Fanconi anemia is an autosomal recessive disorder characterized by aplastic anemia, cancer susceptibility, and cellular sensitivity to mitomycin C. The 6 known Fanconi anemia gene products (FANCA, FANCC, FANCD2, FANCE, FANCF, and FANCG proteins) interact in a common pathway. The monoubiquitination and nuclear foci formation of FANCD2 are essential for the function of this pathway. FANCA, FANCC, FANCG, and FANCF proteins form a multisubunit nuclear complex (FA complex) required for FANCD2 monoubiquitination. Because FANCE and FANCC interact in vitro and FANCE is required for FANCD2 monoubiquitination, we reasoned that FANCE is a component of the FA complex in vivo. Here we demonstrate that retroviral transduction of Fanconi anemia subtype E (FA-E) cells with the FANCE cDNA restores the nuclear accumulation of FANCC protein, FANCA–FANCC complex formation, monoubiquitination and nuclear foci formation of FANCD2, and mitomycin C resistance. Hemagglutinin (HA)-tagged FANCE protein localizes diffusely in the nucleus. In normal cells, HA-tagged FANCE protein coimmunoprecipitates with FANCA, FANCC, and FANCG but not with FANCD2. Our data indicate that FANCE is a component of the nuclear FA complex in vivo and is required for the monoubiquitination of FANCD2 and the downstream events in the FA pathway.
Introduction
Fanconi anemia (FA) is an autosomal recessive cancer susceptibility syndrome characterized by childhood-onset aplastic anemia, multiple congenital anomalies, and cellular sensitivity to DNA cross-linking agents, such as mitomycin C (MMC) and diepoxybutane.1 FA can be divided into at least 8 complementation groups (FA-A, FA-B, FA-C, FA-D1, FA-D2, FA-E, FA-F, and FA-G).2,3 Six FA genes (FANCA, FANCC, FANCD2, FANCE, FANCF, and FANCG) have been cloned.1Products of these 6 FA genes have no homology with other known proteins, and they do not have homology to each other. Because the clinical phenotypes of FA patients for any complementation group are similar, these proteins appear to cooperate in a common pathway.
FANCA, FANCC, FANCF, and FANCG proteins assemble in a multisubunit nuclear complex (FA protein complex).4-7Formation of the FA protein complex is impaired in FA-A, -B, -C, -E, -F, and -G cells but is intact in FA-D1 and FA-D2 cells.5,8 The FA protein complex is required for the monoubiquitination of FANCD2 on lysine 561 (Lys561), and this monoubiquitination is essential for function of the FA pathway and for MMC resistance.9 In non-FA cells or in FA-D1 cells, FANCD2 is monoubiquitinated and translocated to nuclear foci containing BRCA1 in response to DNA damage, such as ionizing radiation (IR), ultraviolet light (UV), or MMC treatment. In FA cells, except for the FA-D1 subtype,7 FANCD2 is not monoubiquitinated and does not form nuclear foci. Thus, the FANCD2 protein is downstream of FA protein complex formation. The product of the FANCD1 gene, which has not yet been cloned, may act further downstream in the pathway or in another pathway. BRCA1 is also required for the efficient monoubiquitination of FANCD2, and BRCA1-deficient cells, like FA cells, are hypersensitive to MMC.9 10 Taken together, these findings suggest that the FA pathway is involved in the DNA damage response in the nucleus.
The recently cloned FANCE gene encodes a protein of 536 amino acids, which has no homology to known proteins but contains 2 putative nuclear localization signals.11 The gene has 10 exons and maps to human chromosome 6p21.2 to 6p21.3. In the yeast 2-hybrid system, FANCE interacts with FANCC strongly, and with FANCA and FANCG weakly.12 Furthermore, in vitro–translated FANCE coimmunoprecipitates with in vitro–translated FANCC, suggesting that FANCE is a member of the FA protein complex.12However, the characterization of FANCE protein in vivo and its possible role in the FA pathway has not yet been described.
In the current study, we examined the FANCE protein in normal and FA cells. We found that hemagglutinin (HA)-tagged FANCE protein is localized predominantly in the nucleus and coimmunoprecipitates with FANCA, FANCC, and FANCG but not with FANCD2. Retroviral transduction of the FANCE cDNA in FA-E cells restored monoubiquitination and nuclear foci formation of the downstream protein, FANCD2. These results are consistent with the hypothesis that FANCE is a component of the multisubunit nuclear FA complex, required for FANCD2 activation.
Materials and methods
Cell lines and culture conditions
Epstein-Barr virus (EBV)–transformed lymphoblasts were maintained in RPMI 1640 medium supplemented with 15% heat-inactivated fetal calf serum (FCS) and grown in a humidified 5% CO2-containing atmosphere at 37°C. A wild-type (normal adult) lymphoblast line (PD7) and FA lymphoblast lines (FA-A [HSC72], FA-B [HSC230], FA-C [PD-4], FA-D1 [HSC62], FA-D2 [PD-20], FA-E [EUFA130, EUFA410, and EUFA622], FA-F [EUFA121], and FA-G [EUFA316]) have been previously described.8,11 13-16Simian virus 40 (SV40)–transformed FA fibroblasts, GM0637 (wild type), GM6914 (FA-A), PD426 (FA-C), FAG326SV (FA-G), and PD20F (FA-D2), as well as HeLa cells, were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 15% FCS.
Retroviral infection and MMC sensitivity assays
The retroviral expression vector, pMMP-puro,17 was described previously. FANCE cDNA11 (generously provided by J. deWinter and H. Joenje, Department of Clinical Genetics and Human Genetics, Free University Medical Center, Amsterdam, The Netherlands) was subcloned into the retroviral vector pMMP-puro.17pMMP-puro-HA-FANCE was generated by adding the influenza HA tag (AYPYDVPDYA) at the amino terminus of FANCE. Production of pMMP retroviral supernatants and infection of fibroblasts or HeLa cells were performed as previously described.18 After 48 hours, cells were trypsinized and selected in medium containing puromycin (1 μg/mL). Dead cells were removed, and surviving cells were grown under continuous selection in puromycin.
FA lymphoblasts were infected with the various pMMP supernatants for 6 hours in the presence of 8 μg/mL polybrene (Sigma Chemical, St Louis, MO). Infected cells were washed free of viral supernatant and resuspended in growth media. After 48 hours the cells were transferred to media containing 1 μg/mL puromycin. Dead cells were removed over Ficoll-Paque Plus (Amersham Pharmacia, Uppsala, Sweden) cushion after 5 days, and the surviving cells were grown under continuous selection in puromycin. MMC sensitivity assays for lymphoblasts were performed as previously described.16
Antibodies
Immunoprecipitation
Whole-cell extracts were prepared in lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% (vol/vol) Triton X-100) supplemented with protease inhibitors (1 μg /mL leupeptin and pepstatin, 2 μg /mL aprotinin, 1 mM phenylmethylsulfonylfluoride [PMSF]) and phosphatase inhibitors (1 mM sodium orthovanadate, 10 mM sodium fluoride). Using the polyclonal antibodies to FANCD2 (E35), FANCA, FANCC, and FANCG, immunoprecipitation (IP) was performed essentially as described,4 except that each IP was normalized to contain 2 mg protein. As a negative control, preimmune rabbit serum was used.
Immunoblotting
Cells were lysed with 1× sample buffer (50 mM Tris-HCl, pH 6.8, 86 mM 2-mercaptoethanol, 2% sodium dodecyl sulfate [SDS]), boiled for 5 minutes, and subjected to 7.0% or 7.5% (10.0% for FANCF detection) SDS–polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, proteins were transferred to nitrocellulose membranes using a submerged transfer apparatus (BioRad, Hercules, CA) filled with 25 mM Tris base, 200 mM glycine, and 20% methanol. After blocking with 5% nonfat dried milk in TBS-T (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% Tween20), the membrane was incubated with the primary antibody diluted in TBS-T (1:1000 dilution), washed extensively, and incubated with the appropriate horseradish peroxidase–linked secondary antibody (Amersham, Piscataway, NJ). Chemiluminescence was used for detection.
Subcellular fractionation
Cells were fractionated into cytoplasmic and nuclear proteins by hypotonic swelling. Cells were incubated in buffer A (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.9, 10 mM KCl, 0.1 mM EDTA [ethylenediaminetetraacetic acid], 0.1 mM EGTA [ethyleneglycoltetraacetic acid], 1 mM PMSF, 1 mM dithiothreitol [DTT]) on ice for 15 minutes, and NP40 (0.6% final concentration) was added. Nuclei were pelleted by centrifugation at 14 000 rpm in a microcentrifuge at 4°C for 5 minutes. The supernatant (cytoplasmic fraction) was transferred to a new tube. Nuclei were washed once with buffer A and were lysed in buffer B (20 mM HEPES, pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF) by repetitive pipetting and were incubated on ice for 15 minutes. Then the nuclear lysates were centrifuged at 14 000 rpm at 4°C for 5 minutes. The supernatant was used as nuclear fraction. Nuclear and cytoplasmic fractions were equalized to 50% buffer A and 50% buffer B. Fractionation was checked by Western blotting with anti–β-tubulin antibody (Ab-1; Oncogene, Boston, MA) and anti–topoisomerase II antibody (Ab-1; Oncogene) as cytoplasmic and nuclear markers, respectively.
Generation of DNA damage
Gamma irradiation was delivered using a Gammacell 40 apparatus (MDS Nordion, Ottawa, Canada). For mitomycin C (Sigma Chemical) treatment, cells were continuously exposed to the drug for the indicated time.
Immunofluorescence microscopy
Cells were fixed with 2% paraformaldehyde in phosphate-buffered saline (PBS) for 20 minutes, followed by permeabilization with 0.2% Triton X-100 in PBS (3 minutes). Specific antibodies were added at the appropriate dilution in 3% bovine serum albumin/0.05% Triton X-100/0.04% sodium azide/PBS and were incubated for 1 hour at room temperature. FANCD2 was detected using the affinity-purified E35 polyclonal antibody (1/200). For HA detection, a commercial monoclonal antibody (HA.11; Babco) (1/200) was used. Cells were subsequently washed 3 times in PBS, and species-specific fluorescein or Texas red-conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA) were diluted in 3% bovine serum albumin/0.05% Triton X-100/0.04% sodium azide/PBS (antimouse 1/500, antirabbit 1/500) and were added. After 30 minutes at room temperature 3 more washes were applied, and the nuclei were counterstained with DAPI (4,6 diamidino-2-phenylindole) diluted in PBS at 1 μg/mL for 5 minutes. Three more washes were applied, and the slides were mounted in Vectashield (Vector Laboratories, Burlingame, CA). Images were captured on a Nikon microscope (Tokyo, Japan) and processed using Adobe Photoshop software (Adobe, San Jose, CA).
Results
FANCE is required for monoubiquitination of FANCD2 and assembly of FANCD2 nuclear foci
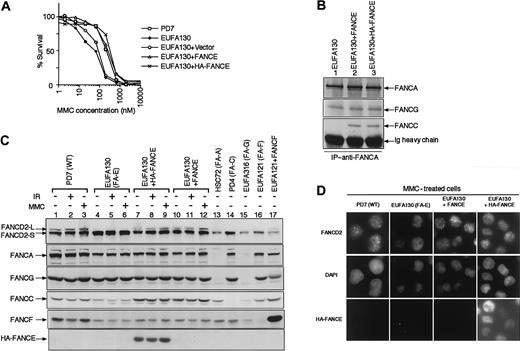
Initially, we tested the function of the FANCE cDNA expressed in FA-E lymphoblasts (Figure 1). Consistent with previous studies,11 retroviral transduction with pMMP-puro FANCE or pMMP-puro HA-FANCE corrected the MMC sensitivity of 3 independent FA-E lymphoblast cell lines—EUFA130 (Figure 1A), EUFA410, and EUFA622 (data not shown).
FANCE is required for function of the Fanconi anemia pathway.
(A) Complementation of MMC sensitivity of an FA-E lymphoblast cell line, EUFA130, with pMMP puro FANCE or pMMP puro HA-FANCE. The indicated retroviral supernatants were generated and used to transduce FA lymphoblast lines. Puromycin-resistant cells were selected, and MMC sensitivity was determined as described in “Materials and methods.” (B) Reintroduction of FANCE or HA-FANCE in FA-E lymphoblasts restores the FANCA/FANCC interaction. Whole-cell extracts were generated from the lymphoblast lines, EUFA130, EUFA130+FANCE, and EUFA130+HA-FANCE. Equal amounts of protein from each extract (2 mg) were used for immunoprecipitation with affinity-purified anti-FANCA antibody. Immune complexes were resolved by SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted with anti-FANCA, anti-FANCC, or anti-FANCG antibody. (C) FANCE is required for the monoubiquitination of FANCD2. Whole-cell extracts were prepared from the indicated lymphoblast cell lines. Some cell lines were harvested 8 hours after treatment with IR (15 Gy) or 24 hours after continuous exposure to MMC (40 ng/mL), as indicated. Cellular proteins were immunoblotted with anti-FANCD2, anti-FANCA, anti-FANCG, anti-FANCC, anti-FANCF, or anti-HA antibody. FANCD2-S (short) is a nonubiquitinated isoform, and FANCD2-L (long) is a monoubiquitinated isoform of FANCD2. (D) FANCE is required for nuclear foci formation of FANCD2. The indicated lymphoblasts treated with MMC (40 ng/mL) for 24 hours were double-immunostained with anti-FANCD2 and anti-HA antibodies. In FA-E lymphoblasts (EUFA130), FANCD2 is expressed in a diffuse nuclear pattern. Correction of FA-E cells with either FANCE cDNA or HA-FANCE cDNA restores FANCD2 nuclear foci. Counterstains for the DNA-specific dye, DAPI, are shown in the middle panels. Expression of HA-FANCE was confirmed, as shown in the lower panels. Original magnification, × 600.
FANCE is required for function of the Fanconi anemia pathway.
(A) Complementation of MMC sensitivity of an FA-E lymphoblast cell line, EUFA130, with pMMP puro FANCE or pMMP puro HA-FANCE. The indicated retroviral supernatants were generated and used to transduce FA lymphoblast lines. Puromycin-resistant cells were selected, and MMC sensitivity was determined as described in “Materials and methods.” (B) Reintroduction of FANCE or HA-FANCE in FA-E lymphoblasts restores the FANCA/FANCC interaction. Whole-cell extracts were generated from the lymphoblast lines, EUFA130, EUFA130+FANCE, and EUFA130+HA-FANCE. Equal amounts of protein from each extract (2 mg) were used for immunoprecipitation with affinity-purified anti-FANCA antibody. Immune complexes were resolved by SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted with anti-FANCA, anti-FANCC, or anti-FANCG antibody. (C) FANCE is required for the monoubiquitination of FANCD2. Whole-cell extracts were prepared from the indicated lymphoblast cell lines. Some cell lines were harvested 8 hours after treatment with IR (15 Gy) or 24 hours after continuous exposure to MMC (40 ng/mL), as indicated. Cellular proteins were immunoblotted with anti-FANCD2, anti-FANCA, anti-FANCG, anti-FANCC, anti-FANCF, or anti-HA antibody. FANCD2-S (short) is a nonubiquitinated isoform, and FANCD2-L (long) is a monoubiquitinated isoform of FANCD2. (D) FANCE is required for nuclear foci formation of FANCD2. The indicated lymphoblasts treated with MMC (40 ng/mL) for 24 hours were double-immunostained with anti-FANCD2 and anti-HA antibodies. In FA-E lymphoblasts (EUFA130), FANCD2 is expressed in a diffuse nuclear pattern. Correction of FA-E cells with either FANCE cDNA or HA-FANCE cDNA restores FANCD2 nuclear foci. Counterstains for the DNA-specific dye, DAPI, are shown in the middle panels. Expression of HA-FANCE was confirmed, as shown in the lower panels. Original magnification, × 600.
We next examined the effect of the FANCE cDNA on the interaction of the FANCA and FANCC proteins in FA-E lymphoblasts (Figure 1B). In an FA-E cell line, EUFA130, FANCA/FANCC binding was not observed (Figure 1B, lane 1). In contrast, in EUFA130 corrected with pMMP-puro FANCE or pMMP-puro HA-FANCE, FANCA/FANCC binding was restored (Figure 1B, lanes 2 and 3). The FANCA/FANCG interaction was observed, irrespective of the presence of (HA-) FANCE expression (lane 1).
We have previously shown that wild-type cells express 2 isoforms of FANCD2 protein: FANCD2-S (nonubiquitinated form) and FANCD2-L (monoubiquitinated form).9 Monoubiquitination of FANCD2 occurs on Lys561. Treatment with IR, UV, or MMC causes an increase of the FANCD2-L isoform. However, Fanconi anemia cells from FA-A, FA-C, FA-F, and FA-G subtypes express only FANCD2-S.9 Therefore, we examined the expression of FANCD2 isoforms in uncorrected and corrected FA-E cells with and without treatment with DNA damaging agents (Figure 1C). Wild-type lymphoblasts (PD7) and corrected FA-E lymphoblasts (EUFA130+HA-FANCE, or EUFA130+FANCE) expressed 2 isoforms of FANCD2 (FANCD2-S and FANCD2-L) (Figure 1C, lanes 1-3, 7-12). After cellular exposure to IR or MMC, a slight increase in FANCD2-L was observed. However, uncorrected FA-E cells (EUFA130) expressed only FANCD2-S (Figure 1C, lanes 4-6).
Correction with the FANCE cDNA did not affect the expression level of FANCA or FANCG protein in FA-E cells, but FANCC levels increased in corrected FA-E cells (Figure 1C, lanes 4-12). The expression of FANCF protein also increased slightly in corrected FA-E cells.
Previously we showed that the assembly of FANCD2 nuclear foci correlates with the existence of the monoubiquitinated isoform of FANCD2 (FANCD2-L). Cellular exposure to DNA-damaging agents causes an increase of the FANCD2 nuclear foci.9 Therefore, we examined FANCD2 nuclear foci formation immunocytochemically in FA-E and corrected FA-E cells. Consistently, FA-E cells showed no FANCD2 foci, and correction of FA-E cells with either pMMP-puro FANCE or pMMP-puro HA-FANCE restored the formation of nuclear FANCD2 foci (Figure 1D). Thus, reintroduction of (HA-) FANCE cDNA in FA-E cells restored MMC resistance, FANCA/FANCC binding, and monoubiquitination and nuclear foci formation of FANCD2.
Nuclear localization of HA-tagged FANCE protein
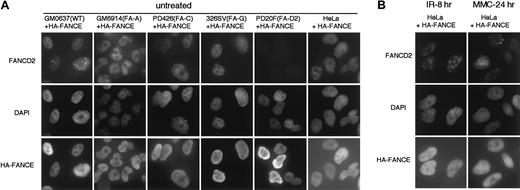
We also confirmed immunocytochemically the expression of HA-tagged FANCE in the corrected cells. HA-FANCE was localized mainly in the nuclei of the corrected cells (Figure 1D). We also generated HA-FANCE stably transfected fibroblasts and HeLa cells and confirmed the nuclear localization of HA-FANCE by immunocytochemistry. In normal fibroblasts (GM0637+HA-FANCE), FA-A fibroblasts (GM6914+HA-FANCE), FA-C fibroblasts (PD426+HA-FANCE), FA-G fibroblasts (326SV+HA-FANCE), FA-D2 fibroblasts (PD20F+HA-FANCE), or HeLa cells (HeLa+HA-FANCE), HA-FANCE stained diffusely in the nucleus (Figure2A). Thus, FANCE was located predominantly in the nucleus, irrespective of the expression of other FA proteins.
Nuclear localization of HA-tagged FANCE protein.
The indicated SV40-transformed fibroblasts (GM0637, GM6914, PD426, 326SV, and PD20F) or HeLa cells stably transfected with HA-FANCE were double-immunostained with anti-FANCD2 and anti-HA antibodies. Counterstains for the DNA-specific dye DAPI are shown in the middle panels. (A) Untreated cells were stained. (B) Cells 8 hours after treatment with IR (15 Gy) or cells treated with MMC (40 ng/mL) continuously for 24 hours are shown. Original magnification, × 600.
Nuclear localization of HA-tagged FANCE protein.
The indicated SV40-transformed fibroblasts (GM0637, GM6914, PD426, 326SV, and PD20F) or HeLa cells stably transfected with HA-FANCE were double-immunostained with anti-FANCD2 and anti-HA antibodies. Counterstains for the DNA-specific dye DAPI are shown in the middle panels. (A) Untreated cells were stained. (B) Cells 8 hours after treatment with IR (15 Gy) or cells treated with MMC (40 ng/mL) continuously for 24 hours are shown. Original magnification, × 600.
Next we examined the effect of DNA-damaging agents on the subcellular localization of HA-FANCE. HeLa cells stably transfected with HA-FANCE were treated with IR or MMC. Treatment with DNA-damaging agents caused an increase in FANCD2 nuclear foci, as previously described.9 HA-FANCE was located diffusely in the nucleus; however, no nuclear FANCE foci were observed, even after exposure to these agents (Figure 2B).
FANCE interacts with FANCA, FANCC, and FANCG in vivo
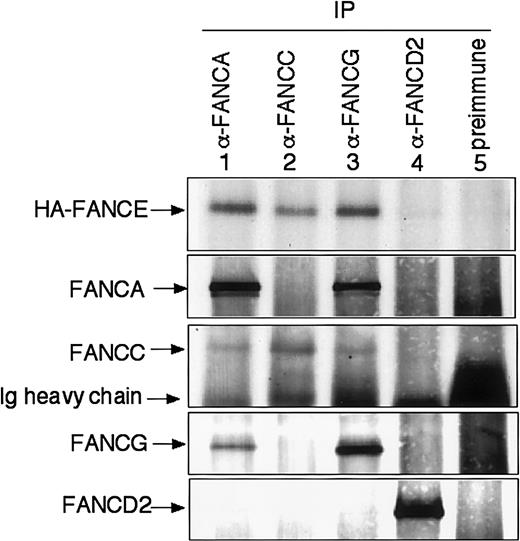
To test whether FANCE interacts with other FA proteins, coimmunoprecipitation experiments were performed using HeLa cells stably transfected with HA-FANCE. HA-FANCE was coimmunoprecipitated by anti-FANCA, anti-FANCC, or anti-FANCG antibodies (Figure 3, lanes 1-3) but not by anti-FANCD2 antibody or preimmune serum (Figure 3, lanes 4 and 5). These results indicate that FANCE interacts with other components of the FA complex in vivo.
HA-FANCE interacts with FANCA, FANCC, and FANCG in vivo.
Whole-cell extracts were generated from HeLa cells stably transfected with HA-FANCE. The same amount of protein from the extract (2 mg) was used for immunoprecipitation with affinity-purified anti-FANCA, anti-FANCC, anti-FANCG, anti-FANCD2 antibody, or preimmune serum, as indicated. Immune complexes were resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-FANCA, anti-FANCC, anti-FANCG, anti-FANCD2, or anti-HA antibody.
HA-FANCE interacts with FANCA, FANCC, and FANCG in vivo.
Whole-cell extracts were generated from HeLa cells stably transfected with HA-FANCE. The same amount of protein from the extract (2 mg) was used for immunoprecipitation with affinity-purified anti-FANCA, anti-FANCC, anti-FANCG, anti-FANCD2 antibody, or preimmune serum, as indicated. Immune complexes were resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-FANCA, anti-FANCC, anti-FANCG, anti-FANCD2, or anti-HA antibody.
FANCE is required for the nuclear accumulation of FANCC
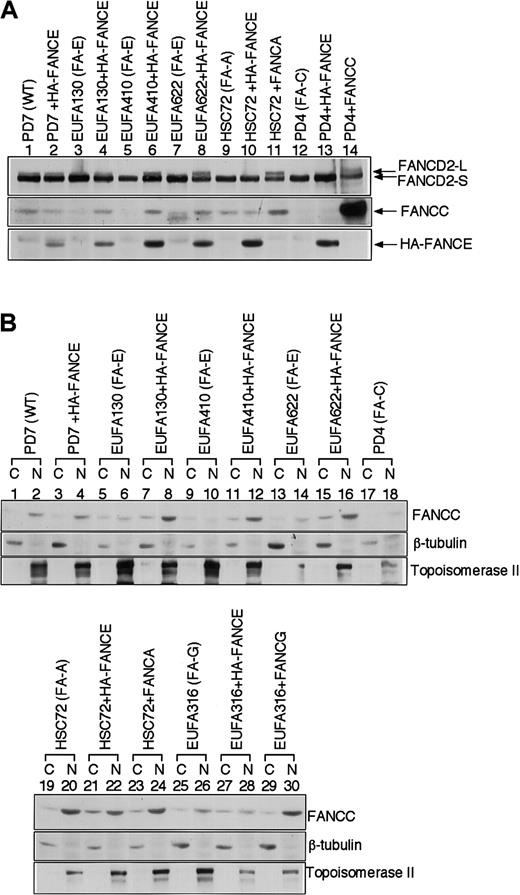
The expression level of FANCC was low in 3 independent FA-E cell lines (EUFA130, EUFA410, and EUFA622), and reintroduction of the (HA-) FANCE cDNA into these cell lines caused an increase of the expression levels of FANCC protein (Figure 1C lanes 4-12, and Figure4A, lanes 3-8). Next we examined the effect of FANCE expression on the subcellular localization of FANCC protein. In the absence of FANCE, FANCC protein levels were low in both the cytoplasm and nucleus (Figure 4B, lanes 5, 6, 9, 10, 13, 14). After reintroduction of HA-FANCE in these FA-E cells, FANCC protein levels increased, especially in the nucleus (Figure 4B, lanes 8, 12, and 16). In contrast, FANCC nuclear accumulation was independent of FANCA expression (Figure 4B, lanes 19-24). Absence of FANCG affected the nuclear accumulation of FANCC to a lesser extent (Figure 4B, lanes 25-30). Thus, FANCE is required for the nuclear accumulation of the FANCC protein.
FANCE is required for the nuclear accumulation of FANCC.
(A) Whole-cell extracts were prepared from the indicated lymphoblast cell lines. Cellular proteins were immunoblotted with anti-FANCD2, anti-FANCC, or anti-HA antibody. (B) Subcellular localization of FANCC in lymphoblast cell lines. Equal amounts of cytoplasmic (C) and nuclear (N) proteins from the indicated lymphoblast lines were immunoblotted with anti-FANCC, anti–β-tubulin, or anti–topoisomerase II antibody, as indicated.
FANCE is required for the nuclear accumulation of FANCC.
(A) Whole-cell extracts were prepared from the indicated lymphoblast cell lines. Cellular proteins were immunoblotted with anti-FANCD2, anti-FANCC, or anti-HA antibody. (B) Subcellular localization of FANCC in lymphoblast cell lines. Equal amounts of cytoplasmic (C) and nuclear (N) proteins from the indicated lymphoblast lines were immunoblotted with anti-FANCC, anti–β-tubulin, or anti–topoisomerase II antibody, as indicated.
Discussion
The 6 cloned FA proteins interact in a common cellular pathway, regulating the DNA damage response. Activation of FANCD2 to a monoubiquitinated isoform is regulated by the upstream multisubunit nuclear FA protein complex containing FANCA, FANCC, FANCF, and FANCG.6,9 FANCE-deficient cells lack the ability to monoubiquitinate FANCD2,9 and direct interaction between FANCE protein and FANCC protein has been shown in vitro.12These findings have led to the hypothesis that FANCE is also a component of the nuclear FA protein complex in vivo.
First we generated pMMP retroviral constructs with wild-type FANCE cDNA or HA-tagged FANCE cDNA. Both retroviruses corrected FA-E cells with respect to MMC sensitivity, the FANCA/FANCC interaction, FANCD2 monoubiquitination, and nuclear foci formation of FANCD2 (Figure 1). Fusing HA to the N terminus of FANCE did not affect FANCE function.
The reintroduction of (HA-) FANCE in FA-E cells restored FANCA/FANCC interaction (Figure 1B). FANCA/FANCC interaction is considered to be indirect and requires FANCA, FANCB, FANCC, FANCE, FANCF, and FANCG, but not FANCD1, or FANCD2.5,8 In contrast, the FANCA/FANCG interaction is direct5 12 and does not require other FA proteins. Our current findings are consistent with these previous observations.
The reintroduction of FANCE in FA-E cells restored the monoubiquitination and nuclear foci formation of FANCD2 (Figure 1C,D). We have previously shown a similar effect for the reintroduction of FANCA, FANCC, FANCF, and FANCG cDNA in FA-A, FA-C, FA-F, and FA-G cells, respectively.7 9 This finding supports a role of FANCE in the monoubiquitination of FANCD2 through its cooperation with other FA proteins.
We showed that HA-FANCE protein is localized to the nucleus, irrespective of the expression of other FA proteins (Figure 2A). Consistently, FANCE has 2 nuclear localization signals.11We examined the effect of DNA damage on the subnuclear localization of FANCE, and HA-FANCE was always detected diffusely in the nucleus. In contrast to FANCD2, we could not detect nuclear foci of HA-FANCE, even after treatment with DNA-damaging agents (Figure 2B). Absence of FANCE foci may result from the overexpression of exogenous HA-FANCE protein.
This study is the first demonstration of the in vivo interaction of FANCE and other components of the FA complex, including FANCA, FANCC, and FANCG (Figure 3). Our findings indicate that FANCE is a component of the FA protein complex. In the yeast 2-hybrid system, a strong interaction of FANCE and FANCC was observed. In addition, in vitro–translated FANCE and FANCC bind directly and coimmunoprecipitate.12 Coimmunoprecipitation of in vitro–translated FANCE and FANCA or FANCE and FANCG was not observed.12 The difference between the in vivo results and the in vitro results can be explained by the presence of other factors required for the interaction of FANCE and FANCA (or FANCG) in the cell lysates. We could not detect the interaction between FANCE and FANCD2 in vivo (Figure 3), consistent with the idea that FANCD2 is not a component but rather a substrate of the FA protein complex.
In 3 independent FA-E cell lines, the expression level of FANCC protein was consistently low. The reintroduction of FANCE into those cell lines caused an increase of the expression level of FANCC protein, especially in the nucleus, suggesting a critical role of the FANCE protein in the accumulation of FANCC in the nucleus (Figure 4).12 Because FANCC localizes to the cytoplasm and to the nucleus,16,19some investigators have proposed cytoplasmic functions of FANCC protein other than the formation of nuclear FA protein complex.20-23 However, our findings suggest that the nuclear accumulation of FANCC is important for its function in the FA pathway, though we cannot exclude other cytoplasmic functions of FANCC. In conclusion, our study indicates that FANCE is a component of the multisubunit nuclear FA protein complex and that it is required for the monoubiquitination and nuclear foci formation of FANCD2 and for MMC resistance.
We thank J. deWinter and H. Joenje for FANCE cDNA plasmid. We thank Maria Stotsky for technical assistance.
Prepublished online as Blood First Edition Paper, June 7, 2002; DOI 10.1182/blood-2002-03-0860.
Supported by National Institutes of Health grants RO1HL52725, RO1DK43889, PO1DK50654, and PO1HL54785 (A.D.D.). T.T. was supported by a grant from the Naito Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alan D. D'Andrea, Dana-Farber Cancer Institute, Department of Pediatric Oncology, Harvard Medical School, 44 Binney St, Boston, MA 02115; e-mail: alan_dandrea@dfci.harvard.edu.