Abstract
We have previously reported that apoptotic tumor cells can be either immunogenic or nonimmunogenic in vivo, depending on whether or not these cells are heat stressed before induction of apoptosis. Stressed apoptotic cells express heat shock proteins on their plasma membranes and dendritic cells are capable of distinguishing them from nonstressed apoptotic cells. Here we provide evidence that when purified heat shock protein 70 or chaperone-rich cell lysate (CRCL) from syngeneic normal tissue is used as an adjuvant with nonimmunogenic apoptotic tumor cells in vaccination, potent antitumor immunity can be generated. This antitumor immunity is mediated by T cells because antitumor effects are not observed in either severe combined immunodeficiency or T cell–depleted mice. We further demonstrate that vaccination of mice with apoptotic tumor cells mixed with liver-derived CRCL as adjuvant were capable of enhancing the production of TH1 cytokines, inducing specific cytotoxic T lymphocytes and eliciting long-lasting antitumor immunity. Stress proteins from autologous normal tissue components therefore can serve as danger signals to enhance the immunogenicity of apoptotic tumor cells and stimulate tumor-specific immunity
Introduction
It is well accepted that antigen-presenting cells (APCs) efficiently acquire antigens from apoptotic tumor cells and present them to T cells.1-4 However, the immunologic consequences of this remain controversial.5,6 Macrophages and dendritic cells (DCs) phagocytose apoptotic tumor cells through a receptor-mediated pathway1-3 that results in tumor antigen access to the cytoplasm and cross- presentation on APC major histocompatibility complex (MHC) class I molecules.1,4Therefore, apoptotic tumor cells induced by chemotherapy or radiotherapy can theoretically be a suitable antigen source for stimulation of antitumor responses. We have previously reported that apoptotic 12B1-D1 (BCR-ABL+) leukemia cells can be either immunogenic or nonimmunogenic in vivo depending on whether or not these cells are heat stressed prior to apoptosis induction.7 Furthermore, we have found that DCs are capable of distinguishing stressed apoptotic tumor cells from nonstressed ones.8 We have also demonstrated that stressed apoptotic tumor cells express heat shock proteins (HSPs) on their surface, which appear to play a critical role in enhancing their immunogenicity.7 8
Tumor-derived HSPs, also called chaperone proteins, when used as vaccines, can induce protective immunity against their tumors of origin.9 In our studies we found that vaccination with multiple HSPs/chaperone proteins enriched from tumor lysate by free solution isoelectric focusing (FS-IEF) induced specific antitumor immunity in various tumor models.10,11 Some important chaperone proteins such as glycoprotein 96 (gp96), HSP90, HSP70, and calreticulum enriched by this method tend to complex together.12 We refer to the FS-IEF–derived preparation as chaperone-rich cell lysate (CRCL). We have previously reported that tumor-derived CRCL has superior abilities to stimulate DCs compared to purified individual HSPs such as HSP70 and gp96 (Y.Z. et al, manuscript submitted), which have been used as tumor vaccines in mice and humans.13 14
Several mechanisms have been proposed to explain how these HSP complexes, which carry tumor-derived peptides as part of their chaperoning functions, can elicit immune responses.15-17One possible hypothesis is that chaperone complexes supply both antigens and danger signals to the immune system.15-17These adjuvant effects/danger signals activate APCs, such as DCs, leading to more efficient processing and presentation of HSP-chaperoned peptides.18,19 We have previously reported that heat stress induces HSP expression on the surface of apoptotic 12B1-D1 cells and increases their immunogenicity.7 8 We therefore reasoned that the immunogenicity of nonstressed apoptotic cells (which do not express HSPs on their surface) may also be enhanced if an exogenous source of HSPs is present at the vaccination site. To test our hypothesis, normal syngeneic liver-derived chaperone proteins (devoid of tumor-specific antigenic peptides) were coinjected with nonstressed apoptotic tumors. This resulted in the reproducible generation of durable and specific T cell–mediated antitumor immunity. Nonstressed apoptotic cells alone or when combined with liver lysate, generated by freeze-thaw and not enriched for chaperone complexes, were ineffective vaccines. Therefore, we have demonstrated that normal tissue (liver) CRCL may function as effective danger signals for the immune system. FS-IEF is a simple and rapid method for the generation of natural vaccine adjuvants active in enhancing immunogenicity of apoptotic tumor cells resulting in the generation of potent antitumor immunity.
Materials and methods
Mice
Six- to 10-week-old female BALB/c (H-2d) mice (Harlan Sprague Dawley, Indianapolis, IN) and C.B-17 severe combined immunodeficiency (SCID) scid/scid (University of Arizona animal breeding facility) were used for the experiments. The animals were housed in a dedicated pathogen-free facility and cared for according to the University of Arizona Institutional Animal Care and Use Committee guidelines.
FS-IEF for chaperone enrichment and conventional purification of HSP70
Methods to enrich CRCL from tumors or naive BALB/c mouse liver have been described previously.11 Briefly, tumor or liver tissue was homogenized in lysis buffer and a 100 000gsupernatant was obtained. The high-speed supernatant was subjected to FS-IEF in a Bio-Rad Rotofor cell (Hercules, CA) for 5 hours at 15 W constant power. Twenty fractions were harvested, and each fraction was analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot probing with specific antibodies for the chaperones HSP60, HSP70, HSP72 (inducible form of HSP70), HSP90, gp96, and calreticulin. Fractions from FS-IEF that contained substantial amounts of the above chaperone proteins, as determined by SDS-PAGE and Western blotting, were pooled and dialyzed stepwise out of urea and detergents. Pooled fractions were then concentrated using Centricon devices (Millipore, Bedford, MA), reconstituted in phosphate-buffered saline (PBS), and stored at −70°C until use. Purification of liver HSP70 was done via conventional and nucleotide-affinity chromatography as previously described.10
Induction of apoptotic cell death in 12B1-D1 cells
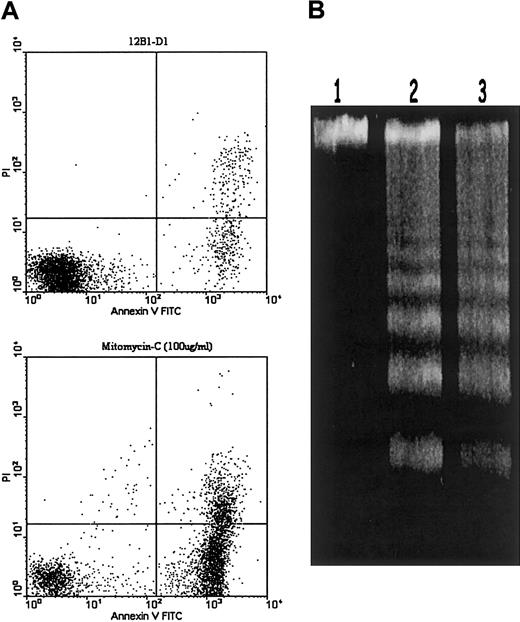
To induce 12B1-D1 cells to undergo apoptosis, cells were treated with 40 nM AP2087 for 6 hours as described previously7 or with 100 μg/mL mitomycin C (Mit-C) for 1 hour. To confirm that 12B1-D1 cells were dying by apoptosis after Mit-C treatment, we performed annexin V–fluorescein isothiocyanate (FITC) and propidium iodide (PI) staining using the annexin-V–FLUOS staining kit (Roche, Indianapolis, IN) followed by flow cytometric analysis of cells. DNA fragmentation analysis was performed using the apoptotic DNA ladder kit from Roche.
In vivo tumor growth experiments
In the prophylactic model, mice were immunized with 2 × 106 Mit-C–treated cells, with or without 20 μg/mouse per vaccination of CRCL or HSP70, by subcutaneous injection into the left groin on days −14 and −7. On day 0, mice were challenged with 2 × 104 (100% lethal dose [LD100]) viable 12B1-D1 cells. Tumor size was measured every other day with calipers once the tumors became palpable. Tumor volume was calculated using the formula: length × width2 × π/6. Differences in mean tumor volume between groups were compared using an unpaired t test. Mice with tumors were euthanized at the end points listed. Tumor-free mice were kept for rechallenge experiments. In rechallenge experiments, 2 × 104 12B1-D1, 103 12B1, or 106 A20 leukemia cells (all LD100 doses as determined by dose titration experiments, data not shown), were injected into the right groin 56 to 80 days after the first challenge.
In the preestablished tumor model, mice were injected with 2 × 104 (LD100) viable 12B1-D1 cells in right groin on day 0. On day +1 or +3, mice were vaccinated as indicated by subcutaneous injection into the left groin. Tumor volume was measured at indicated time points.
ELISPOT assay
Enzyme-linked immunospot (ELISPOT) assays were performed to assess the interferon γ (IFN-γ) production of splenocytes from vaccinated mice following in vitro stimulation with Mit-C–treated apoptotic 12B1-D1 cells. Splenocytes/apoptotic cells (10:1 ratio) were cocultured for 24 hours on Millipore MultiScreen-HA 96 well plates (MAHA S45). The plates had been previously coated overnight with anti–IFN-γ capture antibody (10 μg/mL, clone R4-6A2, rat mAb antimouse IFN-γ; BD Pharmingen, San Diego, CA). Cells were then washed out with copious amounts of PBS plus 0.05% Tween 20 (PBST). Biotinylated anti–IFN-γ antibody (2 μg/mL, clone XMG1.2, rat mAb antimouse IFN-γ; BD Pharmingen) was added for 2 hours. Free antibody was washed out, and the plates were incubated with horseradish peroxidase (HRP)–linked avidin (ABC Elite reagent, 1 drop each of reagent A and reagent B/10 mL PBS; Vector Laboratories, Burlingame, CA) for 1 hour, following extensive washing with PBST, and then washing with PBS. Spots were visualized by the addition of the HRP substrate 3-amino-9-ethylcarbazole (AEC; Sigma Chemical, St Louis, MO) prepared in acetate buffer (pH 5.0) with 0.015% hydrogen peroxide. Spots were counted using a dissecting microscope. Wells of interest were photographed with a microscope-mounted Cool SNAP CCD camera (RS Photometrics, Tucson AZ), and images captured with RS Image, Version 1.07 (Roper Scientific, Tucson, AZ).
T-cell proliferation assays and bioassay to determine IL-2 production
BALB/c mice were immunized with Mit-C–treated 12B1-D1 cells that were mixed with liver lysate, liver-derived HSP70, or liver CRCL (20 μg/mouse) on days −14 and −7. For controls, mice were immunized with an equal number of Mit-C–treated 12B1-D1 cells or 20 μg/mouse liver–derived CRCL or saline. On day −2, splenocytes from the immunized mice were harvested and cocultured with Mit-C–treated apoptotic 12B1-D1 cells. The ratio of splenocytes to apoptotic cells was 10:1. After 72 hours of culture, the supernatant from each group was collected and serially diluted in a 96-well plate. Interleukin 2 (IL-2)–dependent cytotoxic T lymphoid line 2 (CTLL-2) cells were added to each well. Human recombinant IL-2 was used to generate a standard curve. All assays were performed in triplicate wells. After 24 hours culture, [3H]-thymidine (1 μCi/well; 0.037 MBq) was added. For T-cell proliferation assays, splenocytes were cocultured with apoptotic 12B1-D1 cells for 4 days before the addition of [3H]-thymidine. The cells were harvested 18 hours later using a 96-well Packard cell harvester and the radioactivity measured on a Packard beta counter.
Cytotoxicity assay
BALB/c mice were immunized as indicated above. Five days after the second immunization, splenocytes from the immunized mice were harvested. The in vivo–primed splenocytes were cultured for 5 days with Mit-C–treated apoptotic 12B1-D1 cells. The ratio of splenocytes to apoptotic cells was 10:1. Stimulated effector cells were tested for cytolytic activity against 12B1-D1, parental 12B1, or A20 cells by nonradioactive cytotoxicity assay (Promega, Madison, WI) following the instructions provided. The percentage of cytotoxicity was determined according to the formula provided in the kit instructions.
Results
CRCL from normal tissue enhances the immunogenicity of apoptotic 12B1-D1 tumor cells
We have previously reported that 12B1-D1 leukemia cells undergo apoptosis in response to in vitro treatment with AP20187.7A 6-hour exposure to 40 nM AP20187 induces apoptosis in more than 90% of 12B1-D1 cells, whereas 10% of cells remain clonogenic. When we inoculated mice with 5 × 105 AP20187-treated 12B1-D1 cells, rapid tumor growth occurred. This indicated that the apoptotic cells failed to induce an active immune response against the surviving clonogenic cells. To test whether syngeneic naive mouse liver–derived HSP70 or CRCL would provide adjuvant effects that may enhance the immunogenicity of AP20187-induced apoptotic 12B1-D1 tumor cells, we subcutaneously injected mice with AP20187-treated 12B1-D1 cells that were mixed with either liver-derived HSP70 or CRCL (20 μg/mouse, based on previous dose titration experiments, data not shown). We found that coinjection of HSP70 significantly delayed tumor growth compared with mice that were injected with AP20187-treated cells alone (Figure 1A). Moreover, CRCL provided superior adjuvant effects compared to HSP70, resulting in significant delay of tumor growth with rejection of tumors in 75% of mice (Figure 1A). In additional experiments, we compared the adjuvant effects of lipopolysaccharide (LPS) with those of liver-derived CRCL and found that they were comparable (Figure 1B), with both significantly delaying tumor progression when compared to no CRCL adjuvant.
The adjuvant effects of liver-derived CRCL, HSP70, or LPS in enhancing the immunogenicity of AP2087-induced apoptotic 12B1-D1 cells.
12B1-D1 cells were treated with 40 nM AP20187 for 6 hours. (A) Liver-derived CRCL (20 μg/mouse) or HSP70 was added to the cells and the mixture was injected subcutaneously into the groin of BALB/c mice. Control mice were injected with AP20187-treated cells only. (Control versus HSP70 P < .05 from day 16 onward; control versus CRCL P < .05 from day 15 onward in onward; HSP70 versus CRCL P < .05 from day 17 onward; n = 8 mice per group. Representative data from 1 of 2 experiments are shown.) (B) Liver-derived CRCL (20 μg/mouse) or 10 μg/mouse LPS was added to the cells and the mixture was injected subcutaneously into the groin of BALB/c mice. Control mice were injected with AP20187-treated cells only. (Control versus CRCL P < .05 from day 13 onward; control versus LPS P < .05 from day 13 onward; n = 8 mice per group. Representative data from 1 of 3 experiments are shown.) 12B1-D1/AP indicates 12B1-D1 cells treated with AP20187. Error bars indicate SEM.
The adjuvant effects of liver-derived CRCL, HSP70, or LPS in enhancing the immunogenicity of AP2087-induced apoptotic 12B1-D1 cells.
12B1-D1 cells were treated with 40 nM AP20187 for 6 hours. (A) Liver-derived CRCL (20 μg/mouse) or HSP70 was added to the cells and the mixture was injected subcutaneously into the groin of BALB/c mice. Control mice were injected with AP20187-treated cells only. (Control versus HSP70 P < .05 from day 16 onward; control versus CRCL P < .05 from day 15 onward in onward; HSP70 versus CRCL P < .05 from day 17 onward; n = 8 mice per group. Representative data from 1 of 2 experiments are shown.) (B) Liver-derived CRCL (20 μg/mouse) or 10 μg/mouse LPS was added to the cells and the mixture was injected subcutaneously into the groin of BALB/c mice. Control mice were injected with AP20187-treated cells only. (Control versus CRCL P < .05 from day 13 onward; control versus LPS P < .05 from day 13 onward; n = 8 mice per group. Representative data from 1 of 3 experiments are shown.) 12B1-D1/AP indicates 12B1-D1 cells treated with AP20187. Error bars indicate SEM.
We next investigated whether liver-derived CRCL would have similar adjuvant effects in enhancing the immunogenicity of apoptotic 12B1-D1 cells induced by other agents, such as chemotherapy drugs. We used Mit-C (100 μg/mL for 1 hour) to induce apoptosis in 12B1-D1 cells. This resulted in more than 80% cells undergoing apoptosis within 6 hours as determined by annexin V–FITC/PI staining (Figure2A). DNA from Mit-C–treated 12B1-D1 cells displayed electrophoretic ladder patterns typical of apoptotic cells (Figure 2B). We then tested whether liver-derived CRCL can enhance the immunogenicity of Mit-C–induced apoptotic 12B1-D1 cells. To avoid release of cellular components from secondary necrosis that occurs after apoptosis, we injected 12B1-D1 cells into mice immediately following Mit-C treatment of the cells while 12B1-D1 cells maintained their membrane integrity as determined by trypan blue exclusion and flow cytometry (data not shown). Compared to 6 hours of AP20187 treatment, 1 hour of treatment with 100 μg/mL Mit-C generated no clonogenic cells. Mice were immunized on days −14 and −7 and challenged with a lethal dose of live 12B1-D1 cells on day 0 by subcutaneous injection, as described in “Materials and methods.” Vaccination with Mit-C–treated 12B1-D1 cells that were mixed with liver-derived CRCL induced potent antitumor immunity. All mice immunized with CRCL adjuvant-apoptotic cells rejected a lethal dose of 12B1-D1 tumor challenge (Figure 3A). However, immunization with Mit-C–induced apoptotic tumor cells alone did not delay tumor progression, compared to mock vaccinated mice (Figure 3A). Immunization with liver-derived CRCL without a source of antigen, such as apoptotic tumor cells, provided no protection (data not shown).
Apoptosis induction of 12B1-D1 cells by Mit-C treatment.
(A) 12B1-D1 cells were treated with 100 μg/mL Mit-C for 1 hour, washed, and then cultured in complete media for an additional 6 hours. Induction of apoptosis was assessed by annexin V and PI staining. (B) DNA fragmentation analysis. DNA extracted from either 12B1-D1 cells (lane 1) or from 12B1-D1 cells that had been treated with Mit-C for 1 hour and recultured in complete media for additional 6 hours (lane 2) or 24 hours (lane 3).
Apoptosis induction of 12B1-D1 cells by Mit-C treatment.
(A) 12B1-D1 cells were treated with 100 μg/mL Mit-C for 1 hour, washed, and then cultured in complete media for an additional 6 hours. Induction of apoptosis was assessed by annexin V and PI staining. (B) DNA fragmentation analysis. DNA extracted from either 12B1-D1 cells (lane 1) or from 12B1-D1 cells that had been treated with Mit-C for 1 hour and recultured in complete media for additional 6 hours (lane 2) or 24 hours (lane 3).
Both liver-derived and 12B1 tumor–derived CRCL enhance the immunogenicity of Mit-C–induced apoptotic tumor cells.
12B1-D1 cells (2 × 106/mouse) were treated with 100 μg/mL Mit-C for 1 hour and then extensively washed. (A) The 20 μg/mouse liver–derived CRCL was added to the cells and the mixture was subcutaneously injected into the groin of BALB/c mice on days −14 and −7. Control mice were immunized with an equal number of Mit-C–treated 12B1-D1 cells alone or saline. On day 0, mice were challenged with 2 × 104 (LD100) 12B1-D1 cells subcutaneously. Mice that were vaccinated with saline or Mit-C–treated 12B1-D1 alone were killed on day 21, whereas mice that were vaccinated with apoptotic cells plus CRCL as adjuvant survived tumor-free up to day 80. (Saline versus 12B1-D1/Mit-CP = NS; saline, or 12B1-D1/Mit-C versus 12B1-D1/Mit-C + CRCL P < .05 from day 15 onward; n = 8 mice per group. Representative data from 1 of 4 experiments are shown.) (B) The 20 μg/mouse liver– or 12B1 tumor-derived CRCL was added to the cells and the mixture was subcutaneously injected into the groin of BALB/c mice on days −14 and −7. Control mice were immunized with saline. On day 0, mice were challenged with 2 × 104(LD100) 12B1-D1 cells subcutaneously. Mice that were vaccinated with saline or Mit-C–treated 12B1-D1 alone were killed on day 23, whereas mice that were vaccinated with apoptotic cells plus CRCL as adjuvant survived tumor-free up to day 56. (Saline versus CRCLP < .05 from day 15 onward; n = 8 mice per group. Representative data from 1 of 2 experiments are shown.)
Both liver-derived and 12B1 tumor–derived CRCL enhance the immunogenicity of Mit-C–induced apoptotic tumor cells.
12B1-D1 cells (2 × 106/mouse) were treated with 100 μg/mL Mit-C for 1 hour and then extensively washed. (A) The 20 μg/mouse liver–derived CRCL was added to the cells and the mixture was subcutaneously injected into the groin of BALB/c mice on days −14 and −7. Control mice were immunized with an equal number of Mit-C–treated 12B1-D1 cells alone or saline. On day 0, mice were challenged with 2 × 104 (LD100) 12B1-D1 cells subcutaneously. Mice that were vaccinated with saline or Mit-C–treated 12B1-D1 alone were killed on day 21, whereas mice that were vaccinated with apoptotic cells plus CRCL as adjuvant survived tumor-free up to day 80. (Saline versus 12B1-D1/Mit-CP = NS; saline, or 12B1-D1/Mit-C versus 12B1-D1/Mit-C + CRCL P < .05 from day 15 onward; n = 8 mice per group. Representative data from 1 of 4 experiments are shown.) (B) The 20 μg/mouse liver– or 12B1 tumor-derived CRCL was added to the cells and the mixture was subcutaneously injected into the groin of BALB/c mice on days −14 and −7. Control mice were immunized with saline. On day 0, mice were challenged with 2 × 104(LD100) 12B1-D1 cells subcutaneously. Mice that were vaccinated with saline or Mit-C–treated 12B1-D1 alone were killed on day 23, whereas mice that were vaccinated with apoptotic cells plus CRCL as adjuvant survived tumor-free up to day 56. (Saline versus CRCLP < .05 from day 15 onward; n = 8 mice per group. Representative data from 1 of 2 experiments are shown.)
We have previously demonstrated that vaccination with CRCL derived from 12B1 (the parental cell line of 12B1-D1) elicited specific antitumor immunity (Y.Z. et al, manuscript submitted). 12B1-derived CRCL (presumably containing tumor antigenic peptides) has similar adjuvant effects to liver CRCL. Vaccination with either 12B1 tumor CRCL or liver CRCL as adjuvant plus apoptotic 12B1-D1 tumor cells induced potent antitumor immunity that protected 100% of mice from a lethal dose of 12B1-D1 challenge (Figure 3B).
We further investigated whether vaccination with apoptotic 12B1-D1 cells plus CRCL as adjuvant could elicit therapeutic effects on pre-established tumors. Mice received a lethal dose of 12B1-D1 on day 0, and were then immunized with Mit-C–treated apoptotic 12B1-D1 cells plus CRCL as adjuvant in the opposite groin on day +1 or day +3. Vaccination on day +1 with CRCL adjuvant-apoptotic tumor cells significantly delayed tumor growth as compared with mock vaccination (Figure 4). As expected, when vaccination was delayed until day +3, tumor progression was suppressed but to a lesser degree (Figure 4).
CRCL adjuvant plus apoptotic tumor cells provide therapeutic effects in a pre-established tumor model.
BALB/c mice were subcutaneously injected with 2 × 104(LD100) 12B1-D1 cells at right groin on day 0. On day +1 or +3, mice were vaccinated with Mit-C–treated 12B1-D1 cells (2 × 106/mouse) plus 20 μg/mouse liver–derived CRCL adjuvant by subcutaneous injection at the opposite groin. (Saline versus day 3 P = NS; saline versus day1P < .05 from day 17 onward; n = 4 mice per group. Representative data from 1 of 3 experiments are shown.)
CRCL adjuvant plus apoptotic tumor cells provide therapeutic effects in a pre-established tumor model.
BALB/c mice were subcutaneously injected with 2 × 104(LD100) 12B1-D1 cells at right groin on day 0. On day +1 or +3, mice were vaccinated with Mit-C–treated 12B1-D1 cells (2 × 106/mouse) plus 20 μg/mouse liver–derived CRCL adjuvant by subcutaneous injection at the opposite groin. (Saline versus day 3 P = NS; saline versus day1P < .05 from day 17 onward; n = 4 mice per group. Representative data from 1 of 3 experiments are shown.)
Immunity generated by CRCL adjuvant-apoptotic tumor cells is T-cell dependent
We next investigated whether the antitumor response induced by apoptotic tumor cells with CRCL adjuvant was T cell dependent. SCID mice were subcutaneously injected with 5 × 105 AP20187 treated 12B1-D1 cells, with or without liver-derived CRCL adjuvant. Mice developed 12B1-D1 tumors at comparable rates in both groups (Figure 5A), suggesting that CRCL has no adjuvant effects in T cell–deficient mice. Moreover, tumor growth in mice coinjected with apoptotic cells plus LPS as adjuvant was not significantly delayed (Figure 5A).
CRCL adjuvant effects are T cell dependent.
(A) 12B1-D1 cells were treated with 40 nM AP20187 for 6 hours and then washed. Then 20 μg/mouse liver–derived CRCL or 10 μg/mouse LPS was added to the cells and the mixture was subcutaneously injected into the right groin of SCID mice. Control mice were immunized with an equal number of AP20187-treated 12B1-D1 cells alone. (P = NS; n = 8 mice per group; representative data from o1 of 2 experiments are shown.) (B) On days −14 and −7, BALB/c mice were subcutaneously injected with 2 × 106 Mit-C–treated 12B1-D1 cells that were mixed with 20 μg/mouse liver–derived CRCL in the right groin. On days −3, −1, +1, and +7, mice were intraperitoneally injected with 200 μg/mouse anti-CD4 or anti-CD8 mAb (or both), or the same volume of saline. Control mice were immunized with equal number of Mit-C–treated 12B1-D1 cells or saline. On day 0, mice were injected with 2 × 104 (LD100) 12B1-D1 cells subcutaneously. (Saline versus 12B1-D1/Mit-C, or double depletionP = NS; saline versus CRCL P < 005 from day 15 onward; saline versus CD4 or CD8 depletion P < .05 from day 17 onward; CD4 depletion versus CD8 depletionP = NS; n = 8-24 mice per group. Representative data from 1 of 2 experiments are shown.)
CRCL adjuvant effects are T cell dependent.
(A) 12B1-D1 cells were treated with 40 nM AP20187 for 6 hours and then washed. Then 20 μg/mouse liver–derived CRCL or 10 μg/mouse LPS was added to the cells and the mixture was subcutaneously injected into the right groin of SCID mice. Control mice were immunized with an equal number of AP20187-treated 12B1-D1 cells alone. (P = NS; n = 8 mice per group; representative data from o1 of 2 experiments are shown.) (B) On days −14 and −7, BALB/c mice were subcutaneously injected with 2 × 106 Mit-C–treated 12B1-D1 cells that were mixed with 20 μg/mouse liver–derived CRCL in the right groin. On days −3, −1, +1, and +7, mice were intraperitoneally injected with 200 μg/mouse anti-CD4 or anti-CD8 mAb (or both), or the same volume of saline. Control mice were immunized with equal number of Mit-C–treated 12B1-D1 cells or saline. On day 0, mice were injected with 2 × 104 (LD100) 12B1-D1 cells subcutaneously. (Saline versus 12B1-D1/Mit-C, or double depletionP = NS; saline versus CRCL P < 005 from day 15 onward; saline versus CD4 or CD8 depletion P < .05 from day 17 onward; CD4 depletion versus CD8 depletionP = NS; n = 8-24 mice per group. Representative data from 1 of 2 experiments are shown.)
To further evaluate the role of T-cell subsets in the immunity induced by CRCL adjuvant plus apoptotic tumor cells, we performed in vivo T cell–depletion experiments. CD4+, CD8+, or both subsets of T cells were depleted by intraperitoneal injection with 200 μg anti-CD4 or anti-CD8 mAbs or both into mice on days −3, −1, +1, and +7, as described in “Materials and methods.” Mice immunized with apoptotic tumor cells plus CRCL completely rejected a lethal dose of 12B1-D1 tumor challenge (Figure 5B). However, when either CD4+ or CD8+ T cells were depleted (as confirmed by flow cytometry of splenocytes; data not shown), antitumor immunity was partially abrogated, because 12B1-D1 tumor developed, but at significantly slower rates than that of mock vaccinated mice (Figure5B). The antitumor immunity was completely abolished when both subsets of T cells were depleted by antibodies (Figure 5B). These results indicate that the potent antitumor immunity induced by CRCL adjuvant-apoptotic tumor cell immunization is T cell dependent, and that both CD4+ and CD8+ T cells contribute to this immunity.
Vaccination with CRCL adjuvant-apoptotic tumor cells induces tumor-specific CTLs
Cell-mediated immunity, which is particularly important in suppressing tumors, is characterized by production of type I cytokines, activation of macrophages, and induction of cytotoxic T lymphocytes (CTLs). To explore whether vaccination with apoptotic tumor cells plus CRCL as adjuvant can induce type I cytokine secretion by T cells and generate tumor-specific CTLs, we examined IFN-γ and IL-2 production as well as the cytolytic activities of splenocytes derived from vaccinated mice. We found that vaccination with apoptotic tumor cells plus CRCL adjuvant substantially increased the secretion of IL-2 (Figure 6A) and IFN-γ (Figure 6B) by splenocytes on restimulation in vitro as measured by IL-2 bioassay and ELISPOT assay, respectively. Moreover, spleen T-cell proliferation in CRCL adjuvant-apoptotic tumor cell–vaccinated mice also increased dramatically on restimulation with apoptotic tumor cells (Figure 6C), compared to mock immunized mice. The induction of cytokine secretion or T-cell proliferation was not due to nonspecific stimulation because splenocytes from mice immunized with Mit-C–induced apoptotic cells alone or liver CRCL alone produced limited amounts of cytokines and those immunizations resulted in almost no T-cell proliferation (Figure6). We also found that vaccination with apoptotic cells plus liver-derived HSP70 as adjuvant induced IFN-γ and IL-2 secretion by splenocytes, and increased T-cell proliferation, but at lower levels when compared to CRCL as adjuvant (Figure 6). This may explain the weaker in vivo protective effects following immunization with HSP70 adjuvant plus apoptotic 12B1-D1 cells induced by AP20187 (Figure 1A) or Mit-C (data not shown). Because both HSP70 and CRCL were derived from whole liver lysate, we explored whether the liver lysate can also confer adjuvant effects. We found that apoptotic cells coinjected with liver lysate induced minimal cytokine production and T-cell proliferation (Figure 6A-C).
Immunization with apoptotic 12B1-D1 cells plus CRCL adjuvant induces IL-2 and IFN-γ secretion and T-cell proliferation of splenocytes.
12B1-D1 cells were treated with 100 μg/mL Mit-C for 1 hour and then extensively washed. Then 20 μg/mouse liver lysate, liver-derived HSP70, or CRCL was added to the cells and the mixture was injected to BALB/c mice subcutaneously on days −14 and −7. Control mice were immunized with an equal number of Mit-C–treated 12B1-D1 cells or saline. Five days after the secondary immunization, splenocytes of the immunized mice were harvested and restimulated with Mit-C–treated 12B1-D1 cells. (A) CTLL-2 bioassay was used to determine the IL-2 production. (B) IFN-γ secretion was determined by ELISPOT. (C) T-cell proliferation was determined by [3H]-thymidine incorporation. (Representative data from 1 of 3 experiments are shown.)
Immunization with apoptotic 12B1-D1 cells plus CRCL adjuvant induces IL-2 and IFN-γ secretion and T-cell proliferation of splenocytes.
12B1-D1 cells were treated with 100 μg/mL Mit-C for 1 hour and then extensively washed. Then 20 μg/mouse liver lysate, liver-derived HSP70, or CRCL was added to the cells and the mixture was injected to BALB/c mice subcutaneously on days −14 and −7. Control mice were immunized with an equal number of Mit-C–treated 12B1-D1 cells or saline. Five days after the secondary immunization, splenocytes of the immunized mice were harvested and restimulated with Mit-C–treated 12B1-D1 cells. (A) CTLL-2 bioassay was used to determine the IL-2 production. (B) IFN-γ secretion was determined by ELISPOT. (C) T-cell proliferation was determined by [3H]-thymidine incorporation. (Representative data from 1 of 3 experiments are shown.)
CTLs are important in controlling tumor growth.22 Several reports have shown that APCs can acquire antigens from apoptotic bodies and can cross-prime CTLs in vitro.4 23 However, evidence that those apoptotic cells prime CTLs in vivo remains limited. We therefore investigated whether or not vaccination with apoptotic tumor cells plus CRCL as adjuvant induces measurable CTL activity. Splenocytes were collected from immunized mice and restimulated in vitro with Mit-C–treated 12B1-D1 cells for 5 days and then tested for cytolytic function against different tumor targets. Vaccination with apoptotic cells plus CRCL elicited CTL activity (Figure7A). In contrast, vaccination with Mit-C–induced apoptotic tumor cells alone or with liver lysate failed to generate CTL activity, whereas liver-derived HSP70 had less impressive adjuvant effects in eliciting CTL activity (Figure 7A).
Immunization with stressed apoptotic 12B1-D1 cells induces tumor-specific CTLs.
BALB/c were immunized with Mit-C–treated 12B1-D1 cells that were mixed with 20 μg/mouse liver lysate, liver-derived HSP70, or CRCL on days −14 and −7. For controls, mice were immunized with an equal number of Mit-C–treated 12B1-D1 cells or saline. On day −2, splenocytes of the immunized mice were harvested and cocultured with Mit-C–treated 12B1-D1 cells for 5 days. Stimulated effector cells were tested for (A) cytolytic activity against 12B1-D1 cells, (B) parental 12B1 cells, or (C) A20 cells by nonradioactive cytotoxicity assay. Representative data from 1 of 3 experiments are shown.
Immunization with stressed apoptotic 12B1-D1 cells induces tumor-specific CTLs.
BALB/c were immunized with Mit-C–treated 12B1-D1 cells that were mixed with 20 μg/mouse liver lysate, liver-derived HSP70, or CRCL on days −14 and −7. For controls, mice were immunized with an equal number of Mit-C–treated 12B1-D1 cells or saline. On day −2, splenocytes of the immunized mice were harvested and cocultured with Mit-C–treated 12B1-D1 cells for 5 days. Stimulated effector cells were tested for (A) cytolytic activity against 12B1-D1 cells, (B) parental 12B1 cells, or (C) A20 cells by nonradioactive cytotoxicity assay. Representative data from 1 of 3 experiments are shown.
The 12B1-D1 cell line is derived from 12B1 by transfection of a plasmid encoding a death construct as described previously.7 12B1 cells therefore may contain all other tumor antigens of 12B1-D1 except the plasmid products. One of the advantages of using apoptotic cells as a vaccine is that they should contain the whole repertoire of tumor antigens. Theoretically, the CTLs induced by CRCL adjuvant plus apoptotic 12B1-D1 cells should also lyse 12B1 cells. We demonstrated that the CTL activity induced by CRCL adjuvant plus apoptotic 12B1-D1 cells was specific and potent enough to lyse the parental 12B1 targets (Figure 7B). Furthermore, we used another leukemia cell line, A20, as a target for CTLs to confirm the specificity of the CTL activity. Figure7C shows that no lysis above background was detected when A20 cells were used as targets, confirming that the CTL activity induced by CRCL adjuvant-apoptotic cells was tumor specific. In summary, our data provide direct evidence that apoptotic leukemia cells, when combined with syngeneic tissue-derived CRCL as adjuvant, induced potent and specific CTLs in vivo.
CRCL adjuvant-apoptotic tumor cells induce long-term, specific immunity
Finally, we investigated whether the antitumor immunity induced by vaccination with CRCL adjuvant plus apoptotic tumor cells was long lasting. Mice surviving vaccination with either liver-derived or 12B1 tumor-derived CRCL adjuvant plus apoptotic 12B1-D1 cells were rechallenged with a lethal dose of 12B1-D1, 12B1, or A20 tumor cells 56 or 80 days after the initial challenge. All these mice rejected the rechallenge of 12B1-D1 tumor cells (Figure8A). In contrast, age-matched naive mice developed 12B1-D1 tumor rapidly (Figure 8A). The long-term immunity also held true when mice were rechallenged with the parental 12B1 tumor (Figure 8B), but not when challenged with A20 B-cell leukemia (Figure8C), confirming that the antitumor immunity induced by CRCL adjuvant plus 12B1-D1 apoptotic cells was long lasting, potent, and tumor-specific.
Vaccination with stressed apoptotic 12B1-D1 cells induces long-term, specific antitumor immunity.
Naive and surviving mice were rechallenged 56 or 80 days later with 2 × 104 (LD100) 12B1-D1 cells (A), or 103 (LD100) parental 12B1 cells (B), or 106 (LD100) A20 cells (C) in the groin. (In 12B1 or 12B1-D1 rechallenged mice, P < .05 from day 15 onward; in A20 rechallenged mice, P = NS; n = 8-16 surviving and 8 naive mice, pooled data from 2 experiments.)
Vaccination with stressed apoptotic 12B1-D1 cells induces long-term, specific antitumor immunity.
Naive and surviving mice were rechallenged 56 or 80 days later with 2 × 104 (LD100) 12B1-D1 cells (A), or 103 (LD100) parental 12B1 cells (B), or 106 (LD100) A20 cells (C) in the groin. (In 12B1 or 12B1-D1 rechallenged mice, P < .05 from day 15 onward; in A20 rechallenged mice, P = NS; n = 8-16 surviving and 8 naive mice, pooled data from 2 experiments.)
Discussion
The deficiency of self/non-self paradigms led to new hypotheses proposed by Janeway24,25 and Matzinger26,27to explain the immune response. The immune system is thought to react to “danger” associated with certain molecules of infectious organisms, or cell products released during tissue damage or stress.17 In addition to antigens that can be acquired, processed, and presented by APC, these danger signals are needed to activate local APCs, potentiating the immune responses against the antigens. In our studies, we found that 12B1-D1 leukemia cells, induced to undergo apoptosis by either AP20187 or Mit-C, were poorly immunogenic. In vivo immunization using these apoptotic cells induced no detectable antitumor immunity. In vitro, apoptotic 12B1-D1 cells had poor immunostimulatory activities on DCs in terms of inducing IL-12 production and enhancing imunostimulatory functions of DCs.8 Our results are consistent with other studies.28,29 Therefore, our data contribute the notion that apoptotic cells are, by default, nonimmunogenic to the immune system, because normal cell turnover must not induce active immune responses. Although apoptotic tumor cells can be efficiently taken up and their antigens presented by APCs,4,30 an active immune response is seldom generated in vivo because of lack of danger signals. However, when apoptotic cells are under stress, such as heat-stress apoptosis7 or pathogen-induced apoptosis,23,31,32 they are able to induce potent immune responses. How can the immune system recognize “stressful” apoptotic cells? We found that heat-stressed apoptotic 12B1-D1 cells expressed HSPs on their surface7 and these stressed apoptotic cells activated DCs.8 Because HSPs are emerging to be key danger signals to the immune system,17 we hypothesized that, by providing exogenous HSPs, the default (immune silent) pathway of apoptotic cells could be bypassed.
To test our hypothesis, we purified HSP70 (constitutive form) and enriched CRCL from a syngeneic naive mouse liver. In vivo immunization with liver-derived CRCL provided no protection against subsequent autologous 12B1-D1 tumor challenge (data not shown). In addition, repeated injection of these syngeneic tissue components into BALB/c mice resulted in no apparent autoimmune phenomena (data not shown). However, when we coinjected mice with HSP70 and apoptotic tumor cells, antitumor immunity and specific CTLs were generated. We therefore demonstrated that HSP70 derived from naive mouse liver had adjuvant effects enhancing the immunogenicity of apoptotic tumor cells. Danger signals provided by HSP70, together with antigens from apoptotic tumor cells, induced specific antitumor immunity.
Tumor-derived HSP70 has been used as a cancer vaccine against a variety of tumors.13,14 The antigen-chaperoning properties of HSP were highlighted in the tumor vaccine setting by the work of Srivastava.9 How does this soluble tumor-derived HSP70, with its chaperoned peptides, elicit strong cell-mediated antitumor immunity in the absence of adjuvants? Blachere et al showed for different peptide antigens (viral and nonviral CTL epitopes) that vaccination with gp96 or HSP70 induced a peptide-specific CTL response, whereas vaccination with peptides alone did not.33 Ciupitu et al reported that vaccination with HSP70/lymphocytic choriomeningitis virus (LCMV) peptide complexes elicited LCMV-specific CTLs and protective immunity against LCMV.34 Our results demonstrate that autologous naive mouse liver–derived HSP70, devoid of tumor antigens, can serve as an adjuvant to enhance the immunogenicity of apoptotic tumor cells.
We have demonstrated that vaccination with tumor-derived CRCL induced specific antitumor immunity against autologous tumor challenge in a variety of mouse models.11,12 In all models, we found that CRCL was generally more effective than purified individual HSPs in generating antitumor immunity. We also demonstrated that tumor-derived CRCL had a stronger ability to stimulate DCs than purified HSP70 or gp96 (Y.Z. et al, manuscript submitted). Here, we have documented that naive mouse liver–derived CRCL has superior adjuvant effects when compared to HSP70 derived from the same tissue. This may partially explain why tumor-derived CRCL is generally superior to individual HSPs in terms of inducing antitumor immunity.11,12 A secondary “danger signal” is apparently important to activate APCs, which consequently activate specific T cells. Why liver-derived CRCL provides better adjuvant effects than does purified liver HSP70 is an area of active research in our laboratory. One possible explanation is that CRCL contains multiple HSPs or chaperone proteins that synergistically activate APCs. Several studies have documented that APCs bind and internalize gp96 through receptor-mediated endocytosis, which leads to MHC class I–restricted representation of gp96-chaperoned peptides and CTL activation.35,36 The CD91 molecule (α2-macroglobulin receptor), which was initially identified as a protein related to the low-density lipoprotein receptor, has been recently shown to be a cell surface receptor for gp96.37 HSP60 was reported38 to be a putative ligand of the toll-like receptor 4 (TLR-4) complex and triggers TLR-2 and TLR-4 to activate the toll/IL-1 receptor signaling pathway in innate immune cells.39 Exogenous HSP60 has also been shown to stimulate macrophages to express IL-12 and IL-15 and rapidly release tumor necrosis factor α (TNF-α).40HSP70 can assume dual roles as a chaperone and a cytokine41 to activate monocytes and up-regulate the expression of proinflammatory cytokines. CRCL is enriched for multiple stress proteins, including HSP60, 70, and gp96,10 12 which may bind to a distinct set of receptors on APCs and trigger different signaling pathways, resulting in synergistic effects on APCs. In fact, we have demonstrated that tumor-derived CRCL has superior immunostimulatory activities on DCs (compared to HSP70), increasing DC costimulatory molecule expression and production of IL-12 (Y.Z. et al, manuscript submitted).
Cell-mediated immunity, which is particularly important in suppressing tumors, is characterized by production of type I cytokines, activation of macrophages, and induction of CTLs. Previous reports have shown that apoptotic cells are associated with induction of type II immune suppressive cytokines, such as transforming growth factor β (TGF-β) and IL-10.42,43 In our studies, we found that vaccination with CRCL adjuvant plus apoptotic tumor cells induced IL-2 and IFN-γ production. This indicates that CRCL steers the immune system toward a TH1-type response, which is critical in suppressing tumors.44
CTLs are particularly important in tumor immunity.22Several reports have shown that professional APCs can acquire antigens from apoptotic bodies and cross-prime CTLs in vitro.4 23However, evidence that those apoptotic cells prime CTLs in vivo remains limited. We found that vaccination with CRCL adjuvant plus apoptotic tumor cells induced potent and specific CTLs, which appear to play an important role against 12B1-D1 tumor. The CTLs induced by vaccination with CRCL adjuvant plus apoptotic 12B1-D1 cells also lysed parental 12B1 cells. In fact, when we depleted CD8+ cells by using specific antibodies, we found that the antitumor immunity was partially abolished, suggesting that CD8+ T cells played an important role in tumor killing in vivo. This antitumor immunity was not abolished completely by depletion of CD8+ T cells alone, indicating that CD4+ T cells through a direct or indirect action contributed to the immune response. Accordingly, we found that depletion of CD4+ T cells significantly impaired the antitumor immunity, indicating that CTLs require CD4+ help.
It is surprising that coinjection of liver lysate with apoptotic tumor cells did not induce antitumor immunity in vivo (data not shown). The pathologic necrotic cell death has been proposed to be dangerous to the immune system, because it releases cellular components, such as HSPs, mitochondria, double-strained DNA, among others, which may act as danger signals.29,45 It is possible that the lysate, generated simply by several cycles of freeze-thaw, cannot represent the true necrotic cell death occurring in pathologic conditions. In addition, the local concentrations of these “danger signals” released from dying cells may be important.17,46 It has been shown that tumor immunogenicity is associated with increased expression of HSP70 when tumor cells are undergoing necrotic death.47 Tumors that were genetically modified to express HSP or when exogenous HSP70 was provided during tumor cell killing decreased the immunosuppressive cytokine IL-10 expression.48 Furthermore, lysate from primary cells contains less HSP than their transformed counterparts and fail to mature DCs.46 CRCL, which contains at least a 20-fold of enrichment of major HSPs10 12 appears to provide a higher concentration of local danger signals.
Currently, chemotherapy and radiotherapy remain the main treatment modalities for many cancers. Most of these therapies are thought to induce tumor cells to undergo apoptosis.49 These apoptotic tumor cells are attractive tumor antigen sources. However, without proper danger signals, they are largely ignored by the immune system, or may even induce tolerance.4,42,50 We have demonstrated that CRCL, enriched from normal or tumor tissues, provides potent adjuvant effects for enhancing the immunogenicity of apoptotic tumor cells that can induce potent, long-lasting antitumor immunity. Using FS-IEF, a relatively simple and rapid method, one can enrich large quantities of chaperone proteins from tissues in a less laborious and time-consuming manner compared to conventional purification of individual HSPs.10 12 These findings, together with the superior adjuvant effects, confer significant advantages of CRCL in terms of clinical applications.
The authors wish to thank Dr Douglas Lake for his helpful comments.
Prepublished online as Blood First Edition Paper, August 22, 2002; DOI 10.1182/blood-2002-05-1580.
Supported in part by the Leukemia and Lymphoma Society and the Tee up for Tots Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Emmanuel Katsanis, University of Arizona, Department of Pediatrics, 1501 N Campbell Ave, PO Box 245073, Tucson, AZ 85724; e-mail: katsanis@peds.arizona.edu.





![Fig. 6. Immunization with apoptotic 12B1-D1 cells plus CRCL adjuvant induces IL-2 and IFN-γ secretion and T-cell proliferation of splenocytes. / 12B1-D1 cells were treated with 100 μg/mL Mit-C for 1 hour and then extensively washed. Then 20 μg/mouse liver lysate, liver-derived HSP70, or CRCL was added to the cells and the mixture was injected to BALB/c mice subcutaneously on days −14 and −7. Control mice were immunized with an equal number of Mit-C–treated 12B1-D1 cells or saline. Five days after the secondary immunization, splenocytes of the immunized mice were harvested and restimulated with Mit-C–treated 12B1-D1 cells. (A) CTLL-2 bioassay was used to determine the IL-2 production. (B) IFN-γ secretion was determined by ELISPOT. (C) T-cell proliferation was determined by [3H]-thymidine incorporation. (Representative data from 1 of 3 experiments are shown.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/1/10.1182_blood-2002-05-1580/6/m_h80133618006.jpeg?Expires=1765016194&Signature=Wm1bS6fdxZQEqmiY82ZsJuSv6~Jppl20bog18ZrMFC4aq9gAp14zyNXwb90chAbexhh~3wYMEOWoTaLJBUrKJXPkxDfSrkdh6wRz2FhWoxm8jW1ny9Hw~ANxjb0VXyktiRUFHaf7uvuPHbQ2Q7~bhhTodOHPsf5rjyzghfQfMJns1gcAFQfSFJ5sS7RSIY8f-3xiE22hXBWe1LSnoJvmvSH~mwPTaUrrExiCHVYTAsJobvJdJjZKcgOqyMCfEFujoAOd-6Kr-cZwFf7LqEXh67kCtr0yNOxxUAVxUEFH~12ikotDPnw8NHtE9KoINcKpZkvW7wepO8XNAOEitqcbJQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal