mac-1 binds multiple ligands and is important in leukocyte adhesion, chemotaxis, migration, phagocytosis, and cytotoxicity.14 CD18 linkage to the actin cytoskeleton and associated proteins enables Mac-1 signaling.15,16 Furthermore, Mac-1 has been proposed to act as a signaling partner for other leukocyte receptors, including lipopolysaccharide (LPS)/LPS binding protein (LBP) receptors (CD14), formyl-methionyl-leucyl-phenylalanine (FMLP) receptors, urokinase plasminogen activator receptors (CD87), and FcRs.17
Involvement of Mac-1 in phagocyte FcR-mediated phagocytosis and respiratory burst activity has been documented.18-20Phagocytes from leukocyte adhesion deficiency patients lack β2 integrins, and are defective in phagocytosis and ADCC.14,21 An important role has been shown for Mac-1 in FcR-mediated cytotoxicity toward tumor cells, parasites, virus-infected cells, and erythrocytes.22-26 Recently, Mac-1 was shown to be crucial for neutrophil spreading on Ab-coated tumor cells and formation of immunologic synapses. This was postulated to underlie the mechanism of Mac-1 requirement for Ab-mediated tumor cytolysis.27 Although all these studies point to an essential role for Mac-1 in ADCC, Mac-1 involvement in Ab-mediated tumor rejection has not been documented in vivo. Therefore, we established the syngeneic B16F10 melanoma model in Mac-1–deficient mice and studied Ab-mediated protection. This study documents Mac-1 to be required for FcR-mediated immunity to melanoma and, furthermore, supports an active role for neutrophils in antimelanoma responses.
Materials and methods
Antibodies and peg–G-CSF
mAb TA99 (mouse IgG2a), which is directed against the gp75 antigen, was purified from hybridoma HB-8704 (American Type Culture Collection, Manassas, VA) by protein A Sepharose chromatography (Amersham, Uppsala, Sweden). mAb 17-1A (mIgG2a), used as an isotype control, was kindly provided by Dr T. Valerius (Erlangen, Germany). mAb 520C9 (mIgG1, directed against the proto-oncogene product HER-2/neu) was obtained from Medarex (Annandale, NJ). mAb GR-1 (PharMingen, San Diego, CA) and F4/80 (Serotec, Oxford, United Kingdom) were used in immunohistochemistry to examine neutrophil and monocyte/macrophage infiltration, respectively. Human recombinant polyethylene-glycol granulocyte colony-stimulating factor (peg–G-CSF) was kindly provided by Dr J. Andresen (Amgen, Thousand Oaks, CA). Covalent attachment of polyethylene-glycol (peg) to G-CSF extends its half-life.28 Previous work indicated peg–G-CSF to exhibit similar in vivo biologic effects as uncoupled G-CSF.29
Tumor cell lines and gp75 expression
The B16F10 mouse melanoma cell line of C57Bl/6 origin was from NCI (Frederick, MD). Cells were grown in RPMI 1640 medium (Gibco BRL, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum (FCS), penicillin (50 IU/mL), and streptomycin (50 μg/mL). B16F10 cells were detached with 0.02 mM EDTA (ethylenediaminetetraacetic acid) in phosphate-buffered saline (PBS), and washed twice with PBS. Gp75 expression was determined by incubating B16F10 cells with mAb TA99 (25 μg/mL) at 4°C for 30 minutes, followed by staining with fluorescein isothiocyanate (FITC)–labeled F(ab′)2 fragments of goat anti–mouse immunoglobulin G (IgG) (Protos, San Franscisco, CA). Total gp75 expression in B16F10 cells was assayed upon permeabilization with methanol/acetone (1:1) at 4°C for 15 minutes. In addition, B16F10 cells were incubated with control mIgG2a (17-1A) and FITC-labeled goat anti–mouse IgG. FITC-fluorescence intensities were analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA). SK-BR-3 (human breast carcinoma) cells (ATCC, HTB-30) were used as controls in ADCC experiments.
ADCC assay
To increase circulating effector cells, mice were injected subcutaneously with 15 μg peg–G-CSF, and blood was collected from the retro-orbital plexus 3 days later. Erythrocytes were removed by hypotonic lysis, followed by washing remaining leukocytes 3 times with RPMI 1640 medium with 10% FCS. Cell viability determined by trypan blue exclusion was always more than 95%. Fluorescence activated cell sorting (FACS) analyses revealed leukocytes to consist of, approximately, 55% neutrophils, 40% lymphocytes, 3% monocytes, and 1% eosinophils. The capacity of leukocytes to lyse tumor cells was evaluated in 51Chromium (51Cr) release assays.30 Briefly, 51Cr-labeled B16F10 or SK-BR-3 cells were plated in round-bottom 96-well plates (5 × 103 cells/well) in RPMI 1640 medium with 10% FCS. Isolated mouse leukocytes were added in the absence or presence of mAb TA99 (concentrations ranging from 1 μg/mL-100 μg/mL) or 2 μg/mL mAb 520C9, giving different effector-to-target ratios, and incubated for 4 hours at 37°C, after which 51Cr release was measured in supernatants.
Mice
C57BL/6 wild-type (WT) mice were purchased from Harlan (Horst, The Netherlands). CD11b-deficient mice (Mac-1−/−), in the C57BL/6 background, were kindly provided by Dr T. N. Mayadas (Harvard Medical School, Boston, MA).25,27 31 Experiments were performed with 8- to 12-week-old female and male mice. Mice were maintained at the Central Laboratory Animal Institute (Utrecht University) and experiments were approved by the Utrecht University animal ethics committee.
Melanoma model
C57BL/6 WT and Mac-1−/− mice were injected intravenously with 1 × 105 B16F10 tumor cells (in 100 μL saline) on day 0. Mice were treated intraperitoneally with 200 μL saline (control), or with 200 μg mAb TA99 (in 200 μL saline) on days 0, 2, 4, 7, 9, and 11. In other experiments, mice were treated with peg–G-CSF or with mAb TA99 and peg–G-CSF. Peg–G-CSF was administered as a subcutaneous injection of 20 μg (in 150 μL saline) on days −3 and −4. Mice were observed daily and killed when they became seriously ill (inactive/blurred fur) or paralyzed. Surviving mice were killed at day 21. Since metastases of B16F10 melanoma are readily visually detected, they were scored at the macroscopical level by 2 independent investigators, who were blinded for the treatment. Lungs from all mice were excised and scored for (1) the number of surface metastases and (2) tumor load. Tumor load was defined by the sum of the following scores: metastases less than 1 mm were scored as 1; metastases of 1 mm to 2 mm scored as 3; and metastases more than 2 mm scored as 10. Tumor load correlated closely with the number of metastases (Figure 2). Secondary target organs, including thoracic and abdominal lymph nodes, liver, kidneys, spleen, and the central nervous system (CNS) were also examined for the presence of melanoma metastases, and the mean number of metastases per target organ was calculated (n ≥ 6 per group). In additional experiments, mice were killed and lungs were excised at day 7, 11, or 15 after tumor inoculation and frozen in liquid nitrogen for immunohistochemical analyses. Mean numbers of GR-1–positive cells in lungs with detectable metastases were quantified by 2 independent investigators using light microscopy.
Immunohistochemistry
Frozen sections of lungs (6 μm thick) were placed on superfrost slides (Menzel, Braunschweig, Germany), air-dried overnight, and fixed in acetone for 10 minutes at 20°C. Slides were incubated with 0.3% H2O2 to quench endogenous peroxidase activity. After fixation, slides were blocked with 10% normal mouse serum, and incubated with mAb GR-1 (1:250) or mAb F4/80 (1:2) for 1 hour. After repeated washing with PBS 0.05% Tween, sections were incubated with peroxidase-labeled rabbit anti–rat IgG (DAKO, Glostrup, Denmark) (1:1200) for 30 minutes at 20°C. Primary antibodies were diluted in 2% normal mouse serum, and a secondary Ab was diluted in 1% normal mouse and 2% normal rabbit serum. Upon washing with PBS 0.05% Tween and with sodium acetate buffer (0.1 M, pH 5.0), peroxidase activity was detected by incubating slides with 0.4 mg/mL 3-amino-9-ethylcarbazole (Sigma) for 15 minutes. Subsequently, slides were rinsed in distilled water, counterstained with Mayer hematoxylin (Merck, Darmstadt, Germany), and mounted in aquamount (BHD, Poole, England).
Statistical analysis
Unpaired Student t tests and Welch tests were used to determine statistical differences. Significance was accepted at theP < .05 level.
Results
mAb TA99 recognizes gp75 antigen on B16F10 melanoma cells
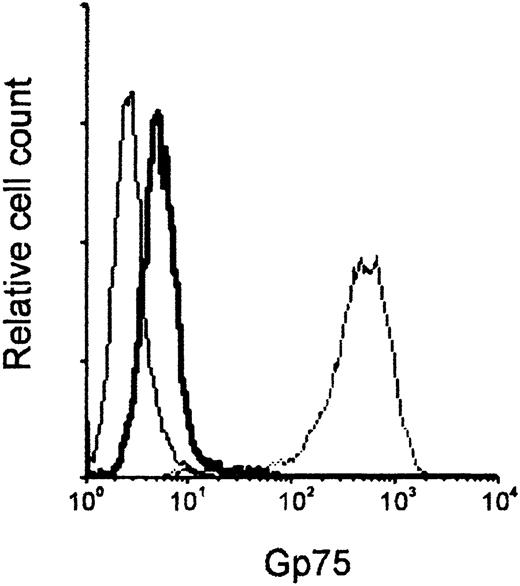
We first examined the binding of mAb TA99 to B16F10 melanoma cells. Low gp75 expression (mean fluorescence intensity [MFI] of 9.94, vs MFI of 3.55 in the control) was found on B16F10 cell membranes, whereas high levels were detectable in permeabilized cells (MFI of 551.3) (Figure 1). Control mIgG2a and FITC-labeled anti–mouse IgG did not bind B16F10 cells. Next, we assessed whether isolated murine leukocytes mediated ADCC of melanoma cells. Leukocytes of WT mice did not mediate Ab-dependent cytotoxicity of B16F10 cells at a range of effector-to-target ratios (data not shown). This was in contrast to breast carcinoma cells (SK-BR-3), which were effectively lysed (56.3% ± 2.5% cytolysis, n = 4) by WT leukocytes in the presence of mAb 520C9. mAb 520C9 recognizes the antigen HER-2/neu on SK-BR-3 cell membranes (MFI of 90.86 vs MFI of 2.9 in the control).
Gp75 expression on B16F10 melanoma cells.
The interaction of mAb TA99, which binds the gp75 melanocyte differentiation antigen, with in vitro–grown B16F10 cells was analyzed by flow cytometry. B16F10 cells were incubated with control mIgG2a (thin solid line) or TA99 (thick solid line), and FITC-conjugated anti–mouse IgG, to assess gp75 membrane expression. Total gp75 expression was assayed by TA99 staining on permeabilized B16F10 cells (dashed line).
Gp75 expression on B16F10 melanoma cells.
The interaction of mAb TA99, which binds the gp75 melanocyte differentiation antigen, with in vitro–grown B16F10 cells was analyzed by flow cytometry. B16F10 cells were incubated with control mIgG2a (thin solid line) or TA99 (thick solid line), and FITC-conjugated anti–mouse IgG, to assess gp75 membrane expression. Total gp75 expression was assayed by TA99 staining on permeabilized B16F10 cells (dashed line).
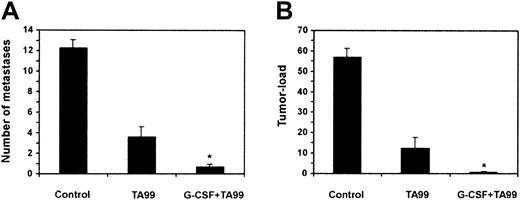
Ab-mediated protection against melanoma is enhanced by G-CSF
Previous work indicating that T and NK cells do not play a direct role in Ab-mediated rejection of B16F10 melanoma10-12 prompted us to study the effect of peg–G-CSF on Ab-induced antitumor responses. Peg–G-CSF mediates in vivo activity similar to G-CSF, but has a prolonged half-life.28 29 WT mice were inoculated with 1 × 105 B16F10 cells, treated with either saline, mAb TA99, peg–G-CSF, or both TA99 and peg–G-CSF, and the number of lung metastases (Figure 2A) and tumor load (Figure 2B) were determined after 21 days. TA99 treatment led to protection against melanoma (61% reduction in number of lung metastases and 78% reduced tumor load, compared with controls). Peg–G-CSF, however, enhanced TA99-mediated antitumor activity significantly (95% reduction in number of metastases and 99% reduced tumor load). Upon combination treatment with TA99 and peg–G-CSF, 64% of mice were tumor-free at day 21. In additional experiments, mice were followed up after TA99/peg–G-CSF combination treatment, and were found to be alive without symptoms at the last observation at day 70. Peg–G-CSF treatment, by itself, did not lead to decreased tumor growth (12.9 ± 1.1% metastases and 61 ± 6.6% tumor load; n = 14).
Peg–G-CSF augments Ab-induced protection against melanoma.
WT mice were challenged intravenously with 1 × 105B16F10 melanoma cells and treated with saline (control), mAb TA99, or TA99, and peg–G-CSF. Number of lung metastases (A) and pulmonary tumor load (B) were determined on day 21. Data are expressed as means ± SEMs from at least 14 mice per group of 2 individual experiments. *Significant difference compared with TA99 treatment (P < .05, determined with unpaired Studentt tests).
Peg–G-CSF augments Ab-induced protection against melanoma.
WT mice were challenged intravenously with 1 × 105B16F10 melanoma cells and treated with saline (control), mAb TA99, or TA99, and peg–G-CSF. Number of lung metastases (A) and pulmonary tumor load (B) were determined on day 21. Data are expressed as means ± SEMs from at least 14 mice per group of 2 individual experiments. *Significant difference compared with TA99 treatment (P < .05, determined with unpaired Studentt tests).
Excitingly, the combination treatment was also protective when started 7 days after tumor cell injection (75% reduction in number of lung metastases and 72% reduced tumor load, compared with controls; n = 6). Moreover, 33% of the treated mice were tumor-free after 21 days in this therapy model.
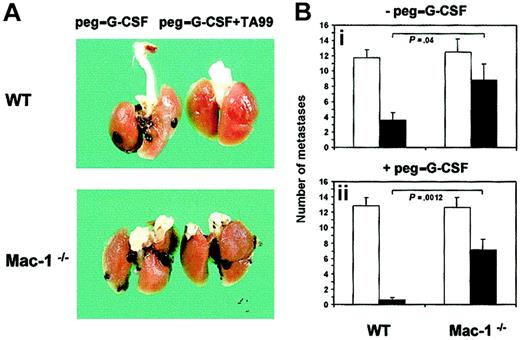
Mac-1 is required for Ab-induced antimelanoma activity
To assess the relevance of Mac-1 in FcR-mediated tumor cytotoxicity in vivo, we established the syngeneic B16F10 melanoma model in CD11b-deficient mice, which were of the same background as WT mice (C57Bl/6). B16F10 cells grew well in Mac-1−/− mice, leading to advanced lung metastases after 3 weeks, similar to WT mice (Figure 3). A striking difference in melanoma growth was observed, however, between Mac-1−/−and WT mice upon treatment. MAb TA99 combined with peg–G-CSF treatment resulted in almost complete tumor remission in lungs of WT mice, whereas Mac-1−/− mice still contained clear melanoma infiltration despite treatment (Figure 3A). Quantification of pulmonary metastases revealed WT mice to be significantly better protected than Mac-1−/− mice by mAb TA99 therapy (Figure 3Bi), as well as TA99 combined with peg–G-CSF (Figure 3Bii). Combination treatment reduced the number of metastases in WT mice by 95%, and in Mac-1−/− mice by only 44%, compared with saline controls. Similarly, pulmonary tumor load was significantly higher in treated Mac-1−/− mice than in WT mice. Treatment with TA99 combined with peg–G-CSF resulted in mean tumor loads of 0.79 (± 0.25, n = 14) in WT mice and 21.3 (± 7.5, n = 12) in Mac-1−/− mice (data not shown). In addition, control experiments revealed peg–G-CSF to increase circulating neutrophil numbers in WT and Mac-1−/− mice with similar kinetics (data not shown).
Mac-1 requirement in Ab-mediated antimelanoma immunity.
The effect of mAb TA99 and peg–G-CSF on melanoma growth was studied in WT and Mac-1−/− mice. Upon tumor inoculation, mice were treated with saline or TA99 combined with or without peg–G-CSF. (A) Lungs were excised at day 21 to analyze surface metastases. (B) Numbers of metastases in saline-treated (■) or TA99-treated mice (▪) combined without (i) or with (ii) peg–G-CSF were quantified. Results represent means ± SEMs from 2 individual experiments (WT: n = 14; Mac-1−/−: n = 12). P values of significant differences were determined using unpaired Welcht tests.
Mac-1 requirement in Ab-mediated antimelanoma immunity.
The effect of mAb TA99 and peg–G-CSF on melanoma growth was studied in WT and Mac-1−/− mice. Upon tumor inoculation, mice were treated with saline or TA99 combined with or without peg–G-CSF. (A) Lungs were excised at day 21 to analyze surface metastases. (B) Numbers of metastases in saline-treated (■) or TA99-treated mice (▪) combined without (i) or with (ii) peg–G-CSF were quantified. Results represent means ± SEMs from 2 individual experiments (WT: n = 14; Mac-1−/−: n = 12). P values of significant differences were determined using unpaired Welcht tests.
To study whether Ab-mediated protection was also diminished in secondary melanoma target organs of Mac-1−/− mice, we evaluated melanoma infiltration into lymph nodes, liver, kidneys, and CNS (Table 1). Similar to the situation in lungs, Ab treatment (with or without peg–G-CSF) was more effective in WT than in Mac-1−/− mice in protecting secondary target organs from melanoma infiltration. Taken together, these results reveal an important role for Mac-1 in Ab-induced antimelanoma immunity in vivo.
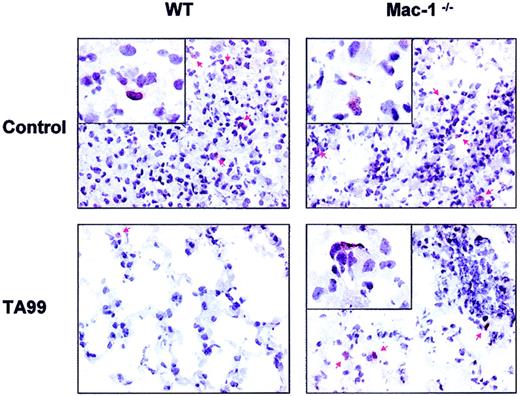
Phagocytic cell migration into melanoma
Because our data pointed to a role for phagocytes in immunity to B16F10 melanoma, we examined the capacity of WT and Mac-1−/− neutrophils and monocytes/macrophages to infiltrate tumor sites. Lungs of WT and Mac-1−/− mice, treated with TA99 and peg–G-CSF, were analyzed 7, 11, and 15 days after tumor challenge. B16F10 cells were distributed as clustered neoplastic cells on days 11 and 15. Histology of lungs of treated WT mice revealed close-to-normal alveolar morphology with sporadic malignant cells and few neutrophils present (Figure4, lower left). Lungs of WT mice not receiving TA99 and lungs of Mac-1−/− mice (treated with saline or TA99), on the other hand, contained large metastatic lesions with occasional neutrophil infiltrates (Figure 4, arrows). We quantified the number of GR-1–positive cells in lung metastases of WT and Mac-1−/− mice not receiving TA99 11 and 15 days after tumor inoculation. Comparable neutrophil infiltration (mean number of cells ± SEM, n = 3) was observed in established tumors of WT and Mac-1−/− mice (26 ± 4.9 vs 40.7 ± 18.7 at day 11, and 118 ± 41.2 vs 115 ± 35.9 at day 15, respectively). Analyzing effector-target cell interactions in more detail revealed both WT and Mac-1−/− neutrophils to be situated in close contact with melanoma cells (Figure 4, inserts). On the contrary, macrophages and monocytes (visualized by F4/80 staining) hardly infiltrated into metastases of WT or Mac-1−/− mice (data not shown). These data show Mac-1 not to be essential for phagocytic cell recruitment into metastatic sites and, furthermore, reveal Mac-1−/− neutrophils capable of binding tumor cells.
Phagocyte infiltration into pulmonary metastases of WT and Mac-1−/− mice.
WT and Mac-1−/− mice were challenged intravenously with 1 × 105 B16F10 cells, and treated with peg–G-CSF and saline (control), or TA99 and peg–G-CSF as detailed in “Materials and methods.” Lungs were removed 15 days after tumor inoculation for immunohistochemistry. GR-1 staining (mouse neutrophil marker, shown in brown) of pulmonary tissue of WT (left panels) and Mac-1−/− (right panels) mice treated with (bottom panels) or without (top panels) TA99. Red arrows point at GR-1–positive cells. Original magnifications: × 400 (main panels) and × 1000 (insets).
Phagocyte infiltration into pulmonary metastases of WT and Mac-1−/− mice.
WT and Mac-1−/− mice were challenged intravenously with 1 × 105 B16F10 cells, and treated with peg–G-CSF and saline (control), or TA99 and peg–G-CSF as detailed in “Materials and methods.” Lungs were removed 15 days after tumor inoculation for immunohistochemistry. GR-1 staining (mouse neutrophil marker, shown in brown) of pulmonary tissue of WT (left panels) and Mac-1−/− (right panels) mice treated with (bottom panels) or without (top panels) TA99. Red arrows point at GR-1–positive cells. Original magnifications: × 400 (main panels) and × 1000 (insets).
Discussion
Melanoma differentiation antigens serve as a hallmark of tumor targets for immune cells. Antibodies directed against the gp75 antigen mediate effective protection in murine melanoma models. The mechanisms by which antibodies initiate antitumor activity remain incompletely understood. In the present study we document a requirement for Mac-1, an important β2 integrin, in FcR-mediated cytotoxicity toward melanoma.
Our data and the data of others6,12 show that the gp75 glycoprotein is predominantly expressed intracellularly in cultured B16F10 melanoma cells, making them resistant to ADCC. However, gp75 membrane expression increases upon in vivo growth, and gp75-specific antibodies induce FcR-dependent melanoma rejection.6,7 In the present study, Mac-1–deficient mice proved significantly less protected against B16F10 melanoma infiltration than WT mice by an antibody targeting gp75, evidenced by higher tumor loads in lungs and secondary target organs. Since Mac-1 also serves as an adhesion molecule, we hypothesized that effector cell infiltration of tumor sites possibly depends on Mac-1. However, our data showed that WT and Mac-1−/− neutrophils exhibit a comparable capability to enter metastases, which was determined by quantifying GR-1–expressing cells. This corresponds with the unaffected capacity of Mac-1−/− neutrophils to migrate in vivo.31 Importantly, Mac-1−/− phagocytes were found to bind well to tumor cells (Figure 4). This is consistent with our earlier data, which showed that the abrogated ADCC capacity of Mac-1−/− neutrophils was not attributable to a defect at the level of FcR-antibody binding. Moreover, Mac-1−/−neutrophils were fully capable of degranulation and oxygen radical production. However, Mac-1 was found to be crucial in neutrophil spreading on tumor cells and the formation of immunologic synapses in vitro.27 Open intercellular clefts between effector cells and tumor cells can result in the leakage of toxic metabolites, and may represent the mechanism by which tumoricidal activity of Mac-1−/− neutrophils is abrogated. We postulate Mac-1 to act as a costimulatory molecule for FcR-mediated cytotoxicity toward malignancies. Although we cannot conclude from the present data whether the Mac-1 requirement is general or restricted to FcR-mediated cytotoxicity of melanoma, a universal role for Mac-1 may be expected given that Mac-1 is crucial in ADCC toward various targets, including a number of tumor cell lines and parasites.23,26,27 The molecular basis of Mac-1/FcR cooperation has not been clarified, albeit that physical interactions between both molecules have been detected.17,32,33 Mac-1 may transmit signals elicited by Fc and other leukocyte receptors via its linkage to the actin cytoskeleton and associated signal transduction proteins.34 The interaction of the cytoplasmic tail of the Mac-1 β-chain with talin and alpha-actinin has a dynamic nature and is dependent on the activation status of neutrophils.15,35Signaling pathways linking β2 integrins to the various neutrophil functions are incompletely understood, but key roles for Syk-2, FAK, and Src-family kinases (Fgr/Hck) and their downstream substrates (Cbl, vav, PLCγ) have been reported.36 37
Mac-1–mediated tumor cytotoxicity was recently shown in therapy models with β-glucan, which was dependent on C3bi deposition on tumor cells.38 However, complement components are unlikely to be involved in our model, because neutrophil ADCC toward tumor targets has been shown independent of active complement.27 Moreover, complement depletion with cobra venom factor does not reduce antibody-induced protection in the B16F10 melanoma model.11 Melanoma cells are known to express intracellular adhesion molecule 1 (ICAM-1),39 enabling direct Mac-1 interactions; in addition, Mac-1 is capable of clustering in the absence of ligand.40 In any case, Ab-mediated melanoma rejection is crucially dependent on FcR and requires Mac-1 for efficient cytotoxicity.
A number of earlier studies focused on identification of the effector cells responsible for Ab-mediated melanoma eradication. An important role for phagocytes seems plausible on the following grounds: (1) enhanced Ab-induced melanoma rejection by macrophage colony-stimulating factor,10,41 (2) intact Ab-mediated protection upon T-cell depletion,10,11 and (3) Ab-mediated immunity in both SCID (lacking lymphocytes) and beige(lacking NK cells) mice.12 Establishing a requirement for Mac-1 in Ab immunity to melanoma is in line with this earlier work and may allude to neutrophil involvement. To further support this, peg–G-CSF was found to augment antimelanoma Ab responses in vivo. Peg–G-CSF proved not tumoricidal by itself, underlining FcR-antibody interactions as a prerequisite. Since peg–G-CSF has a documented capacity to increase circulating neutrophil numbers and to enhance their tissue recruitment,29 the present findings support the involvement of neutrophils in Ab-mediated protection against melanoma. Consistent with our data, G-CSF transduction of adenocarcinoma results in antitumor activity mediated by neutrophils.42,43 Furthermore, phagocytes have been shown to infiltrate tumor sites including melanoma,44-47 and neutrophils have earlier been found important for tumor cell elimination in vivo.8,43 48-50
Ab-mediated protection against melanoma was reduced by approximately 50% in Mac-1−/− mice, pointing to a Mac-1–independent pathway in FcR-mediated tumor cytotoxicity. Since Mac-1 deficiency abrogates neutrophil ADCC capacity,25 we hypothesize that Ab-reliant melanoma destruction results from cross-talk between neutrophils and other cytotoxic effectors.47,51 Although we did not observe apparent macrophage infiltration into tumor sites, Mac-1–deficient macrophages are capable of killing tumor cells, in contrast to neutrophils. This likely reflects differences in cytolytic effector mechanisms between these types of phagocytic cells.27
In summary, the data presented here provide evidence for an important role of Mac-1 in Ab-mediated immunity to melanoma. Furthermore, this study implicates neutrophils to be important for Ab-reliant tumor cytotoxicity. Antibody treatment of human tumors has been studied extensively, with a number of clinical successes reported.5,7,52 FcRs play an important role in direct Ab-mediated tumor cytotoxicity and are, furthermore, important in the induction of vaccine responses.8,53 54 Better insight into the mechanisms of FcR-mediated tumor cytotoxicity may well facilitate the design of more effective therapeutic concepts.
We thank Tanya Mayadas for kindly providing the CD11b-deficient mice, Cora Damen for technical assistance, Theo Thepen and Gerard Groenewegen for critically reading the manuscript, and Toon Hesp, Herma Boere, Anja van der Sar, and Ingrid van den Brink for excellent animal care.
A.B. van S. and H.H. van O. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jan G. J. van de Winkel, Immunotherapy Laboratory, Department of Immunology, University Medical Center Utrecht, Room KC02.085.2, Lundlaan 6, 3584 EA, Utrecht, The Netherlands; e-mail: janvandewinkel@aol.com.